Abstract
The nuclear pore complex (NPC) mediates communication between the cytoplasm and nucleus in eukaryotic cells. Active transport of large polypeptides as well as passive diffusion of smaller (≈10 kD) macromolecules through the NPC can be inhibited by depletion of intracellular Ca2+ stores. However, the physiological relevance of this process for the regulation of nucleocytoplasmic trafficking is not yet clear. We expressed green fluorescent protein (GFP)–tagged glucocorticoid receptor (GR) and mitogen-activated protein (MAP) kinase–activated protein kinase 2 (MK2) to study the effect of Ca2+ store depletion on active transport in HM1 cells, a human embryonic kidney cell line stably transfected with the muscarinic M1 receptor. Dexamethasone-induced nuclear import of GR-GFP and anisomycin-induced nuclear export of GFP-MK2 was monitored by confocal microscopy. We found that store depletion by carbachol, thapsigargin or ionomycin had no effect on GR-GFP import, whereas pretreatment with 1,2-bis-(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid–acetoxymethyl ester (BAPTA-AM) attenuated import significantly. Export of GFP-MK2 was not influenced by any pretreatment. Moreover, carbachol stimulated GFP-MK2 translocation to the cytoplasm in the absence of anisomycin. These results demonstrate that Ca2+ store depletion in intact HM1 cells is not directly linked to the inhibition of active protein transport through the NPC. The inhibition of GR-GFP import but not GFP-MK2 export by BAPTA-AM presumably involves a depletion-independent mechanism that interferes with components of the nuclear import pathway.
Keywords: nuclear transport, nuclear pore complex, Ca2+ store depletion, green fluorescent protein
introduction
The nuclear pore complex (NPC)1 is a supramolecular protein structure spanning the inner and outer nuclear membranes, thereby forming a continuous link between the cyto- and nucleoplasm. The central aqueous channel within the NPC has a functional diameter of <10 nm that allows the passage of small molecules (up to 30–40 kD) by diffusion (Bonner, 1975; Paine et al., 1975). Larger macromolecules possessing specific nuclear localization (NLS) or nuclear export sequences require energy-dependent multi-step processes to enter or exit the nucleus (Görlich, 1997; Nigg, 1997; Weiss, 1998).
As the exclusive transport route for proteins (Feldherr et al., 1984), the NPC is likely to be one of the main checkpoints for the regulation of nucleocytoplasmic trafficking. However, the mechanisms involved in determining NPC permeability are not well understood.
In normal rat kidney cells, the active import of microinjected nucleophilic proteins, as well as the passive diffusion of 10-kD dextran-coupled dyes into the nucleus was prevented by Ca2+ store depletion by thapsigargin, ionomycin, or 1,2-bis-(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid–acetoxymethyl ester (BAPTA-AM; Greber and Gerace, 1995). Likewise, entry of 10 kD dextrans into isolated Xenopus laevis oocyte nuclei was inhibited after emptying of the perinuclear Ca2+ store (Stehno-Bittel et al., 1995a). These observations suggested that loss of store Ca2+ induced structural changes of the NPC that in turn reduced NPC permeability. One possible mechanism, originally proposed by Greber and Gerace (1995), involved binding of endoplasmic reticulum (ER)–Ca2+ to the nuclear pore protein gp210. Similar to Ca2+ depletion, expression of anti–gp210 antibodies decreased active import and passive diffusion into the nucleus (Greber and Gerace, 1992). The large lumenal domain of gp210 contains several putative Ca2+-binding sites and, therefore, interaction with store Ca2+ could control NPC structure and function via gp210.
Further support for a direct regulation of the NPC by store Ca2+ is provided by experiments using field-emission scanning electron microscopy and atomic force microscopy, which revealed distinct changes in NPC structure after Ca2+ depletion of the nuclear envelope. Whereas control NPCs exhibited a characteristic shape with an open central pore region symmetrically surrounded by eight protein complexes, Ca2+-depleted specimens contained a central plug within the NPC (Perez-Terzic et al., 1996).
The release of Ca2+ from intracellular stores occurs via inositol-(1,4,5) trisphosphate (InsP3) and ryanodine receptors. The outer nuclear membrane, which is continuous with the rough ER membrane, contains functional InsP3 receptors (Stehno-Bittel et al., 1995b) and, therefore, the perinuclear Ca2+ store is efficiently emptied by InsP3 in isolated nuclei (Stehno-Bittel et al., 1995a). Even though numerous membrane receptors, such as Gq-coupled receptors, signal through the formation of InsP3 and release of Ca2+ from intracellular stores (Berridge, 1993; Lee and Rhee, 1995), it has not been demonstrated that activation of any of these receptors can decrease nuclear transport by closing the NPC. In contrast, in many cell types, Ca2+ release from stores and the subsequent rise of the cytoplasmic Ca2+ concentration induces gene expression (Menezes et al., 1996; Waser et al., 1997), which requires functional nucleocytoplasmic trafficking.
We investigated how Ca2+ store depletion influences nuclear import and export at the single cell level using green fluorescent protein (GFP)–tagged proteins. Our results demonstrate that active nuclear transport in intact HM1 cells is independent of the filling state of intracellular Ca2+ stores.
materials and methods
Cell Culture and Transfection
HM1 cells, a human embryonic kidney cell line stably expressing the muscarinic M1-receptor (Peralta et al., 1988), were used throughout this study. Cells were grown at 37°C in DMEM/F12 (1:1) medium supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.5 mg/ml G 418, and HT supplement (1:100; GIBCO BRL) under a 5% CO2 atmosphere.
cDNAs were transfected using the Ca2+-phosphate method (Sambrook et al., 1989), 6–8 h after transfection cells were plated onto coverslips, and used for the experiments after 48–72 h. Cells transfected with glucocorticoid receptor–GFP (GR-GFP) were cultured after plating on coverslips in DMEM/F12 without phenol red (GIBCO BRL) supplemented with charcoal-treated FBS (Cocalico Inc.) to remove endogenous glucocorticoid activity.
