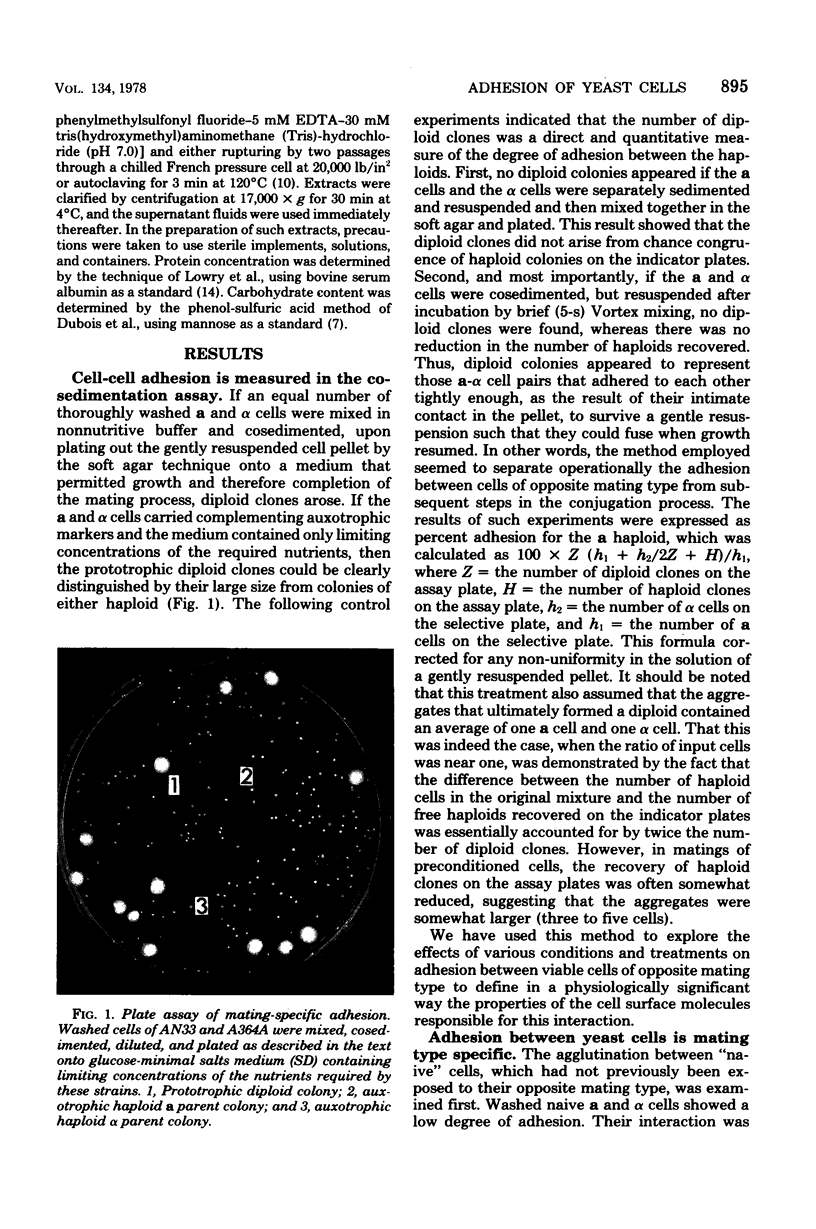
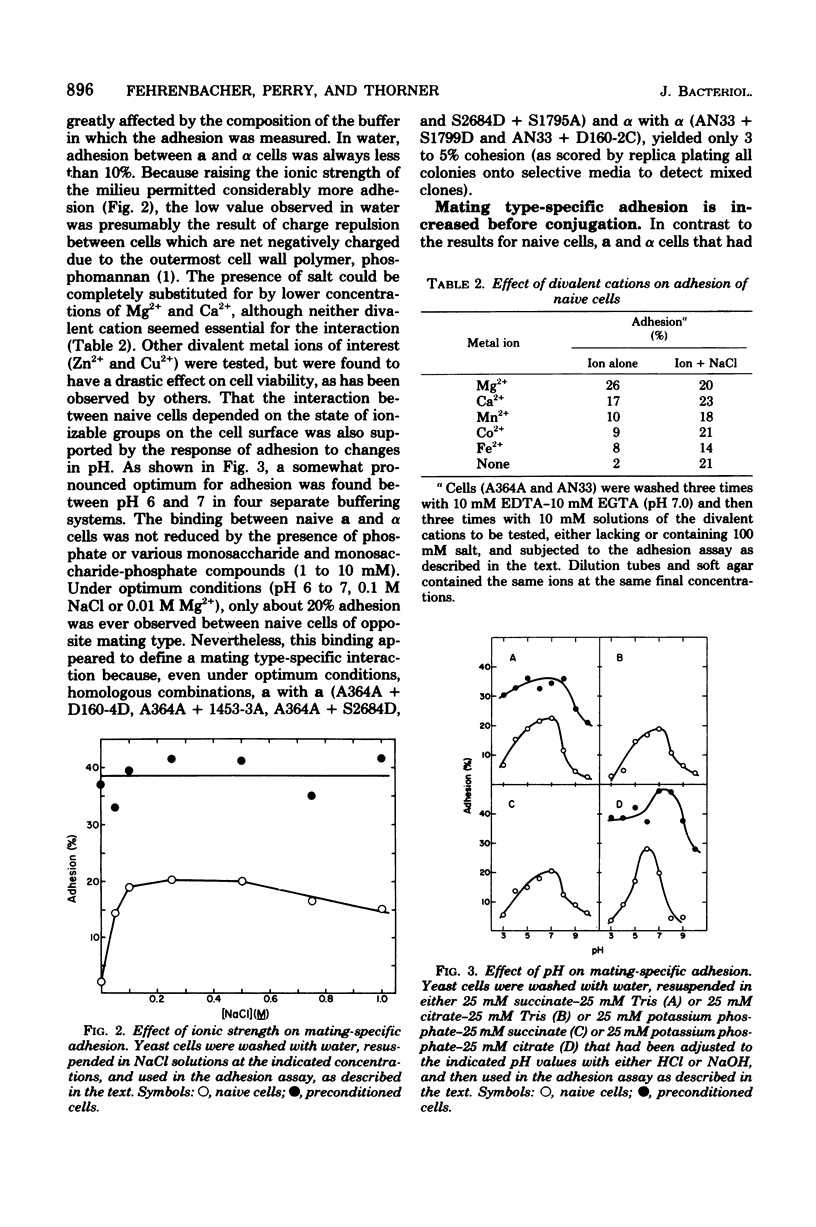
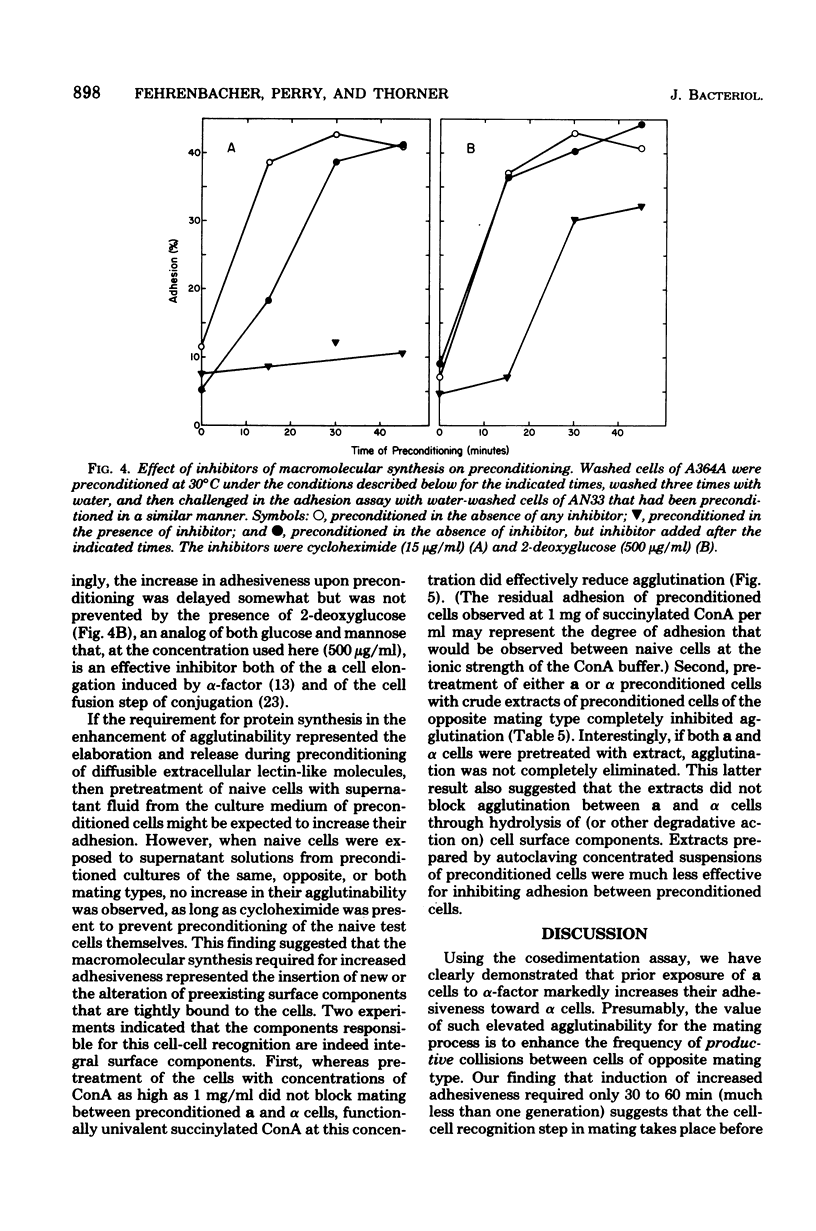
Abstract
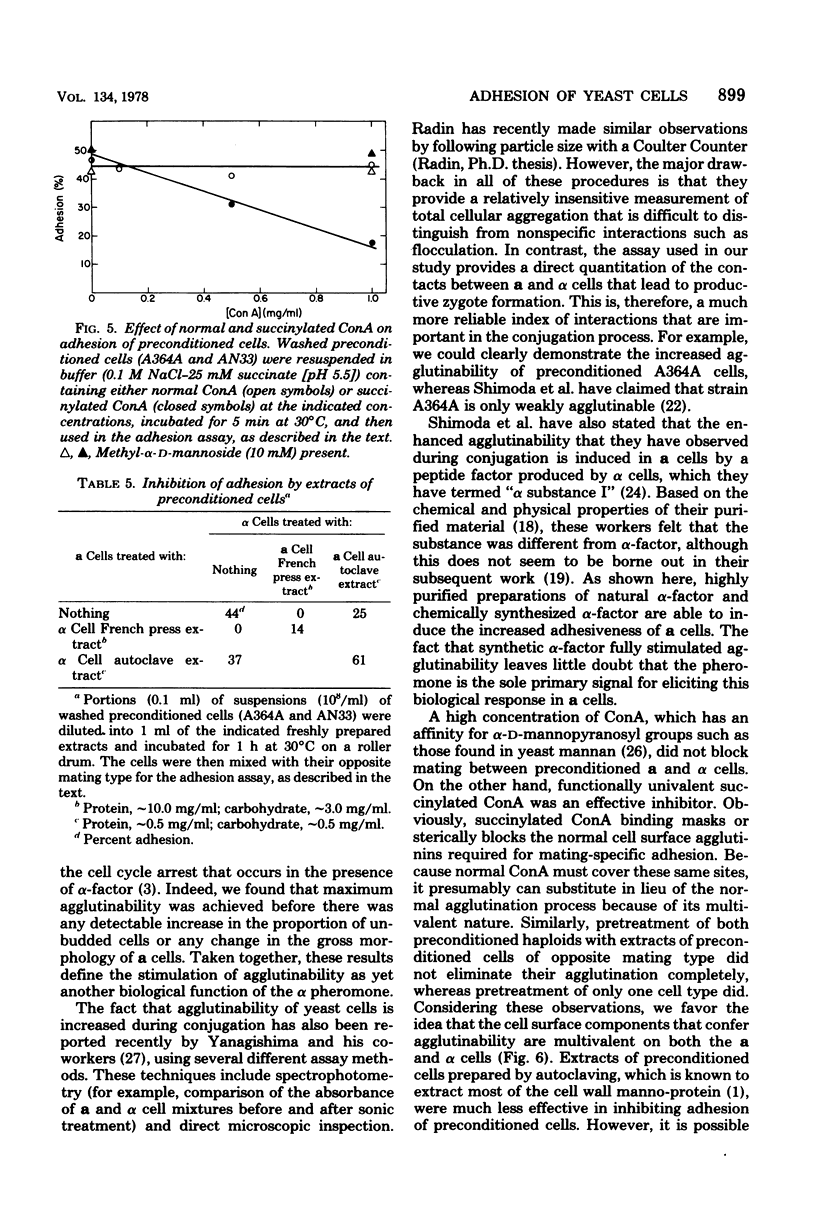
Mating-specific adhesion between haploid yeast cells of opposite mating type (a and α) was studied by using a quantitative agar plate assay. Washed a and α cells that had not previously been exposed to their respective opposite mating type (“naive” cells) adhered relatively weakly. In water, only 5 to 10% of the a cells stuck tightly enough to α cells to give rise subsequently to diploid clones on the assay plates. Under optimum conditions (pH 6 to 7, at least 0.1 M Nacl or 0.01 M Mg2+), there was about 20% adhesion. Nevertheless, this weak binding defined a mating type-specific interaction because, even under optimum conditions, the homologous interactions (a with a and α with α) yielded only 3 to 5% cohesion. In contrast to these results, washed cells that had been preincubated in the cell-free culture medium of their opposite mating type (“preconditioned” cells) adhered quite strongly. The degree of adhesion between preconditioned cells (40 to 50%) was essentially unaffected by extremes of ionic strength, pH, and temperature and by the absence of divalent cation. This strong interaction was also mating type specific since cohesion between preconditioned cells of like mating type was only about 5%. The increase in agglutinability was