Abstract
WISP-2/CCN5 is an estrogen-regulated member of the “connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed” (CCN) family of the cell growth and differentiation regulators. The WISP-2/CCN5 mRNA transcript is undetectable in normal human mammary cells, as well as in highly aggressive breast cancer cell lines, in contrast with its higher level in the breast cancer cell lines characterized by a more differentiated phenotype. We report here that knockdown of WISP-2/CCN5 by RNA interference in estrogen receptor alpha (ERα)-positive MCF-7 breast cancer cells induced an estradiol-independent growth linked to a loss of ERα expression and promoted epithelial-to-mesenchymal transdifferentiation. In contrast, forced expression of WISP-2/CCN5 directed MCF-7 cells toward a more differentiated phenotype. When introduced into the poorly differentiated, estrogen-independent, and invasive MDA-MB-231 breast cancer cells, WISP-2/CCN5 was able to reduce their proliferative and invasive phenotypes. In a series of ERα-positive tumor biopsies, we found a positive correlation between the expression of WISP-2/CCN5 and ID2, a transcriptional regulator of differentiation in normal and transformed breast cells. We propose that WISP-2/CCN5 is an important regulator involved in the maintenance of a differentiated phenotype in breast tumor epithelial cells and may play a role in tumor cell invasion and metastasis.
The conversion of normal breast epithelial cells into tumor cells is a multistep process that involves changes in the activity of a number of different genes and proteins. In normal and malignant mammary cells, the proliferation is stimulated by several important modulators of the cell cycle (45), such as steroid hormones (estrogens and progesterone) and peptide growth factors (insulin, insulin-like growth factors [IGFs], and epithelial growth factor [EGF]). The progression of breast cancer is characterized by cell changes manifested by escape from control mechanisms, increased growth potential, and morphological and biochemical deviations conferring the ability to invade and to metastasize and form new solid tumors at distant sites (1, 23). Numerous genes coding for proteins involved in the regulation of the cell cycle and/or involved in the process of tumorigenesis in the mammary gland, such as c-Myc, cyclin D1, MDM2, c-erbB-2, p53, BRCA-1, and BRCA-2, have been identified (2, 5, 18). Although these factors have been proposed as molecular markers to help predict the prognosis, the identification of genes critical for the development and progression of breast cancer remains one of the major goals of cancer research.
Previous studies have demonstrated that the expression of human WISP-2/CCN5 (Wnt-1-induced signaling pathway protein-2/connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed 5) in MCF-7 cells is enhanced by several stimulators of human breast cancer cell proliferation, such as estrogens, progesterone, epidermal growth factor, and insulin-like growth factor 1 (6, 7, 17, 25). WISP-2/CCN5 has also been reported as a serum- and PMA (phorbol 12-myristate 13-acetate)-induced early response gene in breast tumor cell lines (41, 48). Furthermore, WISP-2/CCN5 knockdown abrogated the mitogenic effects of PMA in MCF-7 cells (41). Although the WISP-2/CCN5 gene was first identified as being upregulated in C57MG mouse mammary epithelial cells transformed by the Wnt-1 retrovirus (35), the role of WISP-2/CCN5 in breast carcinogenesis remains unclear.
WISP-2/CCN5 is a secreted-protein member of the CCN family of growth factors that shares conserved modular domains (11). This family of genes has been implicated in normal physiological and pathological processes, such as cell proliferation, differentiation, migration, angiogenesis, and apoptosis, as well as tumorigenesis (13, 36, 37). Unlike other CCN family members, which encompass four structural modules with sequence homologies with insulin-like growth factor binding proteins, von Willebrand factor, thrombospondin, and cysteine knot (CT), WISP-2/CCN5 contains only three structural modules and lacks the CT domain (11, 12, 35). The CT domain has been shown to be a proliferation module (30). Although the physiological function of WISP-2/CCN5 is not well defined, its domain structure suggests that its function may be different from that of other members of the CCN family. Previously, we showed that estrogen receptor alpha (ERα) directly regulates the WISP-2/CCN5 gene in all ERα-positive cell lines tested (19). We have identified, by using in vitro and in vivo approaches, a functional estrogen response element involved in the regulation of the WISP-2/CCN5 gene promoter by estrogens (19). Furthermore, the WISP-2/CCN5 mRNA transcripts in MCF-7 cells were enhanced by the expression of p21WAF-1/CIP1 in the presence of estrogens, conditions where p21WAF-1/CIP1 plays a positive role in the commitment of MCF-7 cells to differentiate (20, 38). However, the upregulation of WISP-2/CCN5 under estrogen treatment could be independent of DNA synthesis (25), although it has been demonstrated that WISP-2/CCN5 is important for proliferation in MCF-7 cells (6, 7). These data raise questions concerning the role of WISP-2/CCN5 in breast cancer.
In view of these findings, we examined the role of WISP-2/CCN5 in tumorigenesis by ablating or inducing its expression in two different human breast cancer cell lines that exhibit elevated to nearly undetectable expression of WISP-2/CCN5. We show that reducing WISP-2/CCN5 expression enhances the proliferative potential of MCF-7 cancer cells and induces estrogen-independent proliferation of these cells, whereas inducing WISP-2/CCN5 expression inhibits the proliferative potential of both MCF-7 and MDA-MB-231 cells. In addition, we have also attempted to explore signaling molecules associated with these WISP-2/CCN5 activities. These studies suggest a role of WISP-2/CCN5 in epithelial-to-mesenchymal transition (EMT).
MATERIALS AND METHODS
Patients and samples.
To investigate the interrelationships between mRNA levels of genes of interest, we analyzed 48 primary breast tumors, 24 ERα negative and 24 ERα positive, excised from women at Centre René Huguenin (Saint-Cloud, France). Samples containing more than 70% tumor cells as judged by histology were considered suitable for this study. Immediately following surgery, the tumor samples were placed in liquid nitrogen until RNA extraction. The patients met the following criteria: primary unilateral nonmetastatic breast carcinoma; complete clinical, histological and biological information available; no radiotherapy or chemotherapy before surgery; and full follow-up. ERα status was determined at the protein level by biochemical methods (dextran-coated charcoal method until 1988 and enzymatic immunoassay thereafter) and confirmed by ERα real-time quantitative reverse transcription (RT-PCR) assay. The malignancy of infiltrating carcinomas was scored according to Scarff, Bloom, and Richardson's histoprognostic system (10). The characteristics of these 48 patients and their tumors are shown in Table 1.
TABLE 1.