For transfection of pcDIC15nu, the Ca2+-phosphate maximizer kit (Clontech) was used according to the manufacturer's instructions. Cells grown for 6–12 h on coverslips were incubated for 3 h with the transfection mixture, washed twice with Ca2+-free PBS, and either cultured in control medium containing 0.1% DMSO or low Ca2+ medium containing 100 nM thapsigargin, or 100 nM thapsigargin plus 5 μM calmidazolium for another 9–11 h. The low Ca2+ medium was prepared as normal growth medium, except that 2 mM EGTA was added to CaCl2-free DMEM (GIBCO BRL). The resulting free Ca2+ concentration was calculated to be 60 nM (Schubert, 1990).
Cell Processing and Confocal Microscopy
Control cells were treated for 30 min at 37°C with 0.1% DMSO (the highest concentration added to drug-treated cells) in standard external solution before transport was induced by addition of dexamethasone (1 μM) or anisomycin (10 μg/ml). The standard external solution contained (mM): 140 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH adjusted to 7.4 with NaOH. After a 20-min treatment with dexamethasone or anisomycin at 37°C, coverslips were transferred to a confocal microscope and examined immediately.
To deplete intracellular Ca2+ stores, cells were incubated with the respective drugs for 30 min at 37°C in Ca2+-free buffer containing (mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 1 EGTA, 10 glucose, 10 HEPES, pH adjusted to 7.4 with NaOH. All chemicals were purchased from Sigma Chemical Co.
Nuclear transport was induced as described above by addition of dexamethasone or anisomycin. Cells expressing pcDIC15nu were investigated 12–14 h after transfection. As many cells cultured in low Ca2+ medium detached from the coverslips, cells were centrifuged at 100 g for 10 min, resuspended in 100 μl low Ca2+ buffer, and transferred into the microscope chamber.
Confocal microscopy was performed with a laser scanning microscope (LSM 410; Carl Zeiss Inc.) using the 488-nm line of an Ar/Kr laser. Emission light was filtered with a 512-nm bandpass filter (27-nm bandwidth) (Chroma Technology) and fluorescence intensities were analyzed with the LSM 410 software.
Stock solution of thapsigargin, anisomycin, ionomycin, calmidazolium (all from Calbiochem Corp.) and BAPTA-AM (Molecular Probes, Inc.) were prepared in DMSO and stored at −20°C. Dexamethasone (Calbiochem Corp.) was prepared in ethanol and stored at −20°C.
Statistical Data Analysis
In GR-GFP–transfected cells, nuclear/cytoplasmic fluorescence ratios were found to be very reproducible between transfections (<15% variation). If no statistical difference between control values was detected, data from different experimental series were pooled and compared using Student's t test or one-way analysis of variance (ANOVA). Data were presented as means ± SEM. In contrast, anisomycin-induced changes in the nuclear/cytoplasmic fluorescence in GFP-MAP kinase–activated protein kinase 2 (MK2)–transfected cells varied considerably among different transfections (up to 35%). Statistical comparisons were only made between data from individual experiments using the t test or ANOVA. For representation, data were pooled and shown as weighted means ± SEM. All statistical tests were performed with Systat 7.0 (SPSS Inc.). P ≤ 0.05 was considered significant.
results
The localization of GFP-tagged proteins can be precisely traced and quantified in living cells using confocal microscopy. We used this approach to measure the translocation of a GR-GFP chimera that is imported into the nucleus upon glucocorticoid binding (Carey et al., 1996; Htun et al., 1996) and a GFP-MAP kinase-activated protein kinase 2 chimera (GFP-MK2) that is exported from the nucleus after stimulation with anisomycin (Aniso; Engel et al., 1998). Furthermore, the nuclear targeting of pcDIC15nu, a fusion protein between a blue and green fluorescent protein containing an SV 40-NLS (Persechini et al., 1997; M. Badminton, unpublished data), was analyzed.
Nuclear GR-GFP Import Is Inhibited by BAPTA-AM but Not by Ca2+Store Depletion
After transfection of GR-GFP cDNA into HM1 cells, fluorescence localized to the cytoplasm (Fig. 1). Treatment of GR-GFP–expressing cells with 1 μM dexamethasone (Dex), a synthetic GR agonist, in Ca2+-containing control buffer induced translocation of GR-GFP into the nucleus. After 20 min, fluorescence was observed almost exclusively in the nucleus (Fig. 1). To quantify transport activity in GR-GFP–transfected cells, we measured the ratio of nuclear/cytoplasmic fluorescence intensity (NF/CF). We found that NF/CF changed from 0.46 ± 0.02 (n = 135) in untreated cells to 5.23 ± 0.34 (n = 89) after Dex treatment.
Figure 1.
