Abstract
Canonical Wnt signaling is mediated by a molecular “switch” that regulates the transcriptional properties of the T-cell factor (TCF) family of DNA-binding proteins. Members of the myeloid translocation gene (MTG) family of transcriptional corepressors are frequently disrupted by chromosomal translocations in acute myeloid leukemia, whereas MTG16 may be inactivated in up to 40% of breast cancer and MTG8 is a candidate cancer gene in colorectal carcinoma. Genetic studies imply that this corepressor family may function in stem cells. Given that mice lacking Myeloid Translocation Gene Related-1 (Mtgr1) fail to maintain the secretory lineage in the small intestine, we surveyed transcription factors that might recruit Mtgr1 in intestinal stem cells or progenitor cells and found that MTG family members associate specifically with TCF4. Coexpression of β-catenin disrupted the association between these corepressors and TCF4. Furthermore, when expressed in Xenopus embryos, MTG family members inhibited axis formation and impaired the ability of β-catenin and XLef-1 to induce axis duplication, indicating that MTG family members act downstream of β-catenin. Moreover, we found that c-Myc, a transcriptional target of the Wnt pathway, was overexpressed in the small intestines of mice lacking Mtgr1, thus linking inactivation of Mtgr1 to the activation of a potent oncogene.
Canonical Wnt signaling plays a critical regulatory role in development and in stem cell functions and cellular differentiation (41, 43). Wnts initiate a signaling cascade that leads to the nuclear accumulation of β-catenin, which associates with T-cell factor 4 (TCF4), releasing the TCF4-associated transcriptional corepressors and recruiting coactivators to stimulate TCF4-dependent transcription. The intestinal epithelium has been a rich source of information about this pathway. For example, Tcf4 is required for small intestinal stem cell self-renewal; mice lacking this transcription factor exhaust the capacity for continued replenishment of the epithelium in utero (24). Conversely, hyperactive Wnt signaling is closely associated with colorectal carcinoma, most commonly via inactivation of the APC tumor suppressor, which regulates the levels of β-catenin (41, 43). At the end point of the Wnt signaling cascade, β-catenin opposes the action of transcriptional corepressors for binding to TCFs to regulate genes that affect cell fate decisions.
Two members of the myeloid translocation gene (MTG) family of transcriptional corepressors, MTG on chromosome 8 (MTG8; also known as ETO or RUNX1T1) and MTG on chromosome 16 (MTG16; also known as ETO2 or CBFA2T3), are disrupted by chromosomal translocations in acute myeloid leukemia (10, 29), which suggests that these factors are key regulators of cellular proliferation or differentiation. As would be expected of master regulatory factors, these targets of chromosomal translocations in acute leukemia are commonly mutated in other tumor types as well. A genomewide screen to detect mutations of genes in colorectal and breast cancer from human patients identified MTG8 (RUNX1T1) as one of 189 new candidate cancer genes. In addition to RUNX1T1, we noted that mutations were also found in CBFA2T3 (MTG16/ETO2) in 2 of the 11 original colorectal tumor samples. Thus, in 5 of 11 colorectal tumor samples, an MTG family member was mutated (49), suggesting that members of this family of transcriptional corepressors are modifiers of cancer-related phenotypes.
In the mouse, gene deletion studies indicated that MTG factors play critical roles in gut development. Mtg8 is required for the development of the small and large intestines; 25% of the mice lacking this gene contained a deletion of the midgut, and 80% of the mice died in utero or as neonates (5). Although Mtg8-null mice displayed a deletion of the midgut, the enterocytes and the secretory lineages were present in the intestines of these mice in normal ratios, but the villi were often blunted and disorganized (5). By contrast, deletion of Myeloid Translocation Gene Related-1 (Mtgr1, also known as CBFA2T2) indicated that it was not required for the overall establishment of the gut architecture, but it was required for maintenance of the secretory lineage in the small intestine (1).
The inactivation of Mtgr1 appeared to impair lineage allocation in the small intestine at the level of the secretory progenitor or through alterations in intestinal stem cell functions (1, 32). In addition, loss of Mtgr1 sensitized the colonic epithelium to the effects of dextran sodium sulfate and impaired the regeneration of the colonic crypts, suggesting a role for Mtgr1 in the colonic stem cell (32). The loss of goblet, Paneth, and endocrine cells in Mtgr1-null small intestines may be partially attributed to the inactivation of the transcriptional repressor Gfi1 in the secretory progenitor cells, since Gfi1 recruits MTG family members and is required for maintenance of Paneth and goblet cells (1). However, Gfi1-null mice displayed increased numbers of endocrine cells, whereas Mtgr1-null mice showed a reduction in the numbers of these cells (1, 47). This suggested that at least one other transcription factor that mediates cell fate decisions in the small intestine might recruit MTG family proteins to repress transcription.
Here we examined whether the other DNA binding transcription factors that play a role in small intestinal lineage decisions and stem cell functions might recruit Mtgr1. We found that TCF4, but not Hes1 or Math1, associates with Mtgr1 and that Mtgr1 can oppose β-catenin-dependent transcriptional activation. In addition, expression of the Xenopus laevis homologue of Mtgr1 impaired axis formation during the development of frog embryos, which is regulated by Wnt signaling, and epistasis analysis indicated that this effect was downstream of β-catenin. Moreover, the c-Myc oncogene, which is regulated by TCF4 and β-catenin, was activated upon removal of Mtgr-1, which may begin to explain why this gene family is so frequently mutated in human cancer.
MATERIALS AND METHODS
Cell culture and transcription assays.
Cos7 and NIH 3T3 cells were cultured as previously described (2). K562 cells were cultured in RPMI medium containing 10% fetal bovine serum, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 2 mM l-glutamine (all from Mediatech, Inc.). Reporter assays were performed as described elsewhere (2) using NIH 3T3 cells and the quantities of plasmids indicated in the figure legends. Firefly luciferase activities were measured using the luciferase assay system (Promega) and were normalized to secreted alkaline phosphatase activity.
Generation of TCF4 deletion mutants.
Human TCF4 cDNA (Swiss-Prot accession number Q9NQB0) was used as a template for PCR, and primers were designed to amplify the desired regions of TCF4. EcoRI and XbaI restriction sites were engineered in the 5′ and 3′ primers, respectively, as well as an in-frame stop codon in the 3′ primer for each amplicon. A high-fidelity Taq/proofreading polymerase blend (Expand PCR system; Roche Biosciences) was used to generate TCF4 deletion constructs. Constructs were digested with EcoRI/XbaI and subcloned directionally into the pCMV5 Gal-M2 vector. All inserts were sequenced to ensure the fidelity of amplification.
Coimmunoprecipitations and immunoblotting.
Coimmunoprecipitations and immunoblotting were performed as described previously using Cos7 or K-562 cells (2); 0.5% Triton X-100-0.1% sodium deoxycholate-0.1% sodium dodecyl sulfate as the extraction and washing buffer (2); and anti-Mtgr1 (raised against amino acids 46 to 173), anti-TCF4 (U.S. Biologicals), anti-Myc (9E10; Covance), antihemagglutinin (HA) (12CA5; Covance), and anti-FLAG M2 beads (Sigma) or anti-GAL4 beads (Santa Cruz Biotechnology). Immune complexes were washed three times at 4°C with lysis buffer prior to immunoblot analysis (2).
Immunohistochemistry/histology.