Characteristics of the 24 ERα-negative and 24 ERα-positive breast tumors
| Patient or tumor characteristic | No. (%) of patients or tumors
|
|
|---|---|---|
| ERα-negative tumors (n = 24) | ERα-positive tumors (n = 24) | |
| Age (yr) | ||
| ≤60 | 14 (58.3) | 1 (4.2) |
| >60 | 10 (41.7) | 23 (95.8) |
| SBR histological gradea | ||
| I and II | 5 (22.7) | 20 (83.3) |
| III | 17 (77.3) | 4 (16.7) |
| Lymph node status | ||
| Negative | 11 (45.8) | 0 (0) |
| Positive | 13 (54.2) | 24 (100) |
| Macroscopic tumor size | ||
| ≤30 mm | 15 (62.5) | 12 (50) |
| >30 mm | 9 (37.5) | 12 (50) |
Scarff-Bloom-Richardson classification. Information available for 46 patients.
Plasmids.
The entire open reading frame of WISP-2/CCN5 cDNA was inserted in the pCEP4-Flag vector (Invitrogen) and named pCEP4-Flag-WISP-2. Small interfering RNA (siRNA) oligonucleotides for WISP-2/CCN5 were designed by using the Target Finder program (Ambion). The following sequences were used to construct short hairpin RNA interference vectors in pSilencer (Ambion): sh-WISP-2, 5′-AAGGTGCGTACCCAGCTGTG-3′, and sh-scrambled, 5′-AGTACGTGTACAGGCGCCGT-3′, leading to the pSilencer/sh-WISP-2 and the pSilencer/sh-scrambled vector, respectively.
Cell culture and transfection. (i) Human breast epithelial cells.
Breast epithelial tissue was obtained from women between 15 and 25 years of age undergoing reduction mammoplasty. The collected tissues were confirmed to be disease and malignancy free by histopathological examination of tissue sections. Sampling of the tissue was carried out according to the French governmental regulations on clinical experimentation. The healthy-tissue samples from reduction mammoplasty specimens were minced and dissociated with collagenase (1.5 mg/ml; Roche) at 37°C for 6 h and hyaluronidase (0.5 mg/ml; Sigma) at 37°C for 30 min in Ham's F10 medium and then filtered through 300-μm and 150-μm sieves to retain undigested tissue. Cells were grown in Ham's F10 medium supplemented with 0.24% NaHCO3 (Invitrogen), 1% penicillin (10,000 U)-streptomycin (10 mg) (Sigma), 5 ng/ml cortisol (Sigma), 6.5 ng/ml triiodothyronine (Sigma), 10 ng/ml cholera toxin (Sigma), 5 mg/ml transferrin (Sigma), 5% human serum, 0.16 U/ml insulin (Sigma), and 10 ng/ml epidermal growth factor (Sigma) in a humidified atmosphere of 5% CO2 as described previously (31).
(ii) Human breast carcinoma cells.
Human breast carcinoma cells were maintained in Dulbecco's modified Eagle's medium (DMEM) or RPMI 1640 supplemented with 10% fetal bovine serum (FBS). MCF-7 and MDA-MB-231 cells were transfected with pCEP4-Flag vector or pCEP4-Flag-WISP-2 vector expressing full-length human WISP-2/CCN5 by electroporation as previously described (20). The stable transfectants were established by selection in hygromycin-containing medium and designated MCF-7/F3, MCF-7/w1, and MCF-7/w2, respectively, when transfected in MCF-7 cells and MDA-MB-231/F3, WISP-2-w6, and WISP-2-w15 when transfected in MDA-MB-231 cells. For siRNA experiments, MCF-7 cells were transfected with pSilencer/sh-WISP-2 or pSilencer/sh-scrambled vectors by electroporation. Stable cell lines were established by selection in hygromycin-containing media. The stable transfectants were designated MCF-7/sh-WISP-2 and MCF-7/sh-scrambled, respectively.
Proliferation assay.
An MTS-based colorimetric assay was used as instructed by the manufacturer (CellTitre 96 MTS assay; Promega) to estimate the relative cell number. Data are presented as the average absorbance per sample corrected for background.
Anchorage-independent growth assay.
Cells were suspended in 2 ml DMEM containing 10% FBS and 0.33% SeaPlaque low-melting-temperature agarose and 2 × 104 MCF-7/sh-scrambled or MCF-7/sh-WISP-2 cells or 1 × 104 MDA-MB-231/F3, WISP-2-w6, or WISP-2-w15 cells. The cells were plated in a 60-mm dish over a 3-ml layer of solidified DMEM containing 10% FBS and 0.6% agarose. The cells were fed every 3 to 4 days by adding 200 μl of DMEM containing 10% FBS. Colonies were photographed at ×10 and ×30 magnification after 2 to 3 weeks.
Western blot assay.
Cell lysates were made in NTEN lysis buffer (0.5% NP-40, 20 mM Tris-HCl, pH 8, 1 mM EDTA, and 150 mM NaCl) containing 0.5 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, and 1 μM aprotinin and cleared by centrifugation. Fifty micrograms of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with saturating buffer for 1 h at room temperature followed by incubation with appropriate antibodies overnight at 4°C. Immunoblot analyses were performed by using anti-WISP-2 (Abcam); anti-ERα (Ab-15); anti-progestin receptor (anti-PR) (Ab-8) and anti-cyclin D1 (NeoMarkers); anti-p21 and anti-p27 (Pharmingen); anti-Flag and anti-cytokeratin 18 (Sigma-Aldrich); anti-cathepsin D (Upstate); antiactin (I-19) (Santa Cruz); and anti-pS2 (a gift from M. C. Rio). The membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h. The membranes were washed extensively and developed with an enhanced chemiluminescence kit (Amersham Pharmacia).
Real-time RT-PCR.
Total RNA was extracted from all cell lines and frozen tumor samples by using the acid-phenol guanidinium method. The quality of the RNA samples was determined by electrophoresis through agarose gels and staining with ethidium bromide, the 18S and 28S RNA bands being visualized under UV light.
One microgram of total RNA from each sample was reverse transcribed, and real-time RT-PCR measurements were performed as described previously (9, 19) using an Mx3000P apparatus (Stratagene) or an ABI Prism 7900 sequence detection system (Perkin-Elmer Applied Biosystems, Foster City, CA) with the corresponding Sybr green kit, according to the manufacturer's recommendations. The mRNA levels indicated below show the abundance of the target gene relative to that of the endogenous control (TBP) used to normalize the starting amount and quality of total RNA. Similar results were obtained with a second endogenous control, RPLP0 (also known as 36B4).
Statistical analysis.
As the mRNA levels did not fit a Gaussian distribution, correlations between the target gene expression levels (continuous variables) were tested using the nonparametric Spearman test of rank correlation. Differences between two populations were judged significant at confidence levels greater than 95% (P < 0.05).
Morphology analysis.
Cells were cultured in DMEM supplemented with 10% FBS for 48 h and then photographed using a phase-contrast microscope (Nikon Eclipse TE 300).
Microscopic imaging.