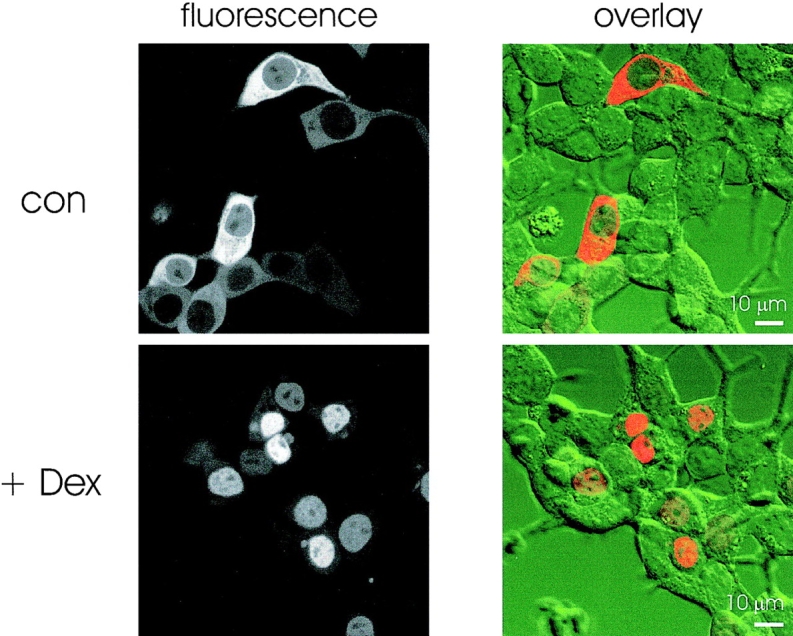
Dexamethasone- induced nuclear translocation of GR-GFP fluorescence in HM1 cells. Confocal images of GR-GFP–transfected HM1 cells incubated for 20 min in control buffer (top) or buffer containing 1 μM Dex (bottom). Fluorescence images (left) were overlaid with the bright field images (right) to illustrate the localization of GFP fluorescence within the cells.
Having established that GR-GFP is efficiently translocated in HM1 cells, we tested the effect of Ca2+ store depletion on nuclear GR-GFP import. When experiments were carried out in Ca2+-free extracellular buffer containing 1 mM EGTA, Dex induced essentially the same change in NF/CF as in control buffer. NF/CF increased from 0.41 ± 0.02 (n = 134) in the absence of Dex to 5.06 ± 0.25 (n = 111) after Dex treatment.
To release Ca2+ from stores, we preincubated cells for 30 min in Ca2+-free buffer with a Ca2+ ionophore (ionomycin, 1 μM), a ER Ca2+-pump inhibitor (thapsigargin, 1 μM), a cell-permeant Ca2+ chelator (BAPTA-AM, 10 μM), or a muscarinic M1-receptor agonist (carbachol, 10 μM). All these treatments completely emptied intracellular Ca2+ stores as subsequent application of ionomycin (10 μM) in Ca2+-free solution did not produce any further rise in the cytosolic Ca2+ concentration in fura-2–loaded cells (data not shown). After store depletion, Dex was added for 20 min to induce nuclear import of GR-GFP. A comparison of the fluorescence ratios showed that incubation of cells with BAPTA-AM induced a significant decrease in GR-GFP import, whereas all other pretreatments were ineffective (Fig. 2 A). Although the blocking effect of BAPTA-AM was incomplete, the decrease in nuclear GR-GFP accumulation could be clearly detected at the single cell level (Fig. 2 B).
Figure 2.
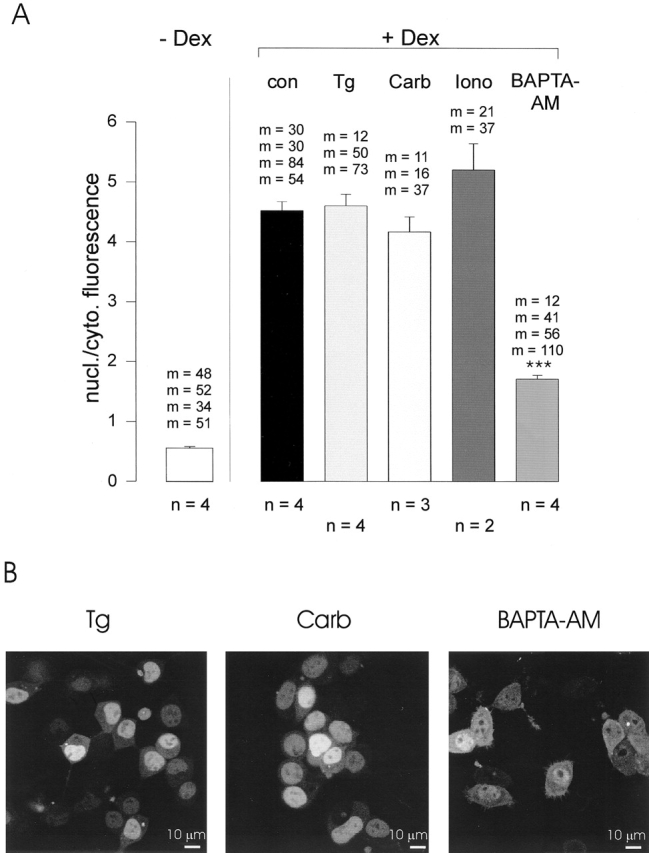
Effect of Ca2+-store depletion on GR-GFP import HM1 cells. (A) Quantification of Dex-induced GR-GFP translocation in transfected HM1 cells. The ratio of nuclear/cytoplasmic fluorescence was determined without treatment (left) or after a 30-min pretreatment with 1 μM thapsigargin (Tg), 10 μM carbachol (Carb), 1 μM ionomycin (Iono), or 10 μM BAPTA-AM and subsequent incubation with 1 μM Dex for 20 min (right). The nuclear/cytoplasmic fluorescence ratio after Dex application was significantly reduced by BAPTA-AM pretreatment (***P ≤ 0.001; analysis of variance). All experiments were performed in Ca2+-free buffer. Bars represent means ± SEM. n, number of experiments; m, number of cells in each experiment. (B) Representative fluorescence images of HM1 cells 20 min after application of 1 μM Dex. Cells were pretreated with 1 μM thapsigargin (Tg), 10 μM carbachol (Carb), or 10 μM BAPTA-AM as in A.