Tissue was fixed in buffered formalin overnight at room temperature prior to embedding in paraffin and sectioning. Antigen retrieval was performed on all of the sections using a neutral pH antigen retrieval agent (DakoCytomation, Carpinteria, CA) before incubation with anti-Ki-67 and anti-bromodeoxyuridine (BrdU) (Novocastra, Newcastle, United Kingdom). Proteins were detected using the rabbit Envision+HRP system (DakoCytomation) and diaminobenzidine. Sections were lightly counterstained with Mayer's hematoxylin. Positive cells were counted, and the differences between wild-type and null cells were assessed using an unpaired Student t test with unequal variance.
RNA analysis.
RNA blot analysis was performed as described elsewhere (26) using the following probes: a 1.4-kb fragment of the c-Myc cDNA (S. Hann, Vanderbilt University), a full-length mouse Cyclin D1 cDNA (C. Sherr, St. Jude Children's Research Hospital), a full-length c-JUN cDNA (L. Matrisian, Vanderbilt University), and a full-length Id2 cDNA isolated by reverse transcription-PCR (RT-PCR). For the analysis of gene expression in the crypt region, RNA was isolated from 300 Mtgr1−/− or wild-type small intestinal crypts using laser capture microdissection. Crypts were identified by modified phase-contrast microscopy. Total RNA was purified using the PicoPure RNA isolation kit (Arcturus, Inc.). RNA was eluted in 14 μl and was used as a template for cDNA synthesis using the iScript system (Bio-Rad, Inc.). Five microliters was used for real-time PCR on a Bio-Rad iCycler using the Bio-Rad Sybr green reagent to track the synthesized product and to determine the threshold cycle. Primers specific for c-Myc were obtained from PrimerBank, and specificity was verified by sequencing the amplified cDNA.
RNA preparation, culture of frog embryos, and microinjection.
Synthetic mRNA molecules encoding the Xenopus homologue of Mtgr-1 (XETOR) were transcribed from pCS2+ XETOR (a gift from H. Grunz and Y. Cao [6]) using the SP6 Message Machine kit (Ambion). Mammalian Mtg16 and MTG8 were cloned into the XbaI site of pCS2+ and transcribed using the SP6 promoter. Xenopus Lef1 (XLef-1) was transcribed from pT7TS-XLLEF-1 (36) and kindly provided by Stefan Hoppler, University of Aberdeen, Aberdeen, United Kingdom) using the T7 Message Machine kit (Ambion). Embryos were fertilized in vitro, dejellied with 2% cysteine (pH 7.8), and subsequently cultured at 14°C in 10% Marc's modified Ringer solution (0.1× MMR) (40). Embryos were staged according to the method of Nieuwkoop and Faber (39). In vitro-transcribed capped XETOR mRNA was injected in the marginal zones of both dorsal blastomeres (3 ng each) at the 4-cell stage, and embryos were photographed at stage 26. Embryos injected with green fluorescent protein or β-galactosidase mRNA were included as a negative control. After injections, any embryos displaying obvious gastrulation defects or lethality were excluded from further analysis.
For determination of TOP-FLASH activity in Xenopus embryos, the TOP-FLASH reporter construct (0.5 ng) was coinjected with β-catenin mRNA (0.5 ng) either alone or together with XETOR mRNA (1.0 ng) into the animal pole region of one cell at the 2-cell stage. Three pools of 5 embryos each were collected at the gastrula stage and homogenized in 200 μl of passive lysis buffer (Promega). Luciferase activity was determined from 20 μl of pooled embryo lysates, and the experiment was repeated four times. Values were normalized to Renilla luciferase activity by using the dual luciferase assay (Promega).
To examine endogenous gene expression, embryos were injected in their animal poles at the 2-cell stage with XETOR (5 ng) and/or β-catenin (10 pg) mRNA. Ectodermal explants (animal caps) from injected and uninjected sibling control embryos were dissected at stage 9 and cultured in 0.75× MMR until stage 11. Total RNA was isolated from animal caps using RNA STAT-60 (Tel-Test) according to the manufacturer's guidelines. Cycling conditions for all genes were as follows: 95°C for 5 min, followed by 25 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. RT-PCR primers were 5′-AAGATAACTGGCATTCCTGAGC and 5′-GGTAGGGCTGTGTATTTGAAGG for Siamois, 5′-CGAGTGCAAGAAGGTGGACA and 5′-ATCTTCATGGGGACACAGGA for Xnr3, and 5′-CAGCTAGCTGTGGTGTGG and 5′-CAACATGGAAACTCACACC for ODC. We determined whether amplification was within the linear range for each primer set by using serial dilutions of reverse-transcribed cDNA as the PCR template.
RESULTS
MTG family members interact with TCF4.
Targeted deletion of Mtgr1 caused a depletion of the small intestinal secretory lineage cells that encompasses Paneth, goblet, and enteroendocrine cells (1). At least three transcription factors play key roles in lineage decisions in the small intestine. Hes1 is required for the development of the enterocyte lineage (21); Math1 is required for the secretory lineage (58); and Tcf4 is required for stem cell self-renewal (3, 24) and Paneth cell maturation (52). If Mtgr1 functions as a transcriptional corepressor, we reasoned that it is likely that Mtgr1 is recruited by transcription factors that regulate lineage allocation or stem cell functions. Therefore, we tested whether Mtgr1 associates with Hes1, Math1, or TCF4. GAL4 epitope-tagged Mtgr1 was coexpressed with FLAG-tagged Hes1, FLAG-tagged Math1, or HA-tagged TCF4, and Mtgr1-associated complexes were immunoprecipitated with anti-GAL4. Immunoblot analysis indicated that although both Hes1 and Math1 were robustly expressed, only TCF4 copurified with Mtgr1 (Fig. 1A). Similarly, immunoprecipitation of TCF4 copurified Mtgr1 (data not shown). We confirmed that endogenous Mtgr1 associates with endogenous TCF4 by using K-562 cells, which express detectable levels of Mtgr1 and TCF4. When TCF4 was immunoprecipitated, MTGR1 specifically copurified with anti-TCF4 but not with control immunoglobulin G (Fig. 1B).
FIG. 1.
Mtgr1 associates with TCF4. (A) Mtgr1 interacts with TCF4 but not with Math1 or Hes1. Cos7 cells were cotransfected with plasmids encoding GAL-Mtgr1 and either FL-Math1, FL-Hes1, or HA-TCF4. Coimmunoprecipitation (IP) analysis using an anti-GAL4 monoclonal antibody linked to agarose beads was followed by immunoblot analysis using a mixture of anti-HA and anti-FLAG, which detected the associated TCF4 (top panel). Immunoblot analysis using anti-GAL4 and anti-HA plus anti-FLAG confirmed the expression of Mtgr1 (middle panel) and of Math1, Hes1, and TCF4 (bottom panel). (B) Endogenous Mtgr1 copurifies with TCF4. Whole-cell lysates (WCL) from K-562 cells were immunoprecipitated with anti-TCF4, and the associated MTGR1 was detected by immunoblot analysis. Lysates in the control lane (Con) were immunoprecipitated with normal mouse immunoglobulin G (IgG). Asterisks indicate background bands that were consistently observed in more than five experiments.
Based on this initial observation, we extended this analysis to the other MTG/ETO and TCF family members. Immunoprecipitation and Western blot analysis indicated that, like Mtgr1, MTG8/ETO and Mtg16 (also known as ETO2) associated with TCF4 (Fig. 2A). In the subsequent analysis, these factors were used interchangeably with similar results. In addition, we tested whether other TCF family proteins might associate with Mtgr1 (Fig. 2B). Although TCF1 was poorly expressed in this experiment, it appeared to associate with Mtgr1 as well as TCF4. By contrast, less Lef1 associated with Mtgr1, and TCF3 failed to associate with Mtgr1 under these conditions (Fig. 2B).
FIG. 2.
