Abstract
Runx1 binds the silencer and represses CD4 transcription in immature thymocytes. In this study, using looping chromatin immunoprecipitation and chromatin conformation capture assays, we demonstrated that interactions between Runx1 and positive elongation factor b (P-TEFb) appose the silencer and enhancer in CD4-negative thymoma cells and double-negative immature thymocytes. This chromatin loop decoys P-TEFb away from the promoter, thus preventing RNA polymerase II from elongating on the CD4 gene. In the absence of Runx1 on the silencer, P-TEFb interacts with the transcription complex, forming a different chromatin loop between the enhancer and the promoter, which leads to the expression of the CD4 gene in CD4-positive hybridoma cells and double-positive thymocytes. Moreover, the knockdown of CycT1 from P-TEFb abolishes both of these chromatin loops. Finally, the selective removal and restoration of Runx1 causes rapid interchanges between these chromatin loops, which reveals the plasticity of this regulatory circuit. Thus, differential looping and decoying of P-TEFb away from the promoter mediate active repression of the CD4 gene during thymocyte development.
Mammalian transcription starts with the formation of a preinitiation complex at the promoter, where cyclin-dependent kinase 7 (Cdk7) from the general transcription factor TFIIH phosphorylates serines at position 5 of the heptapeptide (YSPTSPS) repeats in the C-terminal domain (CTD) of the large subunit of RNA polymerase II (RNAPII). This phosphorylation allows RNAPII to clear the promoter and to initiate transcription. However, RNAPII then comes under the control of the negative elongation factor (NELF) and the DRB sensitivity-inducing factor (DSIF), which render RNAPII susceptible to pausing and arrest. Positive elongation factor b (P-TEFb) phosphorylates NELF, DSIF, and serines at position 2 of the heptapeptide repeats in the CTD, releasing NELF from the arrested complex and turning DSIF into a positive elongation factor, thus leading to productive transcription elongation. P-TEFb is composed of Cdk9 and one of three C-type regulatory cyclin subunits, CycT1, CycT2, or CycK (reviewed in reference 16). Over the past 2 decades, it has become clear that many mammalian genes are regulated at this step of transcription. To this end, P-TEFb can be recruited to the transcription complex by DNA-bound activators such as NF-κB and c-Myc (1, 9), as well as by the RNA-bound transactivator Tat of human immunodeficiency virus (26, 32), the chromatin-bound modifier Brd4 (6, 29), or the coactivator CIITA (8). On the other hand, transcription repressors such as PIE1 and Runx1 can decoy P-TEFb away from the transcription complex, thus inhibiting transcription elongation (7, 30).
Runx1, also called AML1, is a master regulator of hematopoiesis and the most frequent target of translocations and mutations in human leukemias. It belongs to the Runt domain (RD) family of transcription factors, which contain the signature RD that is responsible both for sequence-specific DNA binding and for heterodimerization (reviewed in references 3, 13, and 27). Runx1 is a context-dependent regulator. On certain genes, such as the T-cell antigen receptor (17, 25), it facilitates the assembly of transcription complexes, whereas on others, it acts as a repressor by recruiting mSin3A or Groucho/TLE corepressors (11, 14) and/or by decoying P-TEFb (7).
Newly generated thymocytes do not express CD4 or CD8. These CD4− CD8− double-negative (DN) thymocytes will transition through CD4− CD8low immature single-positive (ISP) and CD4+ CD8+ double-positive (DP) stages and eventually develop into two distinct populations: mature CD4+ CD8− single-positive (CD4 SP) or CD4− CD8+ single-positive (CD8 SP) cells. CD4 expression is actively repressed in DN and ISP cells as well as during the transition from DP to CD8 SP cells (2). However, the maintenance of CD4 silencing is achieved by epigenetic silencing in CD8 SP T cells (20). Active repression requires Runx1 and a silencer located in the first intron of the CD4 gene, which contains Runx-binding sites (21, 22, 28). It had been demonstrated previously that the inhibitory domain in Runx1 is required for the repression of CD4 in thymocytes (10, 24) as well as for effects of Runx1 on the CD4 silencer (7). In addition, we demonstrated that Runx1 not only binds P-TEFb but prevents further transcription elongation (7). In this study, we wanted to determine if these interactions are reflected in different chromatin conformations between cis-acting elements in the CD4 gene.
MATERIALS AND METHODS
Cells.