BAPTA was previously found to inhibit NPC assembly in Xenopus egg extracts by mechanisms possibly involving binding of heavy metal ions (Marshall et al., 1997). We tested the requirement of heavy metal ions for GR-GFP import using tetrakis-[2-pyridylmethyl]-ethylenediamine (TPEN), a chelator with much higher affinity for heavy metal ions than BAPTA (Arslan et al., 1985). At 25–50 μM, concentrations that are sufficient to strongly reduce lumenal heavy metal ion concentration in RBL-1 cells (Hofer et al., 1998), 30-min pretreatment with TPEN did not alter Dex-induced GR-GFP translocation (NF/CF = 5.28 ± 0.27, n = 71) compared with DMSO-treated controls (NF/CF = 4.69 ± 0.23, n = 84).
Persistent Nuclear Import of an SV 40–containing Protein in Ca2+-depleted HM1 Cells
The insensitivity of GR-GFP transport to store depletion contrasts with previous studies of active import in vivo (Greber and Gerace, 1995). We considered the possibility that Ca2+ depletion is not directly acting on the NPC, but inhibiting other, yet unknown, steps during protein transport. In this scenario, it is conceivable that proteins with different NLSs are differentially influenced by store Ca2+. Furthermore, a recently identified auxiliary calmodulin-dependent import pathway (Sweitzer and Hanover, 1996) might be activated in a cell type– and target-specific manner by increases in the cytosolic Ca2+ concentration after Ca2+ release from stores.
To test these hypotheses, we investigated nuclear targeting of pcDIC15nu, which contains an SV40-NLS. Nuclear transport of serum albumin fused to the same NLS was shown by Greber and Gerace (1995) to be blocked in Ca2+-depleted cells. We also employed the calmodulin antagonist, calmidazolium, to inhibit calmodulin-mediated transport (Sweitzer and Hanover, 1996). As pcDIC15nu is constitutively transported to the nucleus, store depletion was induced 3 h after transfection by exchanging the normal growth medium for a low Ca2+ (≈60 nM Ca2+) medium supplemented with 100 nM thapsigargin (or thapsigargin plus 5 μM calmidazolium). As judged from the fluorescence signal, expression of pcDIC15nu was evident in <1% of cells 1 h and <10% of cells 3 h after medium exchange (not shown). When examined 12–14 h after transfection, pcDIC15nu fluorescence was seen in ∼35% of cells. When expressed, pcDIC15nu localized to the nucleus regardless of whether cells were cultured in normal growth medium or low Ca2+ medium containing thapsigargin and calmidazolium (Fig. 3). A statistical evaluation substantiated that import of pcDIC15nu was unaffected by store depletion and calmidazolium treatment. NF/CF in control cells (3.39 ± 0.2, n = 91) was not different from NF/CF in cells treated with thapsigargin (3.1 ± 0.4, n = 56) or thapsigargin plus calmidazolium (3.19 ± 0.28, n = 56). In human embryonic kidney cells stably expressing GR-GFP, we confirmed that nuclear GR import was not inhibited by pretreatment with ionomycin plus calmidazolium or thapsigargin plus calmidazolium (data not shown).
Figure 3.
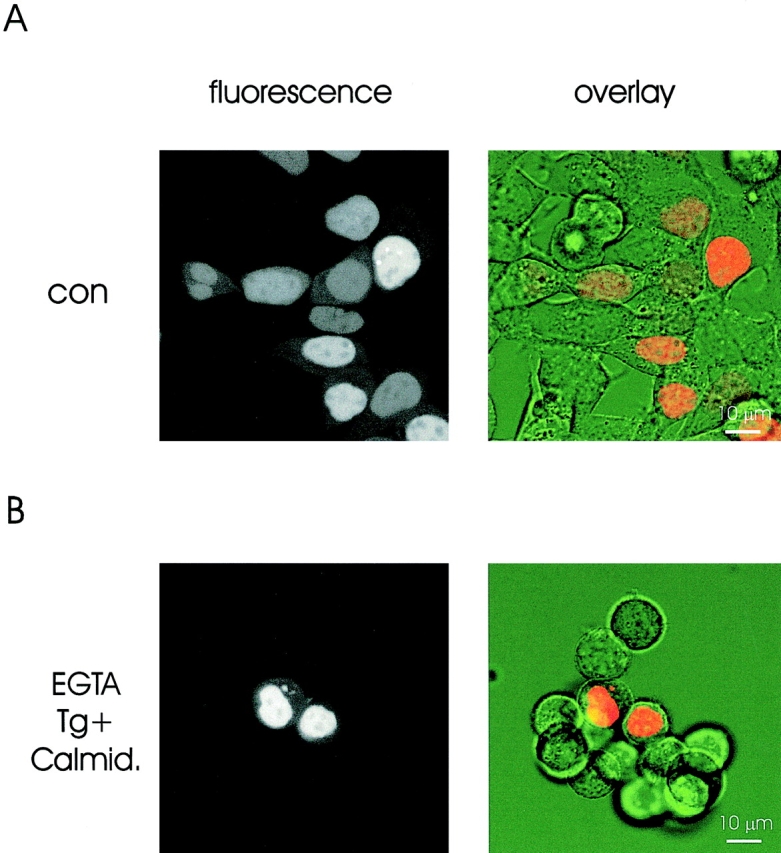
Nuclear localization of a nuclear-targeted fluorescent marker (pcDIC15nu) does not differ between control and Ca2+-depleted HM1 cells. Fluorescence (left) and overlay images (right) of pcDIC15nu-expressing cells kept in normal growth medium (top) or Ca2+-free medium supplemented with 100 nM thapsigargin and 5 μM calmidazolium (bottom) for 12 h after transfection.