(A) TCF4 associates with all of the members of the MTG family. Cos7 cells were cotransfected with pCMV5-HA-TCF4 and either vector alone (control [Con]), pCMV5-GAL-MTG8, pCMV5-GAL-Eto2 (MTG16), or pCMV5-GAL-Mtgr1 and were analyzed as described in the legend to Fig. 3B. An anti-HA immunoblot was used to detect the presence of HA-TCF4 (top panel) in lanes where an MTG family member was coexpressed (middle panel), but not in the vector-only lane. (B) Mtgr1 associates with TCF1, Lef1, and TCF4. GAL-Mtgr1 was coexpressed with the indicated TCF family members. The level of expression of the TCF was determined by immunoblot analysis of whole-cell lysates (WCL) (bottom panel). The expression and purification of Mtgr1 were confirmed by immunoblotting using anti-GAL4 after immunoprecipitation (IP) (middle panel). The Mtgr1-associated TCF family members were detected by immunoblotting (top panel). IgH, immunoglobulin H.
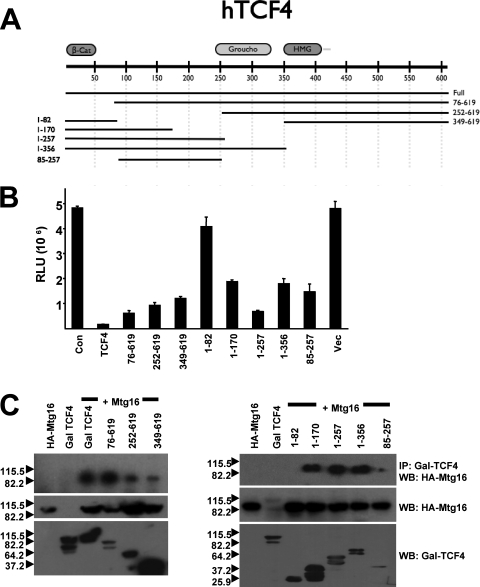
To determine whether the association between MTG family proteins and TCF4 contributes to TCF-mediated repression, we created a series of GAL4-TCF4 fusion proteins containing either N-terminal or C-terminal portions of the largest isoform of TCF4 (Fig. 3A). These fusion proteins were then used to identify the TCF4 domains that are sufficient to mediate transcriptional repression and the domains that contact MTG family members in order to determine whether these activities cosegregate. Although TCF-mediated repression is complex and cell type dependent (14), when the GAL4-TCF4 fusion proteins were cotransfected into NIH 3T3 cells with a promoter consisting of five GAL4 DNA binding sites linked to the thymidine kinase (TK) promoter, full-length GAL4-TCF4 repressed the GAL4-TK reporter gene whereas the GAL4 DNA binding domain had no effect (Fig. 3B). The N-terminal domain of TCF4, which mediates interaction with β-catenin (amino acids 1 to 82), failed to repress transcription, and deletion of this domain (amino acids 76 to 619) did not affect TCF4-mediated repression (Fig. 3B). (Note that amino acids 76 to 619 consistently failed to express as well as full-length GAL-TCF4 [Fig. 3C].) Further N-terminal deletions of TCF4 indicated that the C terminus including the high-mobility group domain was sufficient to mediate repression (Fig. 3B). In addition, serial C-terminal truncation mutants indicated that the first 257 amino acids of TCF4 yielded robust repression and further deletion to residue 170 impaired this repression, yet even residues 1 to 170 reduced expression by approximately 60%. Given the lack of repression by the 1-82 mutant, we constructed the GAL4(85-257) fusion protein, and this small domain of TCF4 repressed transcription, even though it was poorly expressed (Fig. 3B and 3C, bottom panel). Thus, at least two TCF4 domains were capable of mediating repression.
FIG. 3.
Mapping of TCF4 repression domains and TCF4 motifs that mediate MTG binding. (A) Schematic diagram of Gal4-TCF4 fusion proteins used. (B) Identification of TCF4 repression domains. NIH 3T3 cells were transfected with the Gal4-TK-luciferase reporter plasmid (0.2 ng) and Gal4-TCF4 or the indicated mutant (0.15 ng). A plasmid expressing secreted alkaline phosphatase was used to normalize for transfection efficiency. Con, control; Vec, vector. (C) Identification of Mtg16 binding sites on TCF4. Mtg16 and the GAL4-TCF4 deletion mutants were coexpressed in Cos7 cells and immunoprecipitated (IP) from whole-cell lysates using anti-GAL4 agarose beads, followed by Western blot (WB) analysis to detect the associated proteins.
Next, we used the GAL4-TCF4 fusion proteins to identify the domains of TCF4 that are sufficient to contact MTG family members. Coexpression of full-length GAL4-TCF4 and the N-terminal deletions of TCF4 with HA-Mtg16, followed by immunoprecipitation with anti-GAL4 and Western blot analysis with anti-HA, indicated that full-length GAL4-TCF4 and the N-terminal deletion mutants associated with Mtg16, which places the N-terminal boundary of an MTG interaction domain just upstream of the HMG domain (Fig. 3C). Although this is not a highly quantitative method, the signals were consistently weaker for the 252-619 and 349-619 domains, suggesting that an N-terminal domain contributed to MTG binding. Nevertheless, given the lack of binding to the 1-82 mutant, this result indicated that the C-terminal domain was sufficient to mediate an association with Mtg16. Using the C-terminal deletion mutants in a similar analysis, we found that residues 1 to 170 were also sufficient to contact Mtg16 (Fig. 3C). Although the 85-257 mutant was difficult to express, it too associated with Mtg16. Thus, at least two domains of TCF4 are sufficient to mediate association with Mtg16, which is similar to other factors known to associate with MTG family members, such as Gfi1 and N-CoR (2, 12, 30, 33, 54).
β-Catenin disrupts the MTG-TCF4 interaction.
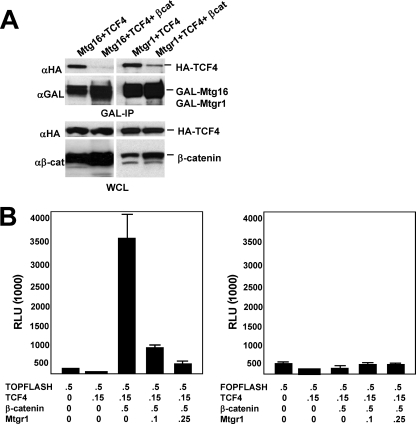
TCF4 functions at the end point of the canonical Wnt signaling pathway to mediate changes in gene expression. Upon activation of the Wnt signaling cascade, β-catenin is stabilized and accumulates in the nucleus, where it binds to the N terminus of TCF4. The association of β-catenin with TCF4 causes a conformational shift that dissociates transcriptional corepressors from TCF4, allowing β-catenin to recruit coactivators and activate transcription (9, 53). Therefore, we coexpressed β-catenin, TCF4, and Mtgr1 or Mtg16 to determine whether β-catenin could affect the interaction between MTG family members and TCF4. In the absence of β-catenin, once again Mtgr1 or Mtg16 associated with TCF4. By contrast, when β-catenin was coexpressed, the association between Mtgr1 or Mtg16 and TCF4 was significantly diminished (Fig. 4A). Given that Mtg16 binds to regions on TCF4 distinct from the β-catenin binding site, β-catenin may cause a conformational shift in TCF4 to release MTG family members.
FIG. 4.