obtained if only the a cells were preconditioned and could be induced by highly purified preparations of natural or synthetically prepared α-factor, an oligopeptide pheromone released by the α cells. The appearance of increased adhesiveness was blocked by an inhibitor of RNA synthesis and by an inhibitor of protein synthesis, but not by an inhibitor of polysaccharide synthesis. Adhesion between preconditioned cells could be inhibited by pretreatment with functionally univalent succinylated concanavalin A or with extracts from preconditioned cells of the opposite mating type. These results confirm in a quantitative manner that the recognition between conjugating cells of S. cerevisiae is a developmentally regulated event that is under the control of the mating type locus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Betz R., MacKay V. L., Duntze W. a-Factor from Saccharomyces cerevisiae: partial characterization of a mating hormone produced by cells of mating type a. J Bacteriol. 1977 Nov;132(2):462–472. doi: 10.1128/jb.132.2.462-472.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- Campbell D. A. Kinetics of the mating-specific aggregation in Saccharomyces cerevisiae. J Bacteriol. 1973 Oct;116(1):323–330. doi: 10.1128/jb.116.1.323-330.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E., Thorner J., Geier M. Solid phase peptide synthesis of alpha-factor, a yeast mating pheromone. Biochem Biophys Res Commun. 1977 Oct 10;78(3):952–961. doi: 10.1016/0006-291x(77)90514-9. [DOI] [PubMed] [Google Scholar]