These results demonstrate that active nuclear import is not regulated by Ca2+ depletion from lumenal stores in our model. However, we found that BAPTA-AM did reduce GR-GFP transport into the nucleus.
Nuclear Export of GFP-MK2 Is Independent of Lumenal Ca2+ Stores
Active nuclear export differs considerably from the nuclear import pathway (Ullman et al., 1997; Görlich, 1998). We were interested in determining how nuclear export is influenced by Ca2+ store depletion. MK2 has recently been shown to exit the nucleus upon phosphorylation by p38/SAP kinase in an exportin 1–dependent manner (Engel et al., 1998). We used GFP-MK2 to examine nuclear export in HM1 cells. After transfection, GFP-MK2 predominantly localized to the nucleus (Fig. 4). To induce GFP-MK2 translocation, cells were treated for 20 min with 10 μg/ml Aniso, which activated the p38/SAP kinase cascade.
Figure 4.
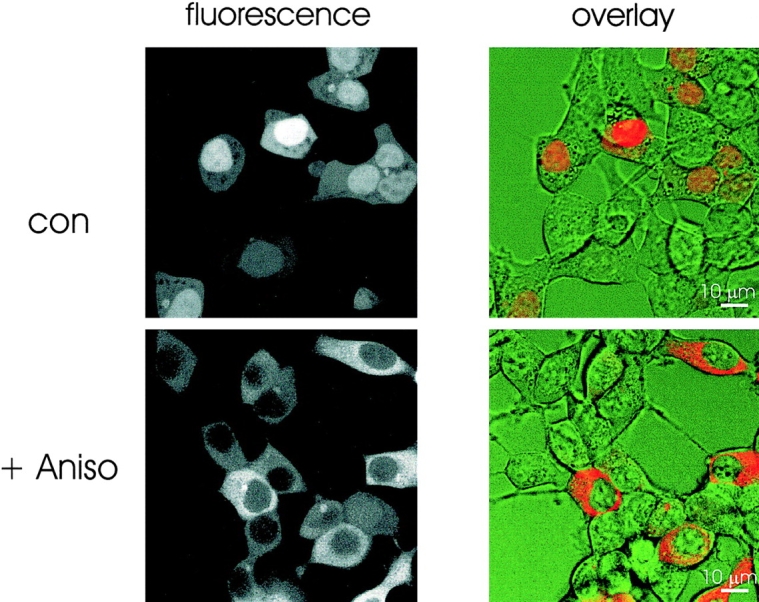
Translocation of GFP-MK2 fluorescence in response to anisomycin stimulation in HM1 cells. Confocal images of GFP-MK2-transfected HM1 cells incubated in control buffer (top) or incubated for 20 min in the presence of 10 μg/ml Aniso (bottom). Fluorescence images (left) and fluorescence/bright field overlay images (right) are shown.
In most cells, GFP-MK2 efficiently translocated to the cytoplasm in response to Aniso (Fig. 4). However, we observed a small population (<15%) of very highly expressing cells in which fluorescence remained higher in the nucleus than in the cytoplasm after Aniso treatment. As GFP tends to form dimers at high concentrations (Ward et al., 1982), dimerization of GFP-MK2 may prevent efficient export in strongly overexpressing cells. We did not study the dependence of GFP-MK2 export on expression levels further and compared export only in cells from the same transfections to reduce variability due to different transfection efficiencies. When measured in control buffer in GFP-MK2-expressing cells, NF/CF changed from 1.58 ± 0.08 (n = 216) in the absence to 0.85 ± 0.08 (n = 228) in the presence of Aniso. These values were not significantly changed when cells were processed in Ca2+-free buffer (NF/CF = 1.6 ± 0.08, n = 274 and 0.82 ± 0.09, n = 244; data from three sets of experiments).
Next, the effect of store depletion on GFP-MK2 export was studied using the same depletion protocols described above for GR-GFP import. In addition, we applied calmidazolium with ionomycin to rule out an involvement of calmodulin in GFP-MK2 transport. As summarized in Fig. 5, GFP-MK2 export was not substantially decreased by any pretreatment that depleted intracellular Ca2+ stores. Essentially the same results were obtained when a stable human embryonic kidney cell line expressing MK2-GFP was pretreated with ionomycin or BAPTA-AM (not shown).
Figure 5.