Mtgr1 is a corepressor for TCF4. (A) Expression of β-catenin disrupts the interaction between Mtgr1 or Mtg16 and TCF4. GAL-Mtgr1 or GAL-Mtg16 and HA-TCF4 were transfected into Cos7 cells with or without a β-catenin expression plasmid. Immunoprecipitation (IP) with anti-GAL was followed by anti-HA (αHA) immunoblotting to detect TCF4 (top panel). Anti-GAL immunoblot analysis was used to detect the expression of GAL-Mtgr1 in the immune complexes, while anti-HA immunoblotting confirmed that equal amounts of TCF4 were present in whole-cell lysates (WCL). The bottom panel shows an immunoblot with anti-β-catenin (αβ-cat), which detects both endogenous and overexpressed protein. (B) Mtgr1 impairs β-catenin-induced activation of a TCF reporter plasmid in a dose-dependent fashion. NIH 3T3 cells were transfected with the TOP-FLASH or FOP-FLASH (containing mutant TCF sites) reporter and either TCF4 alone, TCF4 plus β-catenin, or TCF4 plus β-catenin and either 100 or 250 ng of an Mtgr1 expression plasmid, as indicated. A plasmid expressing cytomegalovirus-secreted alkaline phosphatase was used to normalize for transfection efficiency. Numbers below the graphs indicate the amount of DNA in micrograms.
Based on the association of TCF4 and MTGs, we tested whether Mtgr1 could affect the expression of a TCF-dependent reporter gene. TOP-FLASH is a plasmid containing 4 TCF-consensus-binding motifs upstream of a minimal promoter driving the expression of firefly luciferase. TCF4 repressed the expression of TOP-FLASH, and only minor, nonspecific repression was observed for the sister reporter plasmid that contains mutant TCF binding sites (FOP-FLASH) (Fig. 4B). However, coexpression of TCF4 and β-catenin transactivated TOP-FLASH but not FOP-FLASH (Fig. 4B). When Mtgr1 was coexpressed with TCF4 and β-catenin, Mtgr1 impaired β-catenin-mediated transactivation in a dose-dependent manner (Fig. 4B). However, Mtgr1 had no effect on FOP-FLASH or the internal-control plasmid (pCMV-SEAP), indicating that Mtgr1 can effectively reverse the effects of β-catenin on TCF4-mediated transcription.
Loss of Mtgr1 results in activation of c-Myc.
The association of MTG family members with TCF4 and the ability of β-catenin to disrupt this interaction suggested that MTG family members function as negative regulators of the canonical Wnt signaling pathway. Analysis of data derived from cDNA microarray analysis of wild-type versus Mtgr1-null small intestinal RNA samples (unpublished data) identified a group of modestly up-regulated genes that are regulated by TCFs, including c-Myc, fibroblast growth factor 20, follistatin, and proliferin 2 (7, 17, 28, 55). In addition, a similar analysis of hematopoietic progenitor cells negative for lineage-specific markers and positive for the stem cell markers Sca-1 and c-Kit, which were isolated from the bone marrow of Mtg16-null mice, identified Cyclin D1 and Id2 (44, 48, 51) as activated in the absence of Mtg16 (I. Moreno-Miralles and S. W. Hiebert, unpublished data). Therefore, RNA blot analysis was used to measure the expression of Cyclin D1, c-Myc, and Id2, as well as c-Jun (31), which was not identified in the microarray studies. In RNA isolated from the distal small intestine, the levels of Cyclin D1 and c-Jun RNA were similar for wild-type and Mtgr1-null mice (Fig. 5A). However, Id2 mRNA levels were slightly higher, and c-Myc levels were two- to fourfold higher, in the small intestines of Mtgr1-null mice (Fig. 5A).
FIG. 5.
Loss of Mtgr1 activates the TCF4-regulated gene c-Myc. (A) RNA blot analysis of total RNA isolated from the small intestines of 8-week-old wild-type (WT) and Mtgr1-null littermates using probes for the TCF targets c-Myc, Cyclin D1, Id2, and c-Jun as indicated. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to control for RNA loading (lower panels). (B) c-Myc is overexpressed in the crypts of Mtgr1-null mice. Laser capture microdissection was used to isolate crypt epithelial cells, and quantitative RT-PCR was used to assess the levels of c-Myc mRNA in WT and Mtgr1-null small intestines. CT, threshold cycle; the change in the CT was calculated relative to β-actin. Shown are CT values from two experiments each. The difference in CT is equivalent to a 2.9-fold induction.
To verify that the changes in the expression of c-Myc occurred in the epithelium, we used laser capture microdissection to isolate epithelial cells from the crypts of wild-type and Mtgr1-null mice, prepared RNA, and performed quantitative RT-PCR. The higher c-Myc mRNA levels in the crypts of Mtgr1-null mice mirrored the changes observed in the whole small intestine (2.9-fold increase) (Fig. 5B). Thus, the inactivation of Mtgr1 enhances c-Myc expression in the epithelial compartment of the small intestine.
MTG family members affect axis formation in Xenopus embryos.
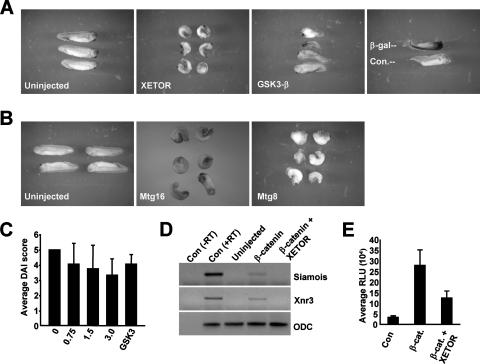
TCFs and Wnt signaling also play a critical role in axis formation during Xenopus development (50). Thus, to determine if MTG family members are involved in axis formation, we microinjected in vitro-transcribed mRNA encoding the Xenopus laevis homologue of Mtgr1 (termed Xenopus ETO-related, or XETOR), which is 72% identical to Mtgr1 and 59% identical to MTG8 (6), into the marginal zones of both dorsal blastomeres at the 4-cell stage, and the embryos were monitored through stage 26. In contrast to control embryos that developed normally (Fig. 6A), the embryos injected with XETOR mRNA were ventralized; they had a shortened body axis, reduced head structures, and defective dorso-anterior patterning, a phenotype that is associated with down-regulation of Wnt signaling. This phenotype was similar to that found for embryos injected with RNA encoding GSK3-β, a negative regulator of Wnt signaling (Fig. 6A) (18). This phenotype was highly penetrant—more than 90% of the embryos were ventralized by XETOR (n = 133; six experiments)—and was not observed when a nonspecific control protein (β-galactosidase or green fluorescent protein) was expressed (Fig. 6A and data not shown, respectively).
FIG. 6.
XETOR affects axis formation in Xenopus embryos. (A) Microinjection of XETOR ventralizes Xenopus embryos. Injection of XETOR RNA into the marginal zones of both dorsal blastomeres at the 4-cell stage (3 ng each) resulted in 92% (123/133; six independent experiments) of the embryos having a ventralized phenotype compared to uninjected sibling controls (left panel). As a positive control, injection of GSK-3β RNA (500 pg each) into the marginal zones of both dorsal blastomeres at the 4-cell stage also resulted in embryos having a ventralized phenotype (82%; 14/17). Embryos were injected with mRNA encoding β-galactosidase (β-Gal) as an injection control. An embryo stained for β-Gal is shown above an uninjected embryo for comparison. (B) Mammalian Mtg16 and MTG8 act similarly to XETOR. Microinjection of 3 ng of mRNA encoding Mtg16 (75%; 12/16 embryos) or MTG8 (61%; 37/60 embryos; four independent experiments) into the marginal zones of both dorsal blastomeres at the 4-cell stage ventralized the embryos as in the experiment for which results are shown in panel A, in contrast to uninjected sibling controls (left panel). (C) Xenopus embryos at the 2- to 4-cell stage were injected with increasing amounts of XETOR mRNA, as indicated, in both dorsal blastomeres, and DAI scores were determined. GSK3β was included as a positive control. The numbers below the graph indicate the amount of mRNA injected (in nanograms). (D) XETOR impairs β-catenin-dependent transactivation in vivo. Embryos at the 2-cell stage were either mock injected, injected with β-catenin alone, or coinjected with mRNAs encoding β-catenin and XETOR, and the levels of the Wnt target genes Siamois and Xnr3 were determined by RT-PCR analysis of the animal caps. Con (−RT), PCR lacking reverse transcriptase, used as a PCR control; Con (+RT), positive control for the PCR. (E) In vivo analysis of TCF-dependent transcription. The TOP-FLASH reporter plasmid was coinjected with mRNA encoding the indicated transcription factors. Luciferase activity was determined, in triplicate, from lysates made from pooled embryos (typically 5 embryos). β-cat., β-catenin. Results of one representative experiment out of four performed are shown.