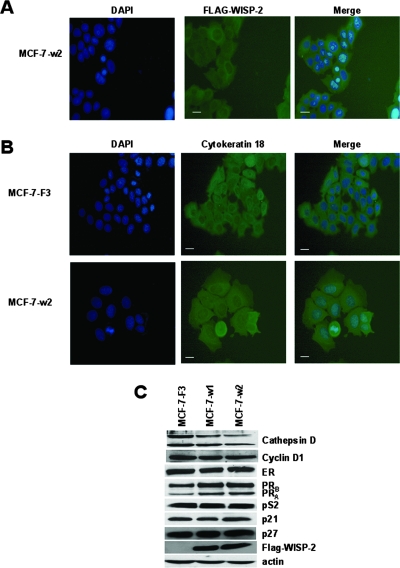
MCF-7/F3 and MCF-7/w2 cells were plated on 60-mm dishes and grown for 48 h in DMEM supplemented with 10% FBS. Cells were fixed in 4% paraformaldehyde-phosphate-buffered saline (PBS) and then treated briefly with 0.1% Triton X-100 in PBS. After three rinses with PBS, cells were incubated for 1 h at room temperature with primary antibodies to Flag or cytokeratin 18 (Sigma-Aldrich), rinsed, and incubated for 1 h at room temperature with Texas Red-conjugated secondary antibody (goat anti-mouse immunoglobulin G; Jackson). Nuclei were stained with 4′,6′-diamino-2-phenylindole (DAPI) at a concentration of 1 μg/ml. Images were obtained with a Leica DMR microscope equipped with a fluorescence imaging system (63× lens objective).
Wound-healing migration assay.
Cells were grown in six-well tissue culture dishes until confluence. Cultures were incubated during 1 min with Moscona's buffer. A scrape was made through the confluent monolayer with a plastic pipette tip. Afterwards, Moscona's buffer was removed, the culture was washed twice, and fresh DMEM containing 10% fetal calf serum was added. At the underside of each dish, marks were made at six arbitrary places where the width of the wound was measured with an inverted microscope (4× lens objective) at time 0 and after 4, 8, and 24 h incubation at 37°C. Migration was expressed as the average ± standard deviation of the difference between the measurements at time 0 and at 4, 8, or 24 h.
Matrigel invasion assay.
Transwell chambers with polycarbonate membrane filters (6.5-mm diameter, 8-μm pore size) were coated with Matrigel. The filter was placed in a six-well plate with conditioned medium from MRC5 human lung fibroblasts as a chemoattractant, and 4 × 105 cancer cells were added to the upper compartment of the Transwell chamber. After 48 h, the cells that invaded to the undersurface of the membrane were stained with DAPI (0.4 mg/ml; Sigma) and were counted on 10 fields/filter.
Spheroid collagen type I invasion assay.
MCF-7 variants were suspended at 1.5 × 105 cells/ml in 6 ml culture medium and were incubated in a 50-ml Erlenmeyer flask on a gyrotory shaker at 37°C and 70 rpm for 2 days. The spheroids were viewed under a microscope equipped with a calibrated ocular grid. Spheroids with a diameter of ±300 μm were used. Spheroids were suspended in collagen type I solution and were poured on a bottom layer of collagen type I gel in a six-well plate. Implantation of the spheroid into the collagen type I gel provides a three-dimensional matrix through which tumor cell invasion can take place. After gelification, 1 ml of culture medium was carefully added. The sample designations were coded such that the experimenter was blind to the treatment type. Invasion was analyzed by determining the invasive distance and the number of invasive cells. The invasive distance is the distance between the periphery of the spheroid and a circle that circumscribes the invasive cells. A mean distance was calculated from more than 10 spheroids. Similarly, we determined the mean number of invasive cells/spheroid calculated from more than 10 spheroids. Representative phase-contrast pictures were taken.
BrdU experiments and flow cytometry analysis.
The cell cycle was analyzed by the bromodeoxyuridine (BrdU)/anti-BrdU method. Briefly, cells were plated (1 × 106/60-mm plate) in serum-supplemented medium for 24 h. Afterward, BrdU was added directly to the culture medium to achieve a final concentration of 10 μM. After 45 min, the cells were harvested and treated for detection of BrdU incorporation by using a 5′-bromo-2-deoxyuridine labeling and detection kit from Roche Diagnostics. Analysis of the cell cycle was performed by flow-cytometric quantitation of nuclear DNA contents after propidium iodide staining as previously described (20).
RESULTS
Expression of WISP-2/CCN5 is elevated in differentiated human breast cancer cells.
WISP-2/CCN5 mRNA expression has been previously reported to be overexpressed in cancerous cells of human breast tissue (19, 39). To further investigate the regulation of WISP-2/CCN5, we examined the mRNA expression levels in a number of cell lines derived from different types of human breast cancer. WISP-2/CCN5 expression was quantified by using RT-PCR and normalized to the 36B4 mRNA within each sample. For this purpose, we used the ER-positive, poorly invasive, and low-metastasizing human breast cancer cell lines MCF-7, T47-D, and ZR-75.1; the ER-negative, highly invasive, and metastatic human breast cancer cell lines MDA-MB-231, MDA-MB-468, BT-20, and DU4475; and the low-metastasizing human breast cancer cell line SKBr3 that is ER negative. Untransformed human mammary epithelial cells were included as normal controls. We found that mRNA levels of WISP-2/CCN5 were strongly elevated in the MCF-7, T47D, ZR-75.1, and SKBr3 cell lines compared to their levels in untransformed cells, which express low levels of WISP-2/CCN5 mRNA (Fig. 1A). We observed that MDA-MB-231, MDA-MB-468, BT-20, and DU-4475 cells express very-low-to-undetectable levels of WISP-2/CCN5 transcripts (Fig. 1A). By Western blot assay, we confirmed the mRNA expression at the protein level (Fig. 1B). Thus, our data indicate that WISP-2/CCN5 expression is more elevated in breast tumor cells with low metastatic potential than in more-aggressive cells.
FIG. 1.