The mouse 3A9 T-cell hybridoma, a gift from Nigel Killeen (University of California at San Francisco [UCSF], San Francisco, CA), and 1200M cells, a gift from Dan Littman (New York University, New York, NY), were maintained at 37°C under 5% CO2 in RPMI medium supplemented with 10% fetal bovine serum, 100 mM l-glutamine, 50 μM β-mercaptoethanol, and 50 μg each of penicillin and streptomycin per ml. To isolate primary wild-type DP cells, thymocytes from C57BL/6 mice were stained with anti-CD4 (RM4-5) and anti-CD8a (53-6.7), and DP cells were sorted. Total thymocytes from Rag2−/− mice were used as wild-type DN thymocytes.
ChIP assays.
Chromatin immunoprecipitation (ChIP) was carried out essentially as described previously (7). Cross-linking was achieved by incubating 60 million cells in medium containing 1% formaldehyde for 10 min at room temperature. Cells were then pelleted and washed once with cold phosphate-buffered saline with freshly added protease inhibitors (Sigma-Aldrich, St. Louis, MO). The cell pellets were then resuspended in 6 ml of homogenization buffer (10 mM HEPES at pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.1% NP-40) with freshly added protease inhibitors, incubated for 10 min on ice, and homogenized with 10 strokes of a B pestle in a Dounce homogenizer. After spinning, the isolated nuclei were lysed in 600 μl of lysis buffer (1% sodium dodecyl sulfate [SDS], 10 mM EDTA, 50 mM Tris-HCl at pH 8.0) with freshly added protease inhibitors for 10 min on ice and were sonicated to obtain DNA fragments averaging approximately 200 to 500 bp. Chromatin-protein complexes from 10 million cells were used in each ChIP. Chromatin solutions were diluted 10-fold in TES-150 buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl at pH 8.0, 150 mM NaCl) with freshly added protease inhibitors, precleared with protein A-Sepharose beads, and then incubated with appropriate antibodies at 4°C overnight. Antibodies used included normal rabbit serum (as a negative control) (Sigma-Aldrich), rabbit polyclonal anti-RNAPII (sc-899; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-cyclin T1 (sc-10750; Santa Cruz Biotechnology), rabbit polyclonal anti-panRunx (a gift from Masanobu Satake, Tohoku University, Japan), and anti-Runx1 (pc285; EMD Biosciences, San Diego, CA) antibodies. Protein A-Sepharose beads were added the next day. After another 2 h of incubation, the beads were washed once in TSE-150, once in TSE-500 (like TSE-150 but with 500 mM NaCl), once in buffer III (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl at pH 8.0), and twice in TE (10 mM Tris, I mM EDTA, pH 8.0) buffer, all with freshly added protease inhibitors. Immunocomplexes were eluted from the beads with elution buffer (1% SDS and 0.5% NaHCO3) for 15 min at room temperature. Reverse cross-linking was performed at 65°C for 4 h and followed by treatment with proteinase K. DNA was extracted with phenol-chloroform, precipitated with ethanol, and dissolved in 30 μl of TE buffer. Two microliters of DNA was used in PCR with appropriate primer sets to amplify specific DNA fragments. Primer sequences are available upon request. PCR products, taken at various cycle numbers to ensure linear amplification, were separated on agarose gels and visualized with Sybr green. Each ChIP was repeated at least twice with similar results.
3C assays.
Chromatin conformation capture (3C) assays were carried out as previously described with modifications (18). Cross-linking was achieved by incubating 8 million cells in 10 ml medium containing 2% formaldehyde for 5 min at room temperature and was stopped by adding glycine to 0.125 M and incubating for another 5 min at room temperature. Cells were then pelleted in a conical tube and lysed in 10 ml of lysis buffer (10 mM Tris-HCl at pH 8.0, 10 mM NaCl, 0.2% NP-40) with freshly added protease inhibitors for 90 min at 4°C with rotation. The nuclei were collected and incubated in 400 μl of 1.2× restriction buffer 2 (New England Biolabs [NEB], Beverly, MA) containing 0.3% SDS at 37°C for 1 h with shaking. The SDS was then sequestered by adding Triton X-100 to 1.8% and incubating at 37°C for another hour with shaking. Three batches of StuI (NEB), a total of 900 U, were added in a 22-h period to achieve >80% digestion. The reaction was stopped by adding SDS to 1.6% and incubating at 65°C for 20 min. The extent of digestion was verified by quantitative real-time PCR using Sybr green detection (Sigma) across the StuI sites. Two micrograms of digested chromatin was diluted in 800 μl ligation buffer (NEB) (final concentration, 2.5 ng/μl, to ensure that only intramolecular ligation would occur). Residual SDS was sequestered by adding Triton X-100 to 2% and incubating at 37°C for 1 h with shaking. The reaction mixture was then cooled to 16°C, and 2,000 U of T4 DNA ligase (NEB) was added. After 4 h of ligation, the chromatin mixture was incubated with 100 μg/ml proteinase K at 65°C overnight to reverse cross-links. RNA was removed by RNase A (0.5 μg/ml) treatment for 30 min at 37°C, and DNA was purified by phenol extraction. Quantitative real-time PCRs were performed, in the presence of Sybr green (Sigma), with appropriate primers (sequences available upon request) from purified DNA as well as the control template. The control template was generated by PCR amplifying sequences around individual StuI sites to be analyzed and mixing them in equal molar amounts, followed by restriction digestion and ligation to include all possible ligation products. The relative cross-linking frequency between two fragments of the CD4 locus (XCD4 locus) is calculated as [(SCD4 locus/SHprt1 locus) cell]/[(SCD4 locus/SHprt1 locus) control], where SCD4 locus is the signal obtained with primer pairs for two restriction fragments of the CD4 locus from cells or the control template and SHprt1 locus is the signal obtained with primer pairs for two restriction fragments of the Hprt1 locus from cells or the control template. This calculation corrects for differences in cross-linking and ligation efficiencies, PCR amplification efficiency, the amount of the initial template used, and the sizes of the PCR products. For negative controls, we performed real-time PCR from StuI-digested, non-cross-linked genomic DNA and cross-linked chromatin, before and after ligation. In these control reactions, the Sybr green signals obtained from the EP and ES amplicons were normalized to signals obtained from a fragment that was not cut by StuI.