Because of the high level of conservation of the Wnt signaling cascade between frogs and mammals, many murine or human genes also function in this assay. Therefore, we tested whether Mtg16 or MTG8 might also impair axis formation when expressed in Xenopus embryos. Like XETOR, Mtg16 and MTG8 impaired dorso-anterior patterning with high penetrance (75% and 61%, respectively) when the mRNA encoding either of these factors was injected into the marginal zones of the dorsal blastomeres (Fig. 6B). To determine if MTG family members ventralize Xenopus embryos in a dose-dependent manner, embryos were injected dorsally at the 2- to 4-cell stage with increasing amounts of XETOR or MTG8 RNA, and the degree of ventralization was determined using the dorsoanterior index (DAI) (normal embryos have a DAI of 5; completely ventralized embryos have a DAI of 0). Both XETOR and MTG8 ventralized embryos in a dose-dependent manner (Fig. 6C and data not shown), with average DAI scores of 3.3 and 3.8 for embryos injected with 3 ng of XETOR or MTG8 RNA, respectively. This was similar to the DAI obtained after injection of GSK3-β mRNA (Fig. 6C). In addition, we performed an RT-PCR analysis to examine the levels of the Xenopus Wnt target genes Siamois and Xnr3 in embryos coinjected with mRNAs encoding β-catenin and XETOR. β-Catenin alone caused up-regulation of both of these genes, but coinjection of β-catenin and XETOR mRNAs blunted the induction of both Siamois and Xnr3 (Fig. 6D).
To further examine the specificity of the effect of MTG family members on TCF-mediated transcription in Xenopus embryos, we measured the activity of a TCF-dependent promoter in vivo in the absence and presence of expressed XETOR. The TCF-dependent reporter TOP-FLASH was microinjected into embryos at the 4-cell stage, and 8 h later, the embryos were pooled and luciferase activity assessed. This procedure yielded easily measurable levels of basal activity from TOP-FLASH, and coinjection of β-catenin further activated the reporter (Fig. 6E). Unfortunately, the companion reporter, FOP-FLASH, containing mutant TCF binding sites, did not yield significant activity either in the absence or in the presence of β-catenin. While this emphasizes that the activity of TOP-FLASH is dependent on the endogenous TCF family members in Xenopus embryos, the FOP-FLASH reporter could not be used for further analysis of XETOR activity (data not shown). Nevertheless, when mRNA encoding XETOR was included in the TOP-FLASH assay, XETOR impaired β-catenin-mediated transactivation in Xenopus embryos (Fig. 6E).
XETOR impairs β-catenin-mediated axis duplication.
Stimulation of Wnt signaling by microinjection of mRNA encoding β-catenin induces axis duplication in Xenopus embryos (11). To further confirm that XETOR acts at the level of TCFs, we performed an epistasis experiment by injecting mRNA encoding β-catenin alone or coinjecting mRNA encoding β-catenin and XETOR to determine if XETOR might impair the action of β-catenin during development. Roughly half (51%; 52/102 embryos; four separate experiments) of the embryos injected with β-catenin displayed a duplicated axis, but this effect was significantly diminished by the coexpression of XETOR mRNA (25%; 29/118 embryos; four separate experiments) (Fig. 7A). Though the ability of XETOR to impair axis duplication differed between experiments, the effect of XETOR on β-catenin activity was dose dependent (Fig. 7B). This epistasis experiment places XETOR at the end point of the Wnt signaling cascade, at the same regulatory node or downstream of β-catenin.
FIG. 7.
XETOR acts at the level of TCFs. (A) Epistasis analysis places XETOR downstream of β-catenin. β-catenin RNA (25 pg) induces secondary-axis formation (51%; 52/102; four independent experiments) when injected into one of the two ventral blastomeres at the 4-cell stage. Secondary-axis induction by β-catenin RNA injection (25 pg) was significantly impaired (25%; 29/118 embryos duplicated; four independent experiments) when XETOR RNA (3 ng) was coinjected. Results of a representative experiment are shown. (B) XETOR impairs β-catenin (β-cat)-mediated axis duplication in a dose-dependent manner. Embryos were injected ventrally at the 2- to 4-cell stage with 25 pg of β-catenin and increasing amounts of XETOR, and the percentage of axis duplication was determined. Expt., experiment. (C) XETOR impairs XLef1-induced axis duplication. Embryos at the 2- to 4-cell stage were injected in both ventral blastomeres with 0.75 ng XLef-1 mRNA either alone or together with 3 ng XETOR. XLef-1 resulted in axis duplication, which was not observed for uninjected sibling controls. Coinjection with XETOR mRNA significantly decreased the number of embryos with a duplicated axis. (D) XETOR impairs XLef-1 axis duplication in a dose-dependent manner. Embryos were injected ventrally with 0.75 ng XLef-1 and increasing amounts of XETOR mRNA as indicated, and the percentage of axis duplication was determined.
β-Catenin acts through TCF family members, and Lef1 induces axis duplication when its mRNA is microinjected ventrally into Xenopus embryos (4, 15, 19, 36). Therefore, we also tested the ability of XETOR to impair Lef1-induced axis duplication. Approximately 57% of the embryos injected with XLef-1 alone exhibited a duplicated axis, while XETOR impaired this XLef-1 activity in a dose-dependent manner (Fig. 7C). The ability of XETOR to impair XLef-1-induced axis duplication is consistent with the finding that Mtgr1 binds TCF family members including Lef1 and can impair β-catenin-mediated transcriptional activation (Fig. 4B).
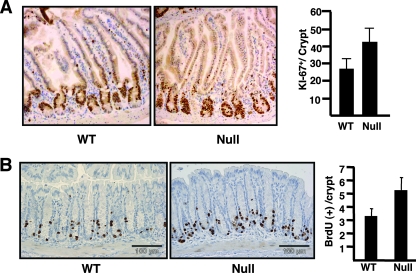
In order to determine whether the inactivation of MTGs might also affect TCF functions, we returned to the small intestines of Mtgr1-null mice rather than attempting to simultaneously inhibit the action of the three MTG homologues that are expressed in Xenopus embryos (6, 25). TCF4 is not required for maintaining the secretory cell lineage in the small intestine, but constitutive activation of Wnt signaling by overexpression of β-catenin caused an expansion of the cycling cells in the crypts of the small intestine (56). Therefore, we used anti-Ki-67 to enumerate the cycling cells in the crypts of the small intestines of Mtgr1-null mice. Compared to those for littermate control mice, immunohistochemical staining with anti-Ki-67 indicated that the numbers of proliferating cells in the crypts of Mtgr1-null mice were increased by approximately 50% (Fig. 8A). Thus, the loss of Mtgr1 allowed the expansion of the proliferative compartment in the crypts of the small intestines, which phenocopies some aspects of β-catenin overexpression (56). Furthermore, we observed activation of the TCF target gene c-Myc in the proliferative compartment of the small intestine crypts (Fig. 5B). Moreover, when BrdU was injected into mice prior to necropsy in order to label cycling cells in the colon, a similar expansion of cycling cells was observed in the crypts of the colon (Fig. 8B), even though there is no loss of secretory cells in the colons of Mtgr1-null mice. Thus, inactivation of Mtgr1 stimulates proliferation in the crypts of the small intestinal and colonic epithelia.