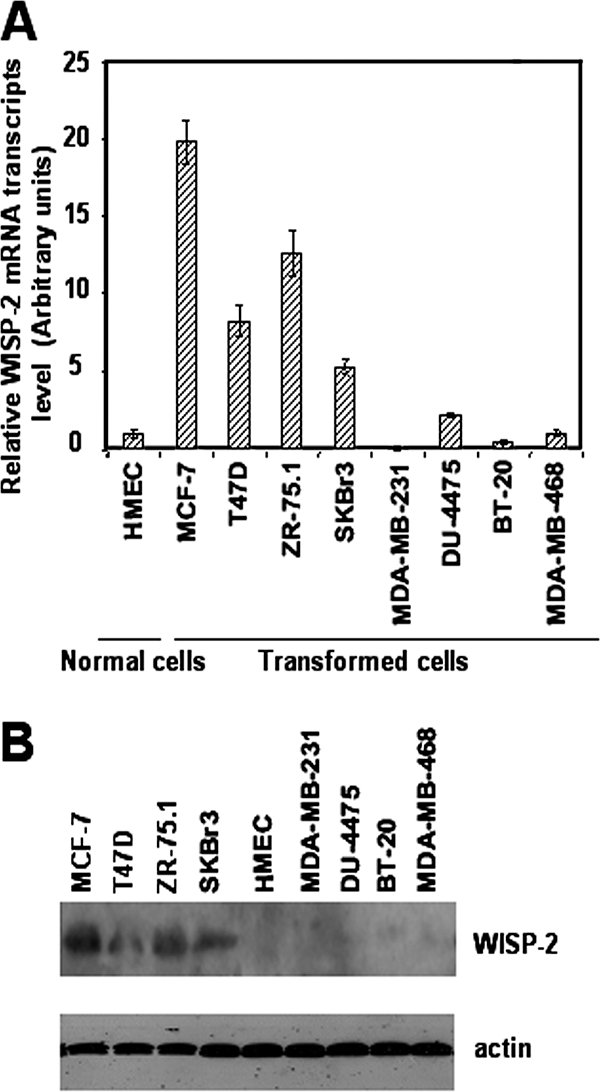
WISP-2/CCN5 mRNA levels showed differences in human cancer cell lines. (A) RNA was isolated from human breast cancer cell lines and untransformed human mammary epithelial cells, and WISP-2/CCN5 expression was analyzed by real-time RT-PCR. The results, after normalization as described in Materials and Methods, represent the relative hWISP-2/CCN5 mRNA transcript levels among these different cell lines tested and are the means ± standard errors of the means of the results of duplicate experiments. (B) Protein extracts of different cell lines were prepared and tested by Western blotting for WISP-2/CCN5 expression. The levels of β-actin in cell lysates were measured by Western blotting and included as a loading control.
WISP-2/CCN5 expression induces morphological and biochemical differentiation of MCF-7 cells.
We have reported previously that increased expression of p21WAF-1/CIP1 in MCF-7 cells in the presence of estrogen led to an increased expression of WISP-2/CCN5 (20). Thus, we evaluated the phenotypic consequences of forced expression of WISP-2/CCN5 in the MCF-7 cells (MCF-7-w2). In this study, we stably transfected MCF-7 cells with a vector expressing full-length human WISP-2/CCN5. These cells expressed WISP-2/CCN5 essentially in the cytoplasm (Fig. 2A). As shown in Fig. 2B, control cells stably transfected with the empty vector (MCF-7-F3) were of the epithelial type, with indistinct cell margins. When WISP-2/CCN5 was expressed, the cells underwent significant morphological changes. The cells increased in size and were flattened. The increase in cell size was predominantly attributable to an abundance of cytoplasm. The cells presented distinct boundaries. In addition, only minimal mitotic activity was present. These morphological changes were suggestive of mammary epithelial differentiation and were very similar to those previously observed in MCF-7 cells stably transfected with p21WAF1/CIP1 (20).
FIG. 2.
WISP-2/CCN5 expression induces morphological and biochemical effects in MCF-7 cells. Cells were fixed, permeabilized, and then incubated with either anti-Flag or anti-cytokeratin 18. Flag-WISP-2 (A) and cytokeratin 18 (B) were detected by using a fluorescein-labeled secondary antibody. Nuclear DNA was stained with DAPI. Images were obtained by microscopy at a magnification of ×63. Scale bars, 7 μm. The results were confirmed by three independent experiments. (C) Protein extracts of stable cell lines corresponding to cells transfected with the empty vector (MCF-7-F3) or with WISP-2 expressing vector (w1 and w2 clones) were prepared and tested by Western blotting for cathepsin D, cyclin D1, ERα, PRB, PRA, pS2, p21WAF1/CIP1, p27KIP1, and Flag-WISP-2. The levels of β-actin in cell lysates were measured by Western blotting and included as a loading control.
To further study the effect of the forced expression of WISP-2/CCN5 on MCF-7 cell cycle progression, cells were pulse labeled with BrdU. The number of BrdU-positive cells expressing WISP-2/CCN5 (MCF-7-w2) was compared with the number of BrdU-positive cells transfected with the empty vector (MCF-7-F3). The results show a significant decrease in BrdU-positive cells after forced expression of WISP-2/CCN5 (data not shown). Cell cycle analyses show decreases in the S phase and increases in the G0/G1 phase of the cell cycle, confirming the results of the BrdU incorporation assay (data not shown).
Next, we evaluated the phenotypic effects of the forced expression of WISP-2/CCN5 in MCF-7 cells on the expression of several estrogen target genes. Western blotting (Fig. 2C) showed that WISP-2/CCN5 enhanced the expression of PRA and PRB, which are differentiation markers of breast epithelial cells, and repressed the expression of cathepsin D. In contrast, WISP-2/CCN5 had no effect on the expression of cyclin D1, pS2, p21WAF1/CIP1, and p27KIP1. The level of ERα was not modulated by WISP-2/CCN5 (Fig. 2C). These results indicated that the expression of WISP-2/CCN5 contributed to directing MCF-7 cells toward a more-differentiated phenotype.
WISP-2/CCN5 downregulation induces loss of estrogen-dependent growth and transdifferentiation in MCF-7 breast cancer cells.
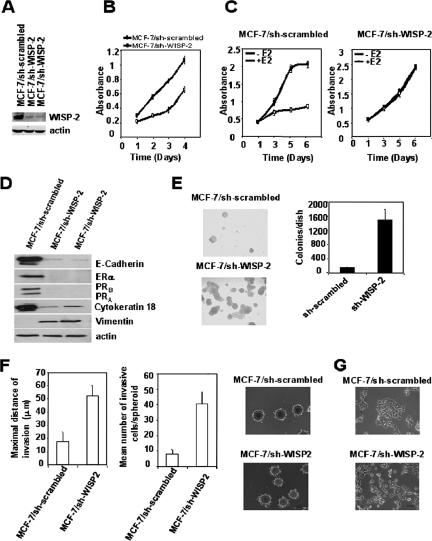
We used the pSilencer vector to produce siRNA duplexes designed to specifically suppress endogenous WISP-2/CCN5 expression. The same vector encoding an siRNA duplex of a scrambled sequence was used as a control in these experiments. MCF-7 cells were stably transfected with these pSilencer vectors. We compared the long-term effect of WISP-2/CCN5 deprivation on cell number over 4 days. Cell lysates were tested in parallel for the expression of the WISP-2/CCN5 and β-actin proteins. In the two clones used in these experiments, the expression of WISP-2/CCN5 was strongly reduced; the scrambled construct had no effect, and the expression of β-actin was not affected by any of these vectors (Fig. 3A). Cells transfected with pSilencer/sh-WISP-2, with a doubling time of approximately 1.5 days, proliferated more quickly than cells transfected with pSilencer/sh-scrambled, with a doubling time of approximately 3 days (Fig. 3B). However, no significant changes in cell cycle distribution or evidence of cell death was detected (data not shown), suggesting that the proliferation is due to an overall faster transit through the cell cycle. Thus, we conclude that deprivation of WISP-2/CCN5 expression in MCF-7 has a major effect on the proliferation rate of the breast carcinoma cell line.