- Crandall M., Caulton J. H. Induction of haploid glycoprotein mating factors in diploid yeasts. Methods Cell Biol. 1975;12:185–207. doi: 10.1016/s0091-679x(08)60957-7. [DOI] [PubMed] [Google Scholar]
- GLEN W. L., BARBER R., MCCONKEY H. M., GRANT G. A. Isolation of beta-dihydroequilin and alpha-dihydroequilenin from the urine of pregnant mares. Nature. 1956 Apr 21;177(4512):753–753. doi: 10.1038/177753a0. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiya M., Yoshida K., Yanagishima N. The release of sex-specific substances responsible for sexual agglutination from haploid cells of Saccharomyces cerevisiae. Exp Cell Res. 1977 Feb;104(2):263–272. doi: 10.1016/0014-4827(77)90090-8. [DOI] [PubMed] [Google Scholar]
- Jayatissa P. M., Rose A. H. Role of wall phosphomannan in flocculation of Saccharomyces cerevisiae. J Gen Microbiol. 1976 Sep;96(1):165–174. doi: 10.1099/00221287-96-1-165. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipke P. N., Taylor A., Ballou C. E. Morphogenic effects of alpha-factor on Saccharomyces cerevisiae a cells. J Bacteriol. 1976 Jul;127(1):610–618. doi: 10.1128/jb.127.1.610-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay V., Manney T. R. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. I. Isolation and phenotypic characterization of nonmating mutants. Genetics. 