Retrovirus production and transduction.
Retroviruses were produced by transfecting Phoenix cells with pSM2 containing a microRNA-adapted short hairpin RNA (shRNAmir) cassette targeting the 3′ untranslated region (3′ UTR) of Runx1 mRNA (OpenBiosystems, Huntsville, AL) or with pMSCV-Runx1-IRES-hCD2 plus the pCL-10A1 packaging plasmid by using Fugene reagents (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Polybrene (4 μg/ml) was added to the viral supernatant, which was then added to 1200M cells in 6-well plates. The cells were spun at 2,500 rpm for 2 h at room temperature. Fresh medium was then added, and cells were incubated at 37°C under 5% CO2 overnight. For RNA interference experiments, transduced cells were selected in 1 μg/ml of puromycin.
Fluorescence-activated cell sorting (FACS).
Half a million cells were stained with fluorescein isothiocyanate- or phycoerythrin-conjugated anti-mouse CD4 (GK1.5; BD Biosciences-Pharmingen, San Diego, CA) or a rat immunoglobulin G2b(κ) isotype control (BD Biosciences-Pharmingen), or with biotin-conjugated anti-human CD2 (B-E2; Diaclone Research, Besancon, France) antibodies in conjunction with allophycocyanin-conjugated streptavidin (Caltag Laboratories, Burlingame, CA), and were analyzed using FACSCalibur flow cytometers and CellQuest software (BD Biosciences-Pharmingen).
Western blotting.
Runx1 and CycT1 protein levels were determined by Western blotting with rabbit polyclonal anti-panRunx and rabbit polyclonal anti-Cyc T1 (sc-10750; Santa Cruz Biotechnology) antibodies, respectively.
RESULTS AND DISCUSSION
Looping ChIP reveals differential interactions between the enhancer, silencer, and promoter in CD4− and CD4+ cells.
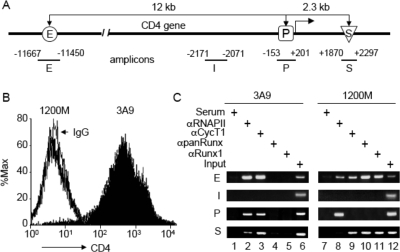
To investigate the role of chromatin structure in the expression of the CD4 gene in thymic development, we first performed ChIP assays and examined the recruitment of RNAPII, P-TEFb, and Runx1 to the CD4 locus. The expression of the mouse CD4 gene in immature T cells is controlled by a proximal promoter (P), an enhancer (E) located about 12 kb upstream of the transcription start site (TSS), and a silencer (S) within the first intron, 2.3 kb downstream from the TSS (Fig. 1A) (reviewed in reference 4). 3A9 is a T-cell hybridoma that represents CD4 SP thymocytes (Fig. 1B) (24). Previously, we demonstrated that RNAPII is present on the CD4 promoter as well as in the coding region (about 1 kb from the TSS) in these cells (7). In Fig. 1C, we demonstrate that RNAPII is also present on the CD4 enhancer in 3A9 cells (lane 2). Although it was reported that the locus control region of the human ɛ-globin locus can deliver RNAPII to the cis-linked promoter via facilitated tracking (31), we did not detect any transcription complexes in the intervening region (I) between the CD4 enhancer and promoter (Fig. 1C, panels I). Thus, this situation does not pertain to the CD4 gene. On the other hand, 1200M is a thymoma that represents ISP thymocytes, which do not express CD4 (Fig. 1B). It has been used extensively to characterize mechanisms of CD4 repression during thymocyte development. In these cells, RNAPII is detected on the enhancer and promoter but not on the silencer (Fig. 1C, lane 8), consistent with our previous finding that RNAPII is arrested at the promoter and cannot elongate in CD4− cells (7). That RNAPII is found on the CD4 enhancer is consistent with reports of sterile transcription from immunoglobulin (19) and major histocompatibility complex (MHC) class II (15) enhancers. The presence of CycT1 on the CD4 enhancer, promoter, and silencer in 3A9 cells (Fig. 1C, lane 3) is consistent with P-TEFb being recruited to proximal and distal sites and traveling with RNAPII during elongation (23). However, in 1200M cells, CycT1 is present at the CD4 enhancer but not at the promoter (Fig. 1C, lane 9), indicating that P-TEFb is recruited only to the distal enhancer in these cells. More surprisingly, the CD4 silencer was immunoprecipitated by anti-CycT1 antibodies in these cells (Fig. 1C, lane 9). This observation suggested that chromatin looping brought the CD4 enhancer close to the silencer in 1200M cells, allowing Runx1 to exert its inhibitory effects on P-TEFb. This hypothesis was confirmed by the presence of both enhancer and silencer fragments in anti-Runx1 immunoprecipitates from 1200M cells (Fig. 1C, lanes 10 and 11). Importantly, no Runx1 binding to the CD4 locus was observed in 3A9 cells (Fig. 1C, lanes 4 and 5).