FIG. 3.
Loss of WISP-2/CCN5 protein induces EMT in MCF-7 cells. (A) Reduction of WISP-2/CCN5 expression in MCF-7 breast cancer cells by siRNA. Stable sh-WISP-2 cell lines expressing the siRNA for human WISP-2 were generated as described in Materials and Methods. WISP-2 expression in two representative stable clones was assessed by Western blotting and compared with that in parental cells. The levels of β-actin in cell lysates were measured by Western blotting and included as a loading control. (B) Cells were cultured in 96-well dishes in medium containing 10% FBS. Relative cell number was estimated using a colorimetric assay 1, 2, 3, and 4 days after replating. Data shown are the means ± standard errors of the means of the results of triplicate experiments. (C) Reducing WISP-2/CCN5 expression affects estrogen-dependent cell growth. Cells were cultured in 96-well dishes in phenol red-free medium supplemented with 5% dextran-charcoal-stripped FBS and 1 μM ICI 182,780 for 48 h. Then, cells were treated with vehicle or 10 nM estradiol (E2). Relative cell number was estimated using a colorimetric assay 1, 3, 5, and 6 days after replating. Data shown are the means ± standard errors of the means of the results of triplicate experiments. (D) Western blot analysis of various epithelial and mesenchymal markers in MCF-7/sh-scrambled and two clones of MCF-7/sh-WISP-2 stable cell lines using specific antibodies. (E) Effect of loss of WISP-2/CCN5 expression on anchorage-independent growth of MCF-7 cells. These cells were assayed for their ability to proliferate and form colonies in soft agar as described in Materials and Methods. Representative fields were photographed at a magnification of ×10. Colonies from three independent experiments were counted and plotted. (F) Spheroid collagen type I invasion assay. Spheroids of MCF-7/sh-scrambled and two clones of MCF-7/sh-WISP-2 cell lines were suspended in collagen type 1 gels. Invasion was scored by counting the number of invasive cells/spheroid and calculating the maximal invasive distance. Error bars indicate standard deviations of measurements derived from 10 spheroids. Spheroid invasion was analyzed by phase-contrast microscopy. A representative image from three experiments is shown. Scale bar, 100 μm. (G) Morphology of MCF-7/sh-scrambled and MCF-7/sh-WISP-2 cells. Cells were plated on tissue culture plastic and were photographed using a phase-contrast microscope. Scale bar, 50 μm.
Next, we investigated the estrogen dependence of the proliferation of the MCF-7/sh-WISP-2 cells (Fig. 3C). The proliferation rates of cells expressing sh-WISP-2 (MCF-7/sh-WISP-2) were compared with those in MCF-7 cells transfected with vector alone (MCF-7sh-scrambed) in response to estradiol. Cells were plated at the same density (3,000 to 5,000 cells/well) in culture medium containing charcoal-stripped serum supplemented with the pure antiestrogen ICI 182,780 which inhibits estrogen-dependent ERα function (24). Then, cells were incubated in the presence of estradiol or vehicle. In contrast to the proliferation of the parental cells, the proliferation of MCF-7/sh-WISP-2 cells was independent of estradiol (Fig. 3C). This effect was highly reproducible, and similar results were obtained with other clones tested. Thus, the loss of WISP-2/CCN5 renders the MCF-7 cells independent of the growth stimulation effect of estradiol.
We then examined protein expression in order to determine if the MCF-7/sh-WISP-2 cells were indeed different from the parental cells in their epithelial markers. Western blotting revealed a decrease in the ERα level that was concomitant with a decrease in the PR level. Thus, the effect of the loss of estrogen dependence of MCF-7/sh-WISP-2 cells on the proliferation rate correlated with the loss of the expression of ERα. In addition, we observed a decrease in epithelial markers E-cadherin and cytokeratin 18 and an increase in the mesenchymal marker vimentin (Fig. 3D). Thus, the attenuation of WISP-2/CCN5 protein in MCF-7 imposes major phenotypic changes.
The acquisition of anchorage-independent growth is generally considered to be one of the in vitro properties associated with the malignancy of cells. To test if MCF-7/sh-WISP-2 cells require a matrix-dependent signal, we assessed the capacity for anchorage-independent growth of these MCF-7/sh-WISP-2 cells by testing their ability to form colonies in soft agar. The MCF-7/sh-WISP-2 cells formed numerous colonies, which were looser and larger in size than those in control cultures, in which tight, round cell aggregates were formed (Fig. 3E). We evaluated the invasive phenotype of cells by spheroid collagen I invasion assay. Invasion is scored by determining two parameters: the mean number of invasive cells/spheroid and the maximal distance of invasion. Invasion is observed with MCF-7/sh-WISP-2 cells which detach from the three-dimensional organized cellular aggregate and migrate as single cells into the matrix. The number of invasive MCF-7/sh-WISP-2 cells was fivefold higher than the number of control cells (Fig. 3F). Furthermore, the maximal invasive distance of MCF-7/sh-WISP-2 cells was threefold higher than that of control cells (Fig. 3F). Together with the increase of invasion following knockdown of WISP-2/CCN5, we observed morphological changes in these cells. The MCF-7/sh-WISP-2 cells showed a scattered morphotype (Fig. 3G). All these observations suggest that the loss of WISP-2/CCN5 expression reduces the estrogen requirement for growth and induces transdifferentiation, leading to EMT (43, 44).
WISP-2 reduces the aggressive phenotype of human metastatic breast cancer cells.