FIG. 8.
Mtgr1−/− mice display an accumulation of cycling cells in the crypts of the small intestines and colons. (A) Proliferation in the small intestine. Proliferating cells were detected in sections of the jejunum from 8-week-old wild-type (WT) and Mtgr1-null mice by immunohistochemistry with anti-Ki-67. Positive staining is brown, with a blue hematoxylin counterstain. Graph shows the quantification of Ki-67-positive cells. Ki-67-positive cells from 100 consecutive histologically complete crypts were counted, and the average number of positive cells per crypt was calculated. Error bars, standard deviations. (B) Inactivation of Mtgr1 leads to the accumulation of cycling cells in the crypts of the colon. Proliferating cells were detected in sections of the colon from 8-week-old WT and Mtgr1-null mice by immunohistochemistry with anti-BrdU 2 h after administration of BrdU. Graph shows the quantification of the average number of positive cells per crypt. Error bars, standard deviations.
DISCUSSION
The chromosomal translocations that are associated with acute leukemia pinpoint key regulators of cellular proliferation and differentiation pathways. We show that all three MTG family members, two of which are targets of chromosomal translocations in acute myeloid leukemia, associate with TCFs in coimmunoprecipitation assays (Fig. 1 and 2). It is notable that an interaction between MTG8/ETO (RUNX1T1) and TCF4 was also detected in a random yeast two-hybrid screen designed to establish global interaction profiles, which provides independent confirmation of our results (45). The association of MTGs with TCFs that mediate Wnt signaling and stem cell functions in multiple tissues provides a key link between disruptions of MTGs in leukemia and colorectal cancer and transcriptional control programs that regulate cell fate decisions. In the Xenopus axis duplication assay and in transcriptional analysis, MTGs acted upon TCFs, as evidenced by the fact that the effects of XETOR were downstream of β-catenin and XLef-1. Thus, MTGs may be required to mediate the repressive action of TCFs in specific situations, and inactivation of MTGs might alter the intensity and/or duration of Wnt signals. The derepression of c-Myc in mice lacking Mtgr1 suggests a molecular mechanism by which inactivation of this gene family can contribute to tumorigenesis.
In addition to MTG family members, TCFs associate with several other corepressors (e.g., Groucho/TLE and CtBP), and these associations are also regulated by β-catenin. MTG factors associate with histone deacetylases and corepressors such as N-CoR/SMRT, mSin3A, and mSin3B but are not known to associate with Groucho family proteins or CtBP (27). Therefore, it is likely that MTGs associate with TCFs independently of Groucho and CtBP. Given the widespread expression of MTG family members, Groucho/TLEs, and other corepressors that contact TCFs, the most likely scenario is that these corepression complexes are recruited in a promoter-specific fashion. Thus, inactivation of any one of the multiple corepressors may have only a limited effect on TCF-dependent transcription in the presence of the other cofactors. However, in specific tissues where one class of corepressors is rate-limiting, inactivation of an MTG family member could have more profound consequences.
The first DNA binding transcriptional repressor shown to recruit an MTG family member was the promyelocytic leukemia zinc finger (PLZF) protein, which is fused to the retinoic acid receptor in t(11;17) in acute promyelocytic leukemia (35). Subsequently, both directed and undirected approaches have been used to identify key regulators of gene expression, including BCL6, a factor closely related to PLZF that is overexpressed due to chromosomal translocation in B-cell lymphoma (8); Gfi1 (33); Gfi1b (46); atrophin (57); and the “E proteins” HEB and TAL1/Scl (13, 46, 59). Remarkably, the majority of these factors are required for lineage allocation and stem cell function in a variety of tissues.
Similarly, Tcf4 is required for stem cell self-renewal in the small intestine (24), and enforced expression of β-catenin stimulated proliferation in the cells lining the crypts of the small intestine (16, 56). If inactivation of Mtgr1 alters stem cell function, this could contribute to the impaired lineage allocation observed for Mtgr1-null mice but would be more likely associated with the expanded cycling population of cells in the crypts (Fig. 8A). In addition, although there were no morphological changes in the colonic epithelia of these mice, the cells lining the colonic crypts were hyperproliferative (Fig. 8B), separating this phenotype from defects in lineage allocation in the small intestine (1, 32). Yet when Mtgr1-null mice were treated with the ulcerative agent dextran sodium sulfate, the colonic epithelium was completely denuded. Upon regeneration of the epithelium, long-term architectural changes remained, suggesting that the colonic stem cells in Mtgr1-null mice were hypersensitive to dextran sodium sulfate and that their ability to faithfully repopulate the colon was impaired (32). Thus, taken together, the data suggest that MTG factors regulate aspects of stem cell or progenitor cell proliferation.
Given that negative regulators of Wnt signaling often act as tumor suppressors (42), it should be noted that not only are two MTG family members disrupted in up to 15% of acute myeloid leukemia cases, but the fusion proteins that are created by these chromosomal translocations may disrupt the function of other family members through physical associations (e.g., MTGR1 [22, 34]). Indeed, the t(8;21) fusion protein can trigger the expression of a “Wnt gene expression signature” in myeloid cell lines (37). This was apparently an indirect effect, because it did not require the ability of the fusion protein to bind to DNA, which is consistent with the hypothesis that RUNX1-MTG8 can impair the action of the endogenous MTG family members (20, 22). In addition to the colorectal cancer-related mutations of MTG8 and MTG16 already discussed (49), MTG16 is frequently deleted in breast cancer. Whereas MTG16 is deleted in the more frequent ductal carcinoma (23), E-cadherin, which resides near MTG16 on chromosome 16, is inactivated in lobular breast carcinoma. Given that loss of E-cadherin activates the Wnt signaling pathway by allowing the nuclear accumulation of β-catenin (38), it is intriguing that the deletions of E-cadherin and MTG16 are mutually exclusive (23). Our data may explain this observation by placing MTG family members at the nuclear apex of the Wnt pathway and suggest a molecular explanation for why MTG family members are so frequently targeted in cancer.
Acknowledgments
We thank Hans Clevers and Lynn Matrisian for cDNAs to TCF family members. We also thank the members of the Hiebert lab for helpful discussions and encouragement and the Vanderbilt-Ingram Cancer Center and Vanderbilt Digestive Diseases Research Center for support and the use of shared resources for DNA sequencing, histology, and immunohistochemistry.
This work was supported by National Institutes of Health (NIH) grants RO1-CA64140 and RO1-CA112005 (S.W.H.) and Center grants from the NCI (CA68485) and NIDDK (5P30DK58404-03).
Footnotes
Published ahead of print on 26 November 2007.