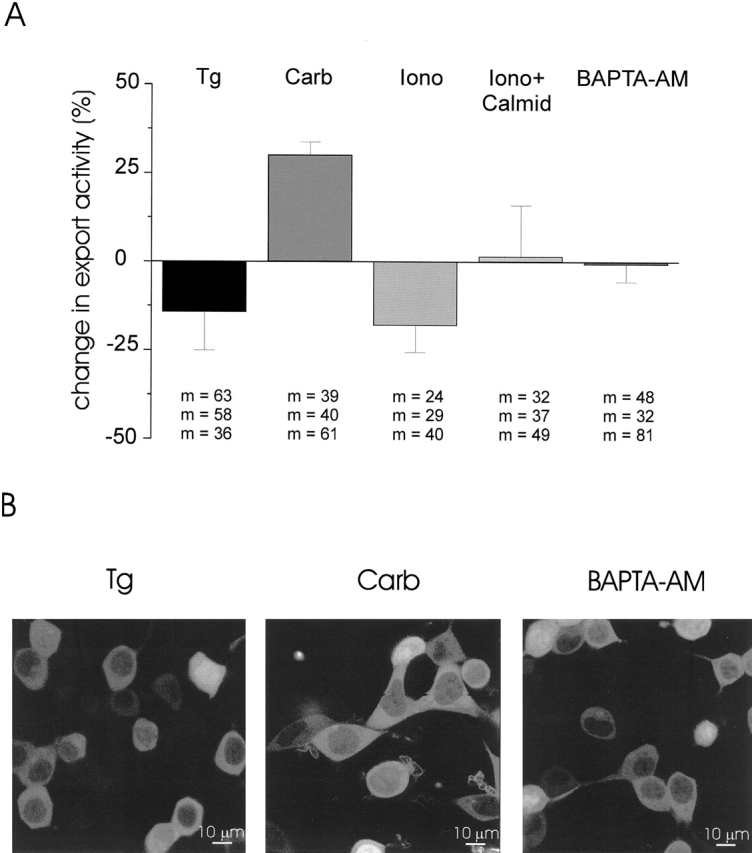
Independence of GFP-MK2 export of Ca2+-store depletion and calmodulin. (A) Effect of store-depleting agents and calmidazolium on Aniso-induced export of GFP-MK2. Cells were pretreated with 1 μM thapsigargin (Tg), 10 μM carbachol (Carb), 1 μM ionomycin (Iono), ionomycin plus 5 μM calmidazolium (Iono + Calmid), or 10 μM BAPTA-AM for 30 min and subsequently incubated with 10 μg/ml Aniso. The Aniso-induced change in NF/CF in control cells was considered as 100% export activity for each experiment. Data represent weighted means ± SEM from three experiments. m, number of cells in each experiment. No pretreatment was found to significantly decrease GFP-MK2 export. In two of three experiments, export was significantly stimulated (P ≤ 0.05, t test) by carbachol pretreatment. All experiments were carried out in Ca2+ -free buffer. (B) Representative fluorescence images of cells 20 min after application of 10 μg/ml Aniso. Cells were pretreated with thapsigargin (Tg), carbachol (Carb), or BAPTA-AM, as in A.
The most pronounced effect in HM1 cells was in fact an increase in export activity when cells were incubated with carbachol before Aniso application (Fig. 5). This prompted us to test whether carbachol per se is able to induce translocation of GFP-MK2 to the cytoplasm. In three independent experiments, we found that carbachol induced export as efficiently as Aniso. The NF/CF change after carbachol treatment in Ca2+-free buffer amounted to 0.65 ± 0.14 (n = 358) in comparison to 0.74 ± 0.18 (n = 258) after Aniso. Under the same conditions, BAPTA-AM, ionomycin, and thapsigargin had little or no effect on export in the absence of Aniso (not shown).
discussion
The objective of this study was to investigate whether there is a physiological link between the release of Ca2+ from the ER lumen and a decrease in the permeability of the NPC in intact cells. Functional inhibition of active import and passive diffusion through the NPC has previously been shown in vitro (Stehno-Bittel et al., 1995a; Sweitzer and Hanover, 1996) and in vivo (Greber and Gerace, 1995; Greber et al., 1997) using pharmacological tools to manipulate store Ca2+. However, it is unclear whether hormones and neurotransmitters coupling to intracellular Ca2+ release similarly regulate nucleocytoplasmic trafficking. As a first approach to answer this question, translocation of GFP fusion proteins undergoing active nuclear import and export was studied in HM1 cells, a human embryonic kidney cell derivative expressing the muscarinic M1 receptor. M1-receptor activation efficiently stimulates InsP3 formation and Ca2+ release from stores (Peralta et al., 1988; Berridge, 1993), thus allowing the investigation of receptor-mediated Ca2+ mobilization on nuclear transport and NPC function.
We found that active nuclear import and export in HM1 cells is independent of store depletion by thapsigargin, ionomycin, and M1-receptor stimulation by carbachol. Pretreatment of cells with BAPTA-AM reduced GR-GFP import but not GFP-MK2 export.
We assume that the import block by BAPTA-AM is due to mechanisms other than or in addition to removal of Ca2+ from stores because thapsigargin, ionomycin, and carbachol, which all decrease the concentration of free lumenal Ca2+ by different mechanisms (Liu and Hermann, 1978; Thastrup et al., 1990; Lee and Rhee, 1995) had no effect on GR-GFP translocation. The cell-permeant BAPTA-AM is converted by intracellular esterases into BAPTA (Tsien, 1980; Tsien et al., 1984), a fast, high affinity Ca2+ chelator, which depletes stores by direct Ca2+ binding. Unlike thapsigargin, ionomycin, and carbachol, BAPTA not only removes lumenal Ca2+ but also binds heavy metal ions (Csermely et al., 1989; Aballay et al., 1995). We therefore used TPEN to investigate the possibility that chelation of heavy metal ions caused import inhibition. TPEN binds Zn2+, Fe2+, and other metal ions with high affinity and was reported to compromise nuclear architecture and NPC assembly in vitro (Shumaker et al., 1998). However, the insensitivity of GR-GFP translocation to TPEN indicates that import inhibition does not involve heavy metal ion chelation. It will be interesting to study whether cation chelation is necessary for BAPTA-induced import inhibition by using structurally related compounds with different binding affinities.