FIG. 1.
Recruitment of RNAPII, P-TEFb (CycT1), and Runx1 to the CD4 locus. (A) Schematic diagram of the CD4 locus and the amplicons used in ChIP assays. The CD4 enhancer (E) is located 12 kb upstream from the promoter (P), and the CD4 silencer (S) is located in the first intron, 2.3 kb downstream from the promoter. I designates the amplicon located between the enhancer and the promoter. (B) FACS analyses of CD4 expression in 1200M (open peak) and 3A9 (solid peak) cells stained with fluorescein isothiocyanate-conjugated anti-mouse CD4 or a rat immunoglobulin G2b(κ) isotype control (thin black line). The intensity of CD4 staining and the relative number of cells are shown on the x and y axes, respectively. (C) ChIP analyses of the CD4 locus in 1200M (CD4−) and 3A9 (CD4+) cells. Anti-RNAPII, anti-CycT1, anti-panRunx, and anti-Runx1 antibodies were used as described in Materials and Methods. Normal rabbit serum served as the negative control for antibody specificity. The presence of the CD4 enhancer, intervening sequence, promoter, and silencer in the immunoprecipitates was examined by PCR with primer sets E, I, P, and S, respectively. PCR analyses with DNA before immunoprecipitation (Input) served as controls for the amplification efficiencies of individual primer sets. PCRs were carried out at various cycle numbers to ensure linear amplification. Representative agarose gels of PCR products within the linear range are presented.
Different chromatin loops form between the enhancer, silencer, and promoter in CD4− and CD4+ cell lines as well as DN and DP thymocytes.
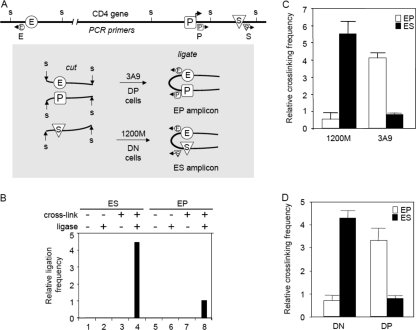
To test our hypothesis further, we performed 3C analyses and examined the chromatin structure at the CD4 locus in 1200M and 3A9 cells as well as in DN and DP thymocytes. In this assay, chromatin segments that are brought into close proximity by protein complexes are cross-linked with formaldehyde. After digestion with appropriate restriction endonucleases (here StuI), chromatin is diluted so that only intramolecular ligation can occur. Ligated DNA is further purified after reverse cross-linking, and ligation products are detected by quantitative PCR with appropriate primer sets as indicated in Fig. 2A. The relative cross-linking frequency of the CD4 enhancer with the silencer and that of the enhancer with the promoter were evaluated by comparing the amounts of quantitative PCR products obtained from the ES and EP amplicons. We first tested the specificity of the assay by comparing quantitative PCR signals from the ES and EP amplicons from StuI-digested, non-cross-linked 1200M genomic DNA or cross-linked chromatin before and after ligation. As shown in Fig. 2B, PCR signals were obtained only from cross-linked chromatin after ligation (Fig. 2B, lanes 4 and 8), not from cross-linked chromatin before ligation (Fig. 2B, lanes 3 and 7) or from genomic DNA with or without ligation (Fig. 2B, lanes 1, 2, 5, and 6). We then performed 3C analyses in 1200M and 3A9 cells. As presented in Fig. 2C, in 1200M cells, the enhancer cross-links more frequently with the silencer, whereas in 3A9 cells, the enhancer cross-links more frequently with the promoter. These observations were confirmed with primary thymocytes. In primary DN thymocytes, the enhancer cross-links more frequently with the silencer. In contrast, in primary DP thymocytes, the enhancer cross-links more frequently with the promoter (Fig. 2D). These results indicate that in CD4− thymocytes, chromatin looping brings the CD4 enhancer into close proximity with the silencer, allowing Runx1 to exert its inhibitory effects on P-TEFb, whereas in CD4+ thymocytes, a different chromatin loop forms between the enhancer and the promoter, allowing P-TEFb to activate transcription elongation.