Our laboratory (19), as well as others (6), reported that the WISP-2/CCN5 mRNA transcripts were nearly undetectable in the aggressive cell line MDA-MB-231. This observation has been confirmed in other cell lines with invasive character in vitro, and it has been confirmed in vivo by the metastasizing potential of breast cancer cell lines. We hypothesize that the absence of WISP-2/CCN5 contributes to the aggressiveness of these cells. In order to test this hypothesis, we have examined the effect of WISP-2/CCN5 expression on anchorage-independent growth of the MDA-MB-231 cells. We established the MDA-MB-231-derived cell lines WISP-2-w6 and WISP-2-w15 overexpressing WISP-2/CCN5. These two clones were chosen because of their different levels of WISP-2/CCN5 expression (Fig. 4A). To investigate the relationship between WISP-2/CCN5 expression and the proliferative activity of these cells, we used a colorimetric assay to estimate relative cell numbers. A significant reduction of the proliferative activity was observed when cells expressed WISP-2/CCN5 (Fig. 4B). In control experiments in which we examined empty-vector-transfected MDA-MB-231 cells, we observed numerous colonies after 4 weeks of growth in soft agar (Fig. 4C), indicating that, like most transformed cells, they do not have an essential requirement for a matrix-derived growth signal. In contrast, under the same conditions, clones expressing WISP-2/CCN5 exhibited a reduced capacity to form colonies in soft agar. This suggests that the attenuation of anchorage-independent growth was mediated specifically by the expression of WISP-2/CCN5 in the transfected cells.
FIG. 4.
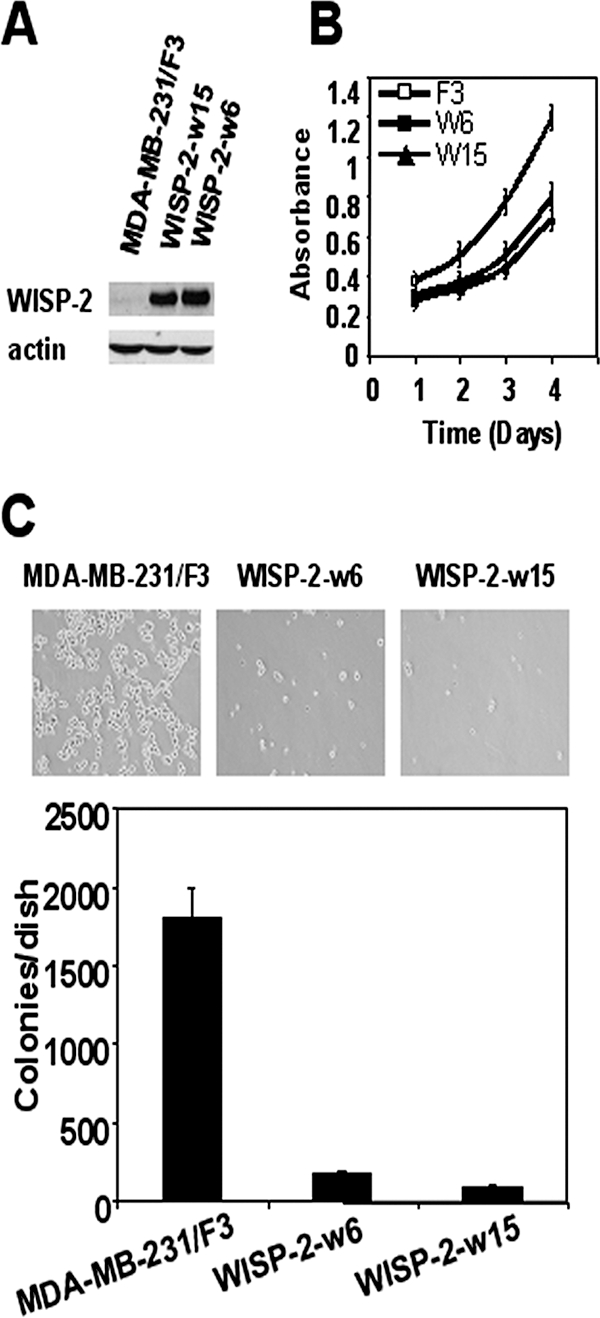
WISP-2/CCN5 expression reduces proliferation and anchorage-independent growth in MDA-MB-231 cells. (A) Stable cell lines expressing Flag-WISP-2 were generated as described in Materials and Methods. WISP-2 expression in two representative stable clones was assessed by Western blotting and compared with that in empty-vector stable transfectant cells. The levels of β-actin in cell lysates were measured by Western blotting and included as a loading control. (B) Cells were cultured in 96-well dishes in medium containing 10% FBS. Relative cell number was estimated by using a colorimetric assay 1, 2, 3, and 4 days after replating. Data are the means ± standard errors of the means of the results of triplicate experiments. (C) Effect of WISP-2/CCN5 expression on anchorage-independent growth of MDA-MB-231 cells. These cells were assayed for their ability to proliferate and form colonies in soft agar as described in Materials and Methods. Representative fields were photographed at a magnification of ×10. Colonies from three independent experiments were counted and plotted.
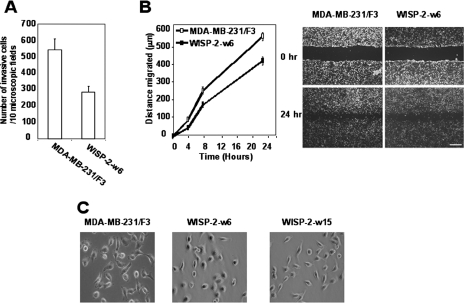
Next, we evaluated the invasive phenotype and motility of cells. In the Matrigel invasion assay, cells expressing WISP-2/CCN5 were twofold less invasive than control cells (Fig. 5A). Then, we performed wound healing-induced migration experiments (Fig. 5B). Cells were forced to migrate through the space created by scraping the monolayer with a tip. After 24 h, WISP-2/CCN5-expressing cells had only partially filled the wound. In correlation with the reduction of cell motility and invasion following WISP-2/CCN5 expression, we observed morphological changes in WISP-2/CCN5-transfected MDA-MB-231 cells. The WISP-2-w15 cells presented an elongated, bipolar shape, whereas the WISP-2-w6 cells, expressing the highest level of WISP-2/CCN5, exhibited a more flattened, stellate, and multipolar shape (Fig. 5C). Collectively, these findings may explain why the cells that have lost the ability to express WISP-2/CCN5 are more aggressive.
FIG. 5.
Effect of WISP-2/CC5 expression on matrix invasion, motility, and morphology of the MDA-MB-231 cells. (A) MDA-MB-231/F3 and WISP-2-w6 cells were plated on Matrigel-coated filters, and the numbers of cells that migrated through the membrane after 48 h were determined as described in Materials and Methods. Error bars indicate standard deviations of the results from 10 independent fields. (B) Confluent cell monolayers of MDA-MB-231/F3 or WISP-2-w6 cells were wounded with a pipette tip. Wound closure was monitored by microscopy at the indicated times. Migration is expressed as the average ± standard deviation of the difference between the measurements at time 0 and at 4, 8, or 24 h. Data are the means ± standard errors of the means of the results of triplicate experiments. Scale bar, 200 μm. (C) Morphology of MDA-MB-231/F3, WISP-2-w6, and WISP-2-w15 cells. Cells were plated on tissue culture plastic and were photographed using a phase-contrast microscope. Magnification, ×30.