REFERENCES
- 1.Amann, J. M., B. J. Chyla, T. C. Ellis, A. Martinez, A. C. Moore, J. L. Franklin, L. McGhee, S. Meyers, J. E. Ohm, K. S. Luce, A. J. Ouelette, M. K. Washington, M. A. Thompson, D. King, S. Gautam, R. J. Coffey, R. H. Whitehead, and S. W. Hiebert. 2005. Mtgr1 is a transcriptional corepressor that is required for maintenance of the secretory cell lineage in the small intestine. Mol. Cell. Biol. 259576-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, J. M., J. Nip, D. K. Strom, B. Lutterbach, H. Harada, N. Lenny, J. R. Downing, S. Meyers, and S. W. Hiebert. 2001. ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. Mol. Cell. Biol. 216470-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batlle, E., J. T. Henderson, H. Beghtel, M. M. van den Born, E. Sancho, G. Huls, J. Meeldijk, J. Robertson, M. van de Wetering, T. Pawson, and H. Clevers. 2002. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111251-263. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382638-642. [DOI] [PubMed] [Google Scholar]
- 5.Calabi, F., R. Pannell, and G. Pavloska. 2001. Gene targeting reveals a crucial role for MTG8 in the gut. Mol. Cell. Biol. 215658-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, Y., H. Zhao, and H. Grunz. 2002. XETOR regulates the size of the proneural domain during primary neurogenesis in Xenopus laevis. Mech. Dev. 11935-44. [DOI] [PubMed] [Google Scholar]
- 7.Chamorro, M. N., D. R. Schwartz, A. Vonica, A. H. Brivanlou, K. R. Cho, and H. E. Varmus. 2005. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2473-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevallier, N., C. M. Corcoran, C. Lennon, E. Hyjek, A. Chadburn, V. J. Bardwell, J. D. Licht, and A. Melnick. 2004. ETO protein of t(8;21) AML is a corepressor for Bcl-6 B-cell lymphoma oncoprotein. Blood 1031454-1463. [DOI] [PubMed] [Google Scholar]
- 9.Daniels, D. L., and W. I. Weis. 2005. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 12364-371. [DOI] [PubMed] [Google Scholar]
- 10.Davis, J. N., L. McGhee, and S. Meyers. 2003. The ETO (MTG8) gene family. Gene 3031-10. [DOI] [PubMed] [Google Scholar]
- 11.Funayama, N., F. Fagotto, P. McCrea, and B. M. Gumbiner. 1995. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J. Cell Biol. 128959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelmetti, V., J. Zhang, M. Fanelli, S. Minucci, P. G. Pelicci, and M. A. Lazar. 1998. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol. Cell. Biol. 187185-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goardon, N., J. A. Lambert, P. Rodriguez, P. Nissaire, S. Herblot, P. Thibault, D. Dumenil, J. Strouboulis, P. H. Romeo, and T. Hoang. 2006. ETO2 coordinates cellular proliferation and differentiation during erythropoiesis. EMBO J. 25357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gradl, D., A. Konig, and D. Wedlich. 2002. Functional diversity of Xenopus lymphoid enhancer factor/T-cell factor transcription factors relies on combinations of activating and repressing elements. J. Biol. Chem. 27714159-14171. [DOI] [PubMed] [Google Scholar]
- 15.Gradl, D., M. Kuhl, and D. Wedlich. 1999. Keeping a close eye on Wnt-1/wg signaling in Xenopus. Mech. Dev. 863-15. [DOI] [PubMed] [Google Scholar]
- 16.Harada, N., Y. Tamai, T. Ishikawa, B. Sauer, K. Takaku, M. Oshima, and M. M. Taketo. 1999. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 185931-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 2811509-1512. [DOI] [PubMed] [Google Scholar]
- 18.He, X., J. P. Saint-Jeannet, J. R. Woodgett, H. E. Varmus, and I. B. Dawid. 1995. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374617-622. [DOI] [PubMed] [Google Scholar]
- 19.Huber, O., R. Korn, J. McLaughlin, M. Ohsugi, B. G. Herrmann, and R. Kemler. 1996. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech. Dev. 593-10. [DOI] [PubMed] [Google Scholar]
- 20.Ibañez, V., A. Sharma, S. Buonamici, A. Verma, S. Kalakonda, J. Wang, S. Kadkol, and Y. Saunthararajah. 2004. AML1-ETO decreases ETO-2 (MTG16) interactions with nuclear receptor corepressor, an effect that impairs granulocyte differentiation. Cancer Res. 644547-4554. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, J., E. E. Pedersen, P. Galante, J. Hald, R. S. Heller, M. Ishibashi, R. Kageyama, F. Guillemot, P. Serup, and O. D. Madsen. 2000. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2436-44. [DOI] [PubMed] [Google Scholar]
- 22.Kitabayashi, I., K. Ida, F. Morohoshi, A. Yokoyama, N. Mitsuhashi, K. Shimizu, N. Nomura, Y. Hayashi, and M. Ohki. 1998. The AML1-MTG8 leukemic fusion protein forms a complex with a novel member of the MTG8(ETO/CDR) family, MTGR1. Mol. Cell. Biol. 18846-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochetkova, M., O. L. McKenzie, A. J. Bais, J. M. Martin, G. A. Secker, R. Seshadri, J. A. Powell, S. J. Hinze, A. E. Gardner, H. E. Spendlove, N. J. O'Callaghan, A. M. Cleton-Jansen, C. Cornelisse, S. A. Whitmore, J. Crawford, G. Kremmidiotis, G. R. Sutherland, and D. F. Callen. 2002. CBFA2T3 (MTG16) is a putative breast tumor suppressor gene from the breast cancer loss of heterozygosity region at 16q24.3. Cancer Res. 624599-4604. [PubMed] [Google Scholar]
- 24.Korinek, V., N. Barker, P. Moerer, E. van Donselaar, G. Huls, P. J. Peters, and H. Clevers. 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19379-383. [DOI] [PubMed] [Google Scholar]
- 25.Koyano-Nakagawa, N., and C. Kintner. 2005. The expression and function of MTG/ETO family proteins during neurogenesis. Dev. Biol. 27822-34. [DOI] [PubMed] [Google Scholar]
- 26.Linggi, B., C. Müller-Tidow, L. van de Locht, M. Hu, J. Nip, H. Serve, W. E. Berdel, B. van der Reijden, D. E. Quelle, J. D. Rowley, J. Cleveland, J. H. Jansen, P. P. Pandolfi, and S. W. Hiebert. 2002. The t(8;21) fusion protein, AML1 ETO, specifically represses the transcription of the p14ARF tumor suppressor in acute myeloid leukemia. Nat. Med. 8743-750. [DOI] [PubMed] [Google Scholar]
- 27.Linggi, B. E., S. J. Brandt, Z. W. Sun, and S. W. Hiebert. 2005. Translating the histone code into leukemia. J. Cell. Biochem. 96938-950. [DOI] [PubMed] [Google Scholar]
- 28.Longo, K. A., J. A. Kennell, M. J. Ochocinska, S. E. Ross, W. S. Wright, and O. A. MacDougald. 2002. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J. Biol. Chem. 27738239-38244. [DOI] [PubMed] [Google Scholar]
- 29.Löwenberg, B., J. R. Downing, and A. Burnett. 1999. Acute myeloid leukemia. N. Engl. J. Med. 3411051-1062. (Erratum, 341:1484.) [DOI] [PubMed] [Google Scholar]