FIG. 2.
3C analyses of the CD4 locus. (A) Diagram of 3C analysis. The locations of StuI restriction sites (s) and PCR primers (arrows) used in 3C analyses, relative to the CD4 enhancer (E), promoter (P), and silencer (S), are indicated. Cross-linked and digested chromatin was ligated at very low concentrations so that only intramolecular ligation could occur. Ligation products were detected by quantitative real-time PCR with appropriate primer sets in the presence of Sybr green. The relative cross-linking frequency of the CD4 enhancer with the silencer and that of the enhancer with the promoter were evaluated by the PCR signals obtained from the ES and EP amplicons, both normalized to Hprt1 and the control template as described in Materials and Methods. (B) Negative controls of 3C analysis. Real-time PCRs were performed from StuI-digested, non-cross-linked 1200M genomic DNA and cross-linked 1200M chromatin before and after ligation. Sybr green signals from the EP and ES amplicons were normalized to signals from a fragment that was not cut by StuI. Since no signal was obtained from non-cross-linked or nonligated samples after 40 cycles (lanes 1 to 3 and 5 to 7), the signal from the EP amplicon from cross-linked and ligated 1200M chromatin (lane 8) was set to 1. (C) 3C analyses of the CD4 locus in 1200M and 3A9 cells. The y axis shows the relative cross-linking frequency of the enhancer with the promoter (EP) and of the enhancer with the silencer (ES) compared to the Hprt1 locus (set to 1; not shown on the graph). At least two independent experiments were performed for each cell population, and more than three measurements were carried out for each experiment. Results of one typical experiment are presented. Error bars, standard deviations. (D) 3C analyses of the CD4 locus in primary DN and DP thymocytes were carried out as described for panel C.
Runx1 and P-TEFb dictate the formation of different chromatin loops in CD4− and CD4+ cells.
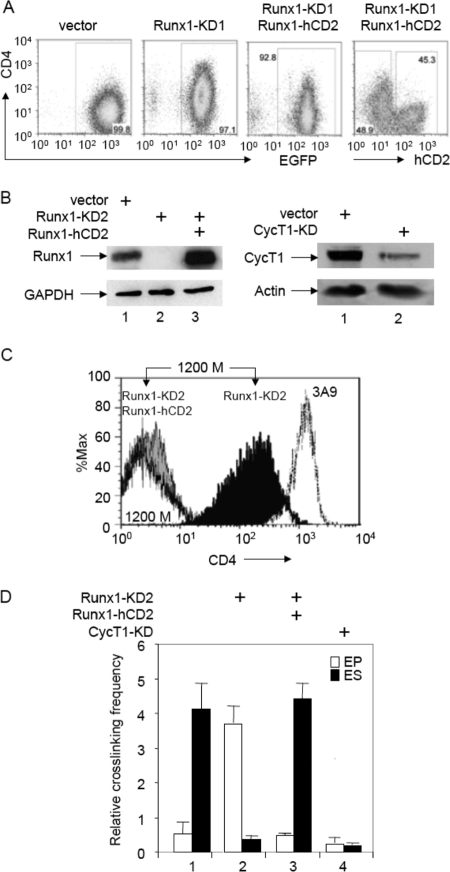
To examine the roles of Runx1 and P-TEFb in this differential looping of the CD4 locus, we next performed RNA interference knockdowns of Runx1 in 1200M cells. 1200M cells transduced with murine stem cell virus (MSCV) vector encoding a shRNA targeting the 3′ UTR of Runx1 (Runx1-KD1, for knockdown) showed 70 to 90% reductions of Runx1 expression levels when examined by quantitative reverse transcription-PCR or Western blotting (data not shown). As presented in Fig. 3A, these cells had higher levels of CD4 expression than those transduced with the empty vector. Reexpression of Runx1 by transducing Runx1-KD1 cells with an MSCV vector containing the Runx1-internal ribosome entry site (IRES)-human CD2 (Runx1-hCD2) sequence returned the expression of CD4 to baseline levels. To confirm that changes in CD4 expression were caused by specific Runx1 knockdown, another MSCV vector, which contained a different hairpin targeting the 3′ UTR of Runx1, was transduced into 1200M cells (Runx1-KD2). Again, protein levels of Runx1 were drastically reduced in Runx1-KD2 cells compared to those in 1200M cells transduced with the empty vector (Fig. 3B, left panel, lanes 1 and 2). This reduction of Runx1 levels led to the derepression of CD4, as revealed by FACS analysis (Fig. 3C; compare the solid black peak on the right to the white peak on the left). Interestingly, the reduction of Runx1 also resulted in a drastic reduction in the relative cross-linking frequency of the enhancer with the silencer and an increase in the relative