The persistent import of GR-GFP in Ca2+-depleted HM1 cells contradicts earlier findings on NPC regulation by store depletion. However, a recent report demonstrated that active nuclear import is independent of ionophore-releasable Ca2+ pools in reconstituted Xenopus nuclear envelopes (Marshall et al., 1997). Thus, the requirement of store Ca2+ for NPC function in vitro can differ depending on the experimental system used. More difficult to reconcile with our data is the inhibition of active transport and passive diffusion by store depletion in intact mammalian cells (Greber and Gerace, 1995). We excluded the possibility that GR-GFP import is a specific, store-independent mechanism by demonstrating that import of another NLS-containing protein, pcDIC15nu, was insensitive to store depletion as well.
We also excluded the possibility that calmodulin-mediated pathways supported store-independent transport in HM1 cells. Sweitzer and Hanover (1996) described calmodulin-mediated import as a new GTP-independent import mechanism activated by elevated Ca2+ concentrations in permeabilized cells. As store depletion can generate transient increases in the cytosolic Ca2+ concentration, this may activate calmodulin-dependent transport pathways. However, when we assayed pcDIC15nu import as well as GFP-MK2 export in the presence of calmidazolium in intact HM1 cells, we found that treatment of cells with high concentrations of the calmodulin antagonist had no effect on import and export.
To our knowledge, the basic components of the nuclear transport machinery are ubiquitously expressed. Nevertheless, cell type–specific mechanisms may confer sensitivity of nucleocytoplasmic transport to store depletion. We have not yet thoroughly tested the effect of store depletion on nuclear transport in other cell lines, but preliminary experiments on wild-type human embryonic kidney cells and neuronal NG-108 cells confirmed that GR-GFP import is insensitive to ionophore treatment (C. Strübing, unpublished observations). Thus, we have no evidence for a cell type–specific regulation of nuclear import by store Ca2+.
The notion that the NPC remains functionally intact after removal of lumenal Ca2+ in HM1 cells is further supported by the study of GFP-MK2 export. When we quantified GFP-MK2 translocation in Ca2+-depleted cells, we found no inhibition of export even by BAPTA-AM treatment. This result suggests that BAPTA interferes with components of the nuclear import pathway. Possible targets may include specific importins that are necessary for the binding and translocation of distinct NLS-containing proteins (Ullman et al., 1997; Görlich, 1998). The site(s) of BAPTA action could also be located within the ER, a possibility, favored by the observation that transport of nucleoplasmin into the nucleus was blocked only by the membrane-permeable BAPTA-AM, but not by cytoplasmic injection of impermeable BAPTA (Greber and Gerace, 1995).
Greber and Gerace (1995) reported that tunicamycin, which induces aberrant protein folding in the ER, is able to mimic the effect of store depletion on nuclear transport in normal rat kidney cells. Based on this finding, they proposed that a stress response initiated by lumenal Ca2+ depletion caused nuclear transport inhibition in intact cells. As MK2 is activated downstream of p38/SAP kinase by several cellular stress stimuli (Freshney et al., 1994; Rouse et al., 1994), it is conceivable that we observed a specific export pathway that was activated rather than inhibited by ER stress. However, GFP-MK2 export was efficiently stimulated only by carbachol, but not by pretreatment with BAPTA-AM, thapsigargin, or ionomycin alone, indicating that release of store Ca2+ is not a stimulus for MK2 export by itself. Moreover, a recent report demonstrated that the nuclear export of the HIV-1 Rev protein, which contained a constitutively active export signal, is not regulated by store depletion or calmodulin in vitro (Love et al., 1998). Despite the independence of the two export cargoes GFP-MK2 and Rev of lumenal Ca2+, it will be interesting to determine whether active nuclear export is generally insensitive to store depletion.
Taken together, the persistent nucleocytoplasmic transport of three different GFP constructs in Ca2+-depleted HM1 cells strongly argues against a direct inhibitory effect of intracellular Ca2+ release on nuclear transport and NPC function. We hypothesize that a complex signaling cascade, perhaps similar to that activated by ER stress, is triggered by store depletion, but requires additional factors to lead to a shut down of nuclear import through the NPC. This may not only explain the differences in nuclear transport regulation by BAPTA-AM and other store-depleting agents in our experiments, but also the discrepancies observed in different model systems. The use of GFP-tagged proteins undergoing nuclear import and export will facilitate the further elucidation of mechanisms controlling transport through the NPC in living cells.
Acknowledgments
We thank Dr. I.G. Macara for GR-GFP, Drs. M. Gaestel and K. Engel for GFP-MK2, and Drs. A. Persechini and M. Badminton for pcDIC15nu. For helpful comments on the manuscript, we thank Drs. S. Sims and M. Badminton.
This work was supported by the Howard Hughes Medical Institute, National Institutes of Health grant 41303 (D.E. Clapham) and by the Deutsche Forschungsgemeinschaft (C. Strübing).