- 30.Lutterbach, B., J. J. Westendorf, B. Linggi, A. Patten, M. Moniwa, J. R. Davie, K. D. Huynh, V. J. Bardwell, R. M. Lavinsky, M. G. Rosenfeld, C. Glass, E. Seto, and S. W. Hiebert. 1998. ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol. Cell. Biol. 187176-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann, B., M. Gelos, A. Siedow, M. L. Hanski, A. Gratchev, M. Ilyas, W. F. Bodmer, M. P. Moyer, E. O. Riecken, H. J. Buhr, and C. Hanski. 1999. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. USA 961603-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez, J. A., C. S. Williams, J. M. Amann, T. C. Ellis, I. Moreno-Miralles, M. K. Washington, P. Gregoli, and S. W. Hiebert. 2006. Deletion of Mtgr1 sensitizes the colonic epithelium to dextran sodium sulfate-induced colitis. Gastroenterology 131579-588. [DOI] [PubMed] [Google Scholar]
- 33.McGhee, L., J. Bryan, L. Elliott, H. L. Grimes, A. Kazanjian, J. N. Davis, and S. Meyers. 2003. Gfi-1 attaches to the nuclear matrix, associates with ETO (MTG8) and histone deacetylase proteins, and represses transcription using a TSA-sensitive mechanism. J. Cell. Biochem. 891005-1018. [DOI] [PubMed] [Google Scholar]
- 34.Melnick, A., G. W. Carlile, M. J. McConnell, A. Polinger, S. W. Hiebert, and J. D. Licht. 2000. AML-1/ETO fusion protein is a dominant negative inhibitor of transcriptional repression by the promyelocytic leukemia zinc finger protein. Blood 963939-3947. [PubMed] [Google Scholar]
- 35.Melnick, A. M., J. J. Westendorf, A. Polinger, G. W. Carlile, S. Arai, H. J. Ball, B. Lutterbach, S. W. Hiebert, and J. D. Licht. 2000. The ETO protein disrupted in t(8;21)-associated acute myeloid leukemia is a corepressor for the promyelocytic leukemia zinc finger protein. Mol. Cell. Biol. 202075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molenaar, M., J. Roose, J. Peterson, S. Venanzi, H. Clevers, and O. Destree. 1998. Differential expression of the HMG box transcription factors XTcf-3 and XLef-1 during early Xenopus development. Mech. Dev. 75151-154. [DOI] [PubMed] [Google Scholar]
- 37.Müller-Tidow, C., B. Steffen, T. Cauvet, L. Tickenbrock, P. Ji, S. Diederichs, B. Sargin, G. Kohler, M. Stelljes, E. Puccetti, M. Ruthardt, S. DeVos, S. W. Hiebert, H. P. Koeffler, W. E. Berdel, and H. Serve. 2004. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol. Cell. Biol. 242890-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson, W. J., and R. Nusse. 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 3031483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwkoop, P. D., and J. Faber. 1967. Normal table of Xenopus laevis (Daudin). North-Holland Publishing Company, Amsterdam, The Netherlands.
- 40.Peng, H. B. 1991. Xenopus laevis: practical uses in cell and molecular biology. Solutions and protocols. Methods Cell Biol. 36657-662. [PubMed] [Google Scholar]
- 41.Pinto, D., and H. Clevers. 2005. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp. Cell Res. 306357-363. [DOI] [PubMed] [Google Scholar]
- 42.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 141837-1851. [PubMed] [Google Scholar]
- 43.Radtke, F., H. Clevers, and O. Riccio. 2006. From gut homeostasis to cancer. Curr. Mol. Med. 6275-289. [DOI] [PubMed] [Google Scholar]
- 44.Rockman, S. P., S. A. Currie, M. Ciavarella, E. Vincan, C. Dow, R. J. Thomas, and W. A. Phillips. 2001. Id2 is a target of the beta-catenin/T cell factor pathway in colon carcinoma. J. Biol. Chem. 27645113-45119. [DOI] [PubMed] [Google Scholar]
- 45.Rual, J. F., K. Venkatesan, T. Hao, T. Hirozane-Kishikawa, A. Dricot, N. Li, G. F. Berriz, F. D. Gibbons, M. Dreze, N. Ayivi-Guedehoussou, N. Klitgord, C. Simon, M. Boxem, S. Milstein, J. Rosenberg, D. S. Goldberg, L. V. Zhang, S. L. Wong, G. Franklin, S. Li, J. S. Albala, J. Lim, C. Fraughton, E. Llamosas, S. Cevik, C. Bex, P. Lamesch, R. S. Sikorski, J. Vandenhaute, H. Y. Zoghbi, A. Smolyar, S. Bosak, R. Sequerra, L. Doucette-Stamm, M. E. Cusick, D. E. Hill, F. P. Roth, and M. Vidal. 2005. Towards a proteome-scale map of the human protein-protein interaction network. Nature 4371173-1178. [DOI] [PubMed] [Google Scholar]
- 46.Schuh, A. H., A. J. Tipping, A. J. Clark, I. Hamlett, B. Guyot, F. J. Iborra, P. Rodriguez, J. Strouboulis, T. Enver, P. Vyas, and C. Porcher. 2005. ETO-2 associates with SCL in erythroid cells and megakaryocytes and provides repressor functions in erythropoiesis. Mol. Cell. Biol. 2510235-10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shroyer, N. F., D. Wallis, K. J. Venken, H. J. Bellen, and H. Y. Zoghbi. 2005. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 192412-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 965522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjöblom, T., S. Jones, L. D. Wood, D. W. Parsons, J. Lin, T. D. Barber, D. Mandelker, R. J. Leary, J. Ptak, N. Silliman, S. Szabo, P. Buckhaults, C. Farrell, P. Meeh, S. D. Markowitz, J. Willis, D. Dawson, J. K. Willson, A. F. Gazdar, J. Hartigan, L. Wu, C. Liu, G. Parmigiani, B. H. Park, K. E. Bachman, N. Papadopoulos, B. Vogelstein, K. W. Kinzler, and V. E. Velculescu. 2006. The consensus coding sequences of human breast and colorectal cancers. Science 314268-274. [DOI] [PubMed] [Google Scholar]
- 50.Sokol, S., J. L. Christian, R. T. Moon, and D. A. Melton. 1991. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67741-752. [DOI] [PubMed] [Google Scholar]
- 51.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398422-426. [DOI] [PubMed] [Google Scholar]
- 52.van Es, J. H., P. Jay, A. Gregorieff, M. E. van Gijn, S. Jonkheer, P. Hatzis, A. Thiele, M. van den Born, H. Begthel, T. Brabletz, M. M. Taketo, and H. Clevers. 2005. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 7381-386. [DOI] [PubMed] [Google Scholar]
- 53.van Noort, M., and H. Clevers. 2002. TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev. Biol. 2441-8. [DOI] [PubMed] [Google Scholar]
- 54.Wang, J., T. Hoshino, R. L. Redner, S. Kajigaya, and J. M. Liu. 1998. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc. Natl. Acad. Sci. USA 9510860-10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willert, J., M. Epping, J. R. Pollack, P. O. Brown, and R. Nusse. 2002. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev. Biol. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong, M. H., B. Rubinfeld, and J. I. Gordon. 1998. Effects of forced expression of an NH2-terminal truncated beta-catenin on mouse intestinal epithelial homeostasis. J. Cell Biol. 141765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood, J. D., F. C. Nucifora, Jr., K. Duan, C. Zhang, J. Wang, Y. Kim, G. Schilling, N. Sacchi, J. M. Liu, and C. A. Ross. 2000. Atrophin-1, the dentato-rubral and pallido-luysian atrophy gene product, interacts with ETO/MTG8 in the nuclear matrix and represses transcription. J. Cell Biol. 150939-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, Q., N. A. Bermingham, M. J. Finegold, and H. Y. Zoghbi. 2001. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2942155-2158. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, J., M. Kalkum, S. Yamamura, B. T. Chait, and R. G. Roeder. 2004. E protein silencing by the leukemogenic AML1-ETO fusion protein. Science 3051286-1289. [DOI] [PubMed] [Google Scholar]