cross-linking frequency of the enhancer with the promoter (Fig. 3D, bars 2), indicating that the loss of Runx1 binding to the silencer disrupted the chromatin looping between the enhancer and silencer, allowing the formation of the loop between the enhancer and promoter, as was observed in CD4+ cells (Fig. 2C and D). Introducing Runx1-hCD2 into Runx1-KD2 cells restored Runx1 expression in these cells (Fig. 3B, left panel, lane 3). Consistent with the repression of CD4 in Runx-1KD2/Runx1-hCD2 cells (Fig. 3C, gray solid peak to the left), the higher relative cross-linking frequency between the enhancer and the silencer indicated that Runx1 actively represses CD4 expression by mediating the formation of the loop between the enhancer and silencer in these cells (Fig. 3D, bars 3). On the other hand, transducing 1200M cells with an MSCV vector that contained a shRNAmir targeting CycT1 (CycT1-KD) led to a reduction of CycT1 levels to less than 10% of those observed with the empty vector (Fig. 3B, right panel). This knockdown of CycT1 resulted in a reduction in the relative cross-linking frequencies of both the enhancer with the silencer and the enhancer with the promoter (Fig. 3D, bars 4). Therefore, P-TEFb is required to establish both loops, that between the enhancer and the silencer and that between the enhancer and the promoter.
FIG. 3.
Runx1 and CycT1 knockdowns disrupt the looping between the CD4 enhancer and silencer in 1200M cells. (A) FACS analyses of CD4 expression in 1200M cells transduced either with the empty vector, with a retroviral vector containing a shRNAmir against Runx1 followed by IRES-green fluorescent protein (GFP) (Runx1-KD1), or with Runx1-KD1 plus MSCV vectors containing Runx1-IRES-human CD2 (Runx1-hCD2). The intensity of CD4 staining and the levels of enhanced GFP (EGFP) or hCD2 are presented on the y and x axes, respectively. (B) Western blotting of Runx1 and CycT1 levels in 1200M cells transduced either with the empty vector, with a retroviral vector containing a different shRNAmir against Runx1 followed by IRES-GFP (Runx1-KD2), with Runx1-KD2 plus Runx1-hCD2, or with a shRNAmir against CycT1 (CycT1-KD). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin were used as controls for equal loading. (C) FACS analyses of CD4 expression in 1200M (white peak to the left), 3A9 (dotted line to the right), Runx1-KD2 (black peak), and Runx1-KD2/Runx1-hCD2 (solid gray peak) cells stained with phycoerythrin-conjugated anti-mouse CD4. The intensity of CD4 staining and the relative numbers of cells are shown on the x and y axes, respectively. (D) 3C analyses of the CD4 locus. The relative cross-linking frequencies of the enhancer with the promoter (EP) and of the enhancer with the silencer (ES) are presented as described for Fig. 2C.
Concluding remarks.
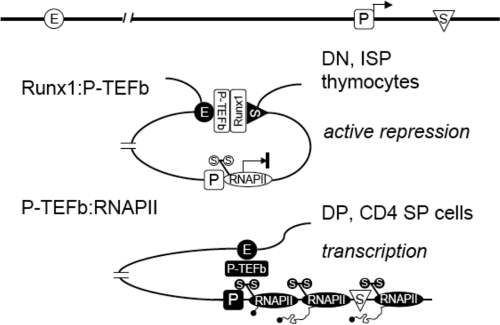
In this study, we provided evidence that (i) interactions between Runx1 and P-TEFb bring the silencer into close proximity with the enhancer in DN and ISP thymocytes, preventing P-TEFb on the enhancer from activating transcription elongation of the CD4 gene and (ii) lack of Runx1-binding to the silencer frees P-TEFb to interact with RNAPII, forming a different chromatin loop between the enhancer and the promoter in DP and CD4 SP thymocytes (Fig. 4). Finally, the knockdown of CycT1 from P-TEFb abolished both chromatin loops. Thus, differential looping by P-TEFb and decoying it away from the promoter mediate active repression of the CD4 gene during thymocyte development.