Taken together, these results suggest that WISP-2/CCN5 is able to reduce the overall aggressiveness of breast cancer cells by reducing not only their invasiveness but also their rate of proliferation and their ability to grow in an anchorage-independent manner.
WISP-2/CCN5 induces gene expression changes in MDA-MB-231 human breast carcinoma cells.
To determine whether these cell phenotypic effects caused by elevation of WISP-2/CCN5 expression could be related to deregulation of specific genes, we used real-time RT-PCR to quantify the mRNA expression of a number of selected genes in both WISP-2-w15 and WISP-2-w6 cells, compared with MDA-MB-231-F3 cells. We assessed the expression level of a panel of 151 genes known to be involved in various cellular and molecular mechanisms associated with tumorigenesis and known to be altered (mainly at the transcriptional level) in various cancers. These genes encode proteins involved in cell cycle control, cell-cell interactions, signal transduction pathways, apoptosis, and angiogenesis (available on request).
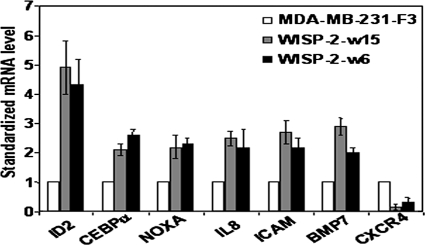
Seven (4.6%) of the 151 genes were expressed at a different level (>2-fold) in both WISP-2-w15 and WISP-2-w6 cells than in the empty vector-transfected MDA-MB-231 cell line; 6 (4.0%) were upregulated, and 1 (0.6%) was downregulated (Fig. 6). Among the six upregulated genes, ID2 is the gene that showed the highest upregulation in both the WISP-2-w15 and WISP-2-w6 cells (i.e., expression levels 4.9- and 4.3-fold higher, respectively, than in the empty-vector-transfected MDA-MB-231 cell line).
FIG. 6.
mRNA levels of seven identified genes in control MDA-MB-231 cell line stably transfected with the empty vector (MDA-MB-231-F3) and in two MDA-MB-231 cell lines overexpressing WISP-2/CCN5 (WISP-2-w15 and WISP-2-w6). For each gene and each cell line, mRNA levels were normalized such that the value of the MDA-MB-231-F3 sample was 1. The mRNA levels indicated are the means ± standard errors of the means of the results from at least three independent experiments.
mRNA expression of WISP-2/CCN5 and of the seven candidate WISP-2/CCN5-regulated genes in 48 human breast tumors.
We then explored whether the correlations identified in our cellular models between the expression of WISP-2/CCN5 and the expression levels of seven identified genes were also observed in human breast tumor biopsies (Table 1). We investigated, by real-time quantitative RT-PCR, the mRNA levels of these genes in 48 human breast tumors. Using the Spearman rank correlation test that compares continuous variables, we found a positive correlation between WISP-2/CCN5 and ID2 mRNA levels (r = 0.442, P = 0.002) and a trend toward correlation with CXCR4 (r = 0.254, P = 0.08) and C/EBPα (r = 0.274, P = 0.06) mRNA levels (Table 2). Surprisingly, CXCR4 was positively linked to WISP-2/CCN5 in these breast tumor biopsies, whereas this gene was found to be downregulated in the two MDA-MB-231 cell lines overexpressing WISP-2/CCN5 (WISP-2-w6 and WISP-2-w15).
TABLE 2.
Relationship between expression of WISP-2 and expression of the seven identified genes in 48 breast tumors
| Gene | WISP-2 mRNA level (r value, P valuea)
|
||
|---|---|---|---|
| Total breast tumors (n = 48) | ERα-negative breast tumors (n = 24) | ERα-positive breast tumors (n = 24) | |
| ID2 | 0.442, 0.002 | 0.369, NS (0.07) | 0.530, 0.007 |
| CXCR4 | 0.254, NS (0.08) | 0.267, NS (0.20) | 0.446, 0.03 |
| CEBPα | 0.274, NS (0.06) | 0.004, NS (0.98) | 0.425, 0.04 |
| ICAM | 0.167, NS (0.26) | 0.007, NS (0.97) | 0.380, NS (0.06) |
| IL-8 | 0.148, NS (0.32) | 0.311, NS (0.14) | 0.225, NS (0.29) |
| NOXA | 0.015, NS (0.92) | −0.225, NS (0.29) | 0.041, NS (0.84) |
| BMP7 | −0.118, NS (0.43) | −0.188, NS (0.38) | 0.131, NS (0.55) |
| MKI67 | −0.167, NS (0.26) | −0.064, NS (0.78) | −0.157, NS (0.50) |
r value, Spearman correlation coefficient; P value, Spearman rank correlation test. NS, not significant.
In the same set of 48 tumors, we also examined the expression of the proliferation-associated gene MKI67 that encodes the proliferation-related antigen Ki-67. We found no correlation between WISP-2/CCN5 and MKI67, suggesting that ID2, CXCR4, and C/EBPα were associated with the WISP-2/CCN5 gene independently of the proliferation status.
It is noteworthy that, among the 48 tumors, 24 tumors were ERα positive and 24 ERα negative. When we examined the correlation between the mRNA levels of WISP-2/CCN5 and the mRNA levels of the other genes in these two subgroups, we observed that WISP-2 was mainly linked to ID2, CXCR4, and C/EBPα in the ERα-positive tumors.
DISCUSSION
Although WISP-2/CCN5 has been identified as being located downstream of the wnt-1 signaling pathway that is relevant to the transformed cell phenotype in C57MG mouse mammary epithelial cells transformed by wnt-1 (35), the role of WISP-2/CCN5 in mammalian carcinogenesis has not been well defined. Both oncogenic and tumor suppressor activities have been attributed to WISP-2/CCN5. In this study, we used an siRNA-mediated reduction of WISP-2/CCN5 in the MCF-7 breast cancer cell line in order to examine the role of WISP-2/CCN5 in mammalian oncogenesis. We found that WISP-2/CCN5 knockdown induced an estradiol-independent growth of these cells, linked to a loss of ERα expression, and promoted epithelial-to-mesenchymal transdifferentiation.
Consistent with this result, in breast cancer, loss of E-cadherin expression and EMT have been associated with lack of ERα expression and a more-aggressive phenotype with poor clinical prognosis. The absence of ER leads to aberrant expression of the transcriptional repressor Snail, a master regulator of EMT (21). Aberrant Snail expression results in loss of expression of the cell adhesion molecule E-cadherin and cytokeratin 18 (8, 14, 16), events associated with changes in epithelial architecture and invasive growth. Interestingly, the WISP-2/CCN5-deficient MCF-7 cells showed an anchorage-independent growth that is suggestive of a more-invasive phenotype in vivo.