Abbreviations used in this paper
- AM
acetoxymethyl ester
- Aniso
anisomycin
- BAPTA
1,2-bis-(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid
- Dex
dexamethasone
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- GR
glucocorticoid receptor
- InsP3
inositol- (1,4,5) trisphosphate
- MK2
MAP kinase–activated protein kinase 2
- NF/CF
ratio of nuclear/cytoplasmic fluorescence intensity
- NLS
nuclear localization sequence
- NPC
nuclear pore complex
- TPEN
tetrakis-[2-pyridylmethyl]-ethylenediamine
references
- Aballay A, Sarrouf MN, Colombo MI, Stahl PD, Mayorga LS. Zn2+depletion blocks endosome fusion. Biochem J. 1995;312:919–923. doi: 10.1042/bj3120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+ . J Biol Chem. 1985;260:2719–2727. [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bonner WM. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975;64:431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KL, Richards SA, Lounsbury KM, Macara IG. Evidence using a green fluorescent protein-glucocorticoid receptor chimera that the Ran/TC4 GTPase mediates an essential function independent of nuclear protein import. J Cell Biol. 1996;133:985–996. doi: 10.1083/jcb.133.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P, Sandor P, Radics L, Somogyi J. Zinc forms complexes with higher kinetical stability than calcium, 5-F-BAPTA as a good example. Biochem Biophys Res Commun. 1989;165:838–844. doi: 10.1016/s0006-291x(89)80042-7. [DOI] [PubMed] [Google Scholar]
- Engel K, Kotlyarov A, Gaestel M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO (Eur Mol Biol Organ) J. 1998;17:3363–3371. doi: 10.1093/emboj/17.12.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldherr CM, Kallenbach E, Schultz AN. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984;99:2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Görlich D. Nuclear protein import. Curr Opin Cell Biol. 1997;9:412–419. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- Görlich D. Transport into and out of the cell nucleus. EMBO (Eur Mol Biol Organ) J. 1998;17:2721–2727. doi: 10.1093/emboj/17.10.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Gerace L. Nuclear protein import is inhibited by an antibody to a lumenal epitope of a nuclear pore complex glycoprotein. J Cell Biol. 1992;116:15–30. doi: 10.1083/jcb.116.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Gerace L. Depletion of calcium from the lumen of endoplasmic reticulum reversibly inhibits passive diffusion and signal-mediated transport into the nucleus. J Cell Biol. 1995;128:5–14. doi: 10.1083/jcb.128.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Suomalainen M, Stidwill RP, Boucke K, Ebersold MW, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO (Eur Mol Biol Organ) J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Fasolato C, Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+] J Cell Biol. 1998;140:325–334. doi: 10.1083/jcb.140.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun H, Barsony H, Renyi I, Gould DL, Hager GL. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci USA. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Rhee SG. Significance of PIP2hydrolysis and regulation of phospholipase C isozymes. Curr Opin Cell Biol. 1995;7:183–189. doi: 10.1016/0955-0674(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- Love DC, Sweitzer TD, Hanover JA. Reconstitution of HIV-1 Rev nuclear export: independent requirements for nuclear import and export. Proc Natl Acad Sci USA. 1998;95:10608–10613. doi: 10.1073/pnas.95.18.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall ICB, Gant TM, Wilson KL. Ionophore- releasable lumenal Ca2+ stores are not required for nuclear envelope assembly or nuclear protein import in Xenopusegg extracts. Cell Calc. 1997;21:151–161. doi: 10.1016/s0143-4160(97)90039-7. [DOI] [PubMed] [Google Scholar]
- Menezes A, Zeman R, Sabban E-H. Involvement of intracellular or extracellular calcium in activation of tyrosine hydroxylase gene expression in PC12 cells. J Neurochem. 1996;67:2316–2324. doi: 10.1046/j.1471-4159.1996.67062316.x. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Paine PL, Moore LC, Horowitz SB. Nuclear envelope permeability. Nature. 1975;254:109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Peralta EG, Ashkenazi A, Winslow JW, Ramachandran J, Capon DJ. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988;334:434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C, Pyle J, Jaconi M, Stehno-Bittel L, Clapham DE. Conformational states of the nuclear pore complex induced by depletion of nuclear Ca2+stores. Science. 1996;273:1875–1877. doi: 10.1126/science.273.5283.1875. [DOI] [PubMed] [Google Scholar]
- Persechini A, Lynch JA, Romoser VA. Novel fluorescent indicator proteins for monitoring free intracellular Ca2+ . Cell Calc. 1997;22:209–216. doi: 10.1016/s0143-4160(97)90014-2. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY. 16.30–16.37.
- Schubert R. A program for calculating multiple metal-ligand solutions. Comput Methods Programs Biomed. 1990;33:93–94. doi: 10.1016/0169-2607(90)90065-h. [DOI] [PubMed] [Google Scholar]
- Shumaker DK, Vann LR, Goldberg MW, Allen TD, Wilson KL. TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro. Cell Calc. 1998;23:151–164. doi: 10.1016/s0143-4160(98)90114-2. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L, Perez-Terzic C, Clapham DE. Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+store. Science. 1995a;270:1835–1838. doi: 10.1126/science.270.5243.1835. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L, Lückhoff A, Clapham DE. Calcium release from the nucleus by InsP3receptor channels. Neuron. 1995b;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Sweitzer TD, Hanover JA. Calmodulin activates nuclear protein import: a link between signal transduction and nuclear transport. Proc Natl Acad Sci USA. 1996;93:14574–14579. doi: 10.1073/pnas.93.25.14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup OP, Cullen J, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–3404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien RY, Pozzan T, Rink TJ. Calcium activities and fluxes inside small intact cells as measured with intracellularly trapped chelators. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:535–541. [PubMed] [Google Scholar]
- Ullman K, Powers M, Forbes D. Nuclear export receptors: from importin to exportin. Cell. 1997;90:967–970. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- Ward WW, Prentice HJ, Roth AF, Cody CW, Reeves SC. Spectral pertubations of the Aequorea green-fluorescent protein. Photochem Photobiol. 1982;35:803–808. [Google Scholar]
- Waser M, Mesaeli N, Spencer C, Michalak M. Regulation of calreticulin gene expression by calcium. J Cell Biol. 1997;138:547–557. doi: 10.1083/jcb.138.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C. Importins and exportins: how to get in and out of the nucleus. TIBS (Trends Biochem Sci) 1998;23:185–189. doi: 10.1016/s0968-0004(98)01204-3. [DOI] [PubMed] [Google Scholar]