FIG. 4.
A model for active repression by Runx1. In DN and ISP thymocytes, the interaction between Runx1 and P-TEFb brings the enhancer (E) into the close proximity of the silencer (S) and prevents P-TEFb from activating RNAPII, which is arrested at the promoter (P), thus actively repressing transcription elongation. In DP and CD4 SP thymocytes, the lack of Runx1 binding to the CD4 silencer frees P-TEFb to interact with RNAPII and to activate transcription elongation. Only RNAPII on the CD4 promoter is depicted in the model.
This type of silencing represents a very dynamic state, where the appearance or loss of any one player changes the transcriptional program. Although other modifiers, insulators and/or boundary elements, which are expected to play critical roles in epigenetic silencing of the CD4 gene in mature CD8 SP cells, could also participate in chromatin remodeling and loop formation, our study demonstrates that P-TEFb is required for nonproductive (looping between the enhancer and silencer) as well as productive (looping between the enhancer and promoter) interactions between cis-acting elements in the CD4 locus in DN and DP thymocytes, respectively. Considering that P-TEFb can mediate the effects of other enhancers from proximal as well as distal sites (12, 23), such chromatin looping by P-TEFb could represent a general mechanism for the regulation of eukaryotic transcription. In support of this notion, similar intrachromosomal interactions have been demonstrated in the activation of the MHC class II genes (5), which require CIITA that acts via P-TEFb (8).
Acknowledgments
We thank Dan Littman and Takeshi Egawa for fruitful discussions and helping us to isolate primary thymocytes.
This work was supported by grants from the NIH and the Nora Eccles Treadwell Foundation.
We declare no conflict of interest with any aspects of this work.
Footnotes
Published ahead of print on 26 November 2007.
REFERENCES
- 1.Barboric, M., R. M. Nissen, S. Kanazawa, N. Jabrane-Ferrat, and B. M. Peterlin. 2001. NF-κB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell 8327-337. [DOI] [PubMed] [Google Scholar]
- 2.Bosselut, R. 2004. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat. Rev. Immunol. 4529-540. [DOI] [PubMed] [Google Scholar]
- 3.de Bruijn, M. F., and N. A. Speck. 2004. Core-binding factors in hematopoiesis and immune function. Oncogene 234238-4248. [DOI] [PubMed] [Google Scholar]
- 4.Ellmeier, W., S. Sawada, and D. R. Littman. 1999. The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Annu. Rev. Immunol. 17523-554. [DOI] [PubMed] [Google Scholar]
- 5.Gomez, J. A., P. Majumder, U. M. Nagarajan, and J. M. Boss. 2005. X box-like sequences in the MHC class II region maintain regulatory function. J. Immunol. 1751030-1040. [DOI] [PubMed] [Google Scholar]
- 6.Jang, M. K., K. Mochizuki, M. Zhou, H. S. Jeong, J. N. Brady, and K. Ozato. 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19523-534. [DOI] [PubMed] [Google Scholar]
- 7.Jiang, H., F. Zhang, T. Kurosu, and B. M. Peterlin. 2005. Runx1 binds positive transcription elongation factor b and represses transcriptional elongation by RNA polymerase II: possible mechanism of CD4 silencing. Mol. Cell. Biol. 2510675-10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanazawa, S., T. Okamoto, and B. M. Peterlin. 2000. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity 1261-70. [DOI] [PubMed] [Google Scholar]
- 9.Kanazawa, S., L. Soucek, G. Evan, T. Okamoto, and B. M. Peterlin. 2003. c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis. Oncogene 225707-5711. [DOI] [PubMed] [Google Scholar]
- 10.Kawazu, M., T. Asai, M. Ichikawa, G. Yamamoto, T. Saito, S. Goyama, K. Mitani, K. Miyazono, S. Chiba, S. Ogawa, M. Kurokawa, and H. Hirai. 2005. Functional domains of Runx1 are differentially required for CD4 repression, TCRβ expression, and CD4/8 double-negative to CD4/8 double-positive transition in thymocyte development. J. Immunol. 1743526-3533. [DOI] [PubMed] [Google Scholar]
- 11.Levanon, D., R. E. Goldstein, Y. Bernstein, H. Tang, D. Goldenberg, S. Stifani, Z. Paroush, and Y. Groner. 1998. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc. Natl. Acad. Sci. USA 9511590-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lis, J. T., P. Mason, J. Peng, D. H. Price, and J. Werner. 2000. P-TEFb kinase recruitment and function at heat shock loci. Genes Dev. 14792-803. [PMC free article] [PubMed] [Google Scholar]