1974 Feb;76(2):255–271. doi: 10.1093/genetics/76.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavletich K., Kuo S. C., Lampen J. O. Chelation of divalent cations by lomofungin: role in inhibition of nucleic acid synthesis. Biochem Biophys Res Commun. 1974 Oct 8;60(3):942–950. doi: 10.1016/0006-291x(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Sena E. P., Radin D. N., Welch J., Fogel S. Synchronous mating in yeasts. Methods Cell Biol. 1975;11:71–88. doi: 10.1016/s0091-679x(08)60317-9. [DOI] [PubMed] [Google Scholar]
- Shimoda C., Kitano S., Yanagishima N. Mating reaction in Saccharomyces cerevisiae. VII. Effect of proteolytic enzymes on sexual agglutinability and isolation of crude sex-specific substances responsible for sexual agglutination. Antonie Van Leeuwenhoek. 1975;41(4):513–519. doi: 10.1007/BF02565093. [DOI] [PubMed] [Google Scholar]
- Shimoda C., Yanagishima N., Sakurai A., Tamura S. Mating reaction in Saccharomyces cerevisiae. IX. Regulation of sexual cell agglutinability of a type cells by a sex factor produced by alpha type cells. Arch Microbiol. 1976 May 3;108(1):27–33. doi: 10.1007/BF00425089. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen J. O. Wall replication in saccharomyces species: use of fluorescein-conjugated concanavalin A to reveal the site of mannan insertion. J Gen Microbiol. 1972 Sep;72(2):243–247. doi: 10.1099/00221287-72-2-243. [DOI] [PubMed] [Google Scholar]