- 13.Lutterbach, B., and S. W. Hiebert. 2000. Role of the transcription factor AML-1 in acute leukemia and hematopoietic differentiation. Gene 245223-235. [DOI] [PubMed] [Google Scholar]
- 14.Lutterbach, B., J. J. Westendorf, B. Linggi, S. Isaac, E. Seto, and S. W. Hiebert. 2000. A mechanism of repression by acute myeloid leukemia-1, the target of multiple chromosomal translocations in acute leukemia. J. Biol. Chem. 275651-656. [DOI] [PubMed] [Google Scholar]
- 15.Masternak, K., N. Peyraud, M. Krawczyk, E. Barras, and W. Reith. 2003. Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat. Immunol. 4132-137. [DOI] [PubMed] [Google Scholar]
- 16.Peterlin, B. M., and D. H. Price. 2006. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell 23297-305. [DOI] [PubMed] [Google Scholar]
- 17.Redondo, J. M., J. L. Pfohl, C. Hernandez-Munain, S. Wang, N. A. Speck, and M. S. Krangel. 1992. Indistinguishable nuclear factor binding to functional core sites of the T-cell receptor δ and murine leukemia virus enhancers. Mol. Cell. Biol. 124817-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spilianakis, C. G., M. D. Lalioti, T. Town, G. R. Lee, and R. A. Flavell. 2005. Interchromosomal associations between alternatively expressed loci. Nature 435637-645. [DOI] [PubMed] [Google Scholar]
- 19.Su, L. K., and T. Kadesch. 1990. The immunoglobulin heavy-chain enhancer functions as the promoter for Iμ sterile transcription. Mol. Cell. Biol. 102619-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniuchi, I., and D. R. Littman. 2004. Epigenetic gene silencing by Runx proteins. Oncogene 234341-4345. [DOI] [PubMed] [Google Scholar]
- 21.Taniuchi, I., M. Osato, T. Egawa, M. J. Sunshine, S. C. Bae, T. Komori, Y. Ito, and D. R. Littman. 2002. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 111621-633. [DOI] [PubMed] [Google Scholar]
- 22.Taniuchi, I., M. J. Sunshine, R. Festenstein, and D. R. Littman. 2002. Evidence for distinct CD4 silencer functions at different stages of thymocyte differentiation. Mol. Cell 101083-1096. [DOI] [PubMed] [Google Scholar]
- 23.Taube, R., X. Lin, D. Irwin, K. Fujinaga, and B. M. Peterlin. 2002. Interaction between P-TEFb and the C-terminal domain of RNA polymerase II activates transcriptional elongation from sites upstream or downstream of target genes. Mol. Cell. Biol. 22321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Telfer, J. C., E. E. Hedblom, M. K. Anderson, M. N. Laurent, and E. V. Rothenberg. 2004. Localization of the domains in Runx transcription factors required for the repression of CD4 in thymocytes. J. Immunol. 1724359-4370. [DOI] [PubMed] [Google Scholar]
- 25.Wang, S., Q. Wang, B. E. Crute, I. N. Melnikova, S. R. Keller, and N. A. Speck. 1993. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol. Cell. Biol. 133324-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92451-462. [DOI] [PubMed] [Google Scholar]
- 27.Westendorf, J. J., and S. W. Hiebert. 1999. Mammalian runt-domain proteins and their roles in hematopoiesis, osteogenesis, and leukemia. J. Cell. Biochem. Suppl. 32-33:51-58. [DOI] [PubMed]
- 28.Woolf, E., C. Xiao, O. Fainaru, J. Lotem, D. Rosen, V. Negreanu, Y. Bernstein, D. Goldenberg, O. Brenner, G. Berke, D. Levanon, and Y. Groner. 2003. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc. Natl. Acad. Sci. USA 1007731-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang, Z., J. H. Yik, R. Chen, N. He, M. K. Jang, K. Ozato, and Q. Zhou. 2005. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19535-545. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, F., M. Barboric, T. K. Blackwell, and B. M. Peterlin. 2003. A model of repression: CTD analogs and PIE-1 inhibit transcriptional elongation by P-TEFb. Genes Dev. 17748-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu, X., J. Ling, L. Zhang, W. Pi, M. Wu, and D. Tuan. 2007. A facilitated tracking and transcription mechanism of long-range enhancer function. Nucleic Acids Res. 355532-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu, Y., T. Pe'ery, J. Peng, Y. Ramanathan, N. Marshall, T. Marshall, B. Amendt, M. B. Mathews, and D. H. Price. 1997. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 112622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]