On the other hand, overexpression of WISP-2/CCN5 in the poorly differentiated, hormone-independent, and aggressive MDA-MB-231 cells led to inhibition of proliferation, reduction of anchorage-independent growth, and morphological changes. Besides these effects, WISP-2/CCN5 also reduced cell migration and invasion. In conclusion, we have shown that WISP-2/CCN5 inhibits metastatic breast cancer cell proliferation and invasion.
Our results are in contrast with the report that WISP-2/CCN5 is important for proliferation in MCF-7 cells (6, 7) but are in accordance with the data showing that WISP-2/CCN5 acts as a growth arrest-specific gene in normal human vascular and uterine smooth muscle cells (28, 32). WISP-2/CCN5 was found to be underexpressed in human colon tumors (47), in leiomyoma (32), and in pancreatic adenocarcinoma (17), suggesting that loss of WISP-2/CCN5 may contribute to malignant transformation.
Thus, WISP-2/CCN5 may have a dual role in breast cancer progression: it could be oncogenic at early stages of tumor development but act as a suppressor of invasive behavior at later stages. WISP-2/CCN5 participates in the mitogenic action of PMA on noninvasive, WISP-2/CCN5-positive breast tumor cells through protein kinase Cα-dependent multiple signal transduction pathways (41), and at the same time, it protects the preneoplastic cells from progressing to an invasive phenotype through estrogen-dependent signaling. It is possible that WISP-2/CCN5 expression varies during different stages of breast cancer progression and that epigenetic or other posttranslational modifications of WISP-2/CCN5 play an important role in the regulation of its activity or expression during malignant progression.
In the present study, we found that WISP-2/CCN5 expression in the MDA-MB-231 cells leads to changes in gene expression. Thus, the ID2 transcript showed the strongest upregulation. This is particularly interesting because it has been previously shown that ID2 is highly expressed in differentiated mammary epithelial cells in vivo, that ID2 antisense transcripts block mammary epithelial cell differentiation (34), and finally, that ID2 is expressed at low levels in undifferentiated breast cancer cells (26). ID2 is also upregulated by BMP7 (27). BMP7 is an important regulator of cell development and differentiation in various organs. The expression of BMP7 was found to be highly correlated with ER and PR levels (40). Thus, the upregulation of BMP7 suggests a more-differentiated phenotype. All other WISP-2/CCN5-modulated genes that we identified are involved in regulating cell proliferation and are implicated in an antiproliferative effect in breast cancer cell lines. The upregulation of ICAM by all-trans-retinoic acid inhibits the growth of breast cancer cells (4). It is known that upregulation of C/EBPα in ERα- and PR-negative breast cancer cell lines is associated with reduction of proliferation, reduced anchorage-independent cell growth, downregulation of c-Myc, and upregulation of p21WAF1, PPARγ, and the breast epithelial differentiation marker maspin (22). Interestingly, the expression of CXCR4 is downregulated in cells expressing WISP-2/CCN5. The chemokine receptor CXCR4 plays an active role in the metastasis of breast cancer (29, 33).
In addition, we showed in human breast tumor biopsies that WISP-2/CCN5 is mainly linked to ID2 and C/EBPα in the ERα-positive subgroup. A surprising result obtained in this study is that one gene (i.e., CXCR4) was negatively linked to WISP-2/CCN5 in our in vitro model (downregulation in the two MDA-MB-231 cell lines overexpressing WISP2/CCN5) but positively linked to WISP-2/CCN5 in the breast tumor series. One possible explanation for this finding is that cultured cell lines have lost many features that characterize tumor specimens in vivo (15, 46). Indeed, it is known that myoepithelial and various stromal cells are implicated in gene expression changes (3, 42). The mechanism that leads to in vivo overexpression of WISP2-inducible genes in breast tumors involves several factors, including WISP2/CCN5 and several known or unknown transcriptional coactivators, not all of which are present in classical in vitro models.
In summary, we propose that the WISP-2/CCN5 protein is an important regulator involved in the maintenance of a differentiated phenotype in breast tumor epithelial cells and suggest that WISP-2/CCN5 may play a role in regulating tumor cell metastasis and invasion.
Acknowledgments
We thank the staff of the Centre René Huguenin for assistance in specimen collection and patient care. We thank J. Mester and A. Zimber for critical review of the manuscript. We thank D. Catala for technical assistance. We thank A. Gompel and A. Courtin for human breast epithelial cells.
This study was supported by the Centre National de la Recherche Scientifique and the Ligue Nationale contre le Cancer, Comité de Paris.
Footnotes
Published ahead of print on 18 December 2007.
REFERENCES
- 1.Albertson, D. G., C. Collins, F. McCormick, and J. W. Gray. 2003. Chromosome aberrations in solid tumors. Nat. Genet. 34369-376. [DOI] [PubMed] [Google Scholar]
- 2.Al-Kuraya, K., P. Schraml, J. Torhorst, C. Tapia, B. Zaharieva, H. Novotny, H. Spichtin, R. Maurer, M. Mirlacher, O. Kochli, M. Zuber, H. Dieterich, F. Mross, K. Wilber, R. Simon, and G. Sauter. 2004. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 648534-8540. [DOI] [PubMed] [Google Scholar]
- 3.Allinen, M., R. Beroukhim, L. Cai, C. Brennan, J. Lahti-Domenici, H. Huang, D. Porter, M. Hu, L. Chin, A. Richardson, S. Schnitt, W. R. Sellers, and K. Polyak. 2004. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 617-32. [DOI] [PubMed] [Google Scholar]
- 4.Baj, G., A. Arnulfo, S. Deaglio, E. Tibaldi, N. Surico, and F. Malavasi. 1999. All-trans retinoic acid inhibits the growth of breast cancer cells by up-regulating ICAM-1 expression. J. Biol. Regul. Homeost. Agents 13115-122. [PubMed] [Google Scholar]
- 5.Balmain, A., J. Gray, and B. Ponder. 2003. The genetics and genomics of cancer. Nat. Genet. 33(Suppl.)238-244. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee, S., N. Saxena, K. Sengupta, O. Tawfik, M. S. Mayo, and S. K. Banerjee. 2003. WISP-2 gene in human breast cancer: estrogen and progesterone inducible expression and regulation of tumor cell proliferation. Neoplasia 563-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee, S., K. Sengupta, N. K. Saxena, K. Dhar, and S. K. Banerjee. 2005. Epidermal growth factor induces WISP-2/CCN5 expression in estrogen receptor-alpha-positive breast tumor cells through multiple molecular cross-talks. Mol. Cancer Res. 3151-162. [DOI] [PubMed] [Google Scholar]
- 8.Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 284-89. [DOI] [PubMed] [Google Scholar]
- 9.Bieche, I., B. Parfait, V. Le Doussal, M. Olivi, M. C. Rio, R. Lidereau, and M. Vidaud. 2001. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: an outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 611652-1658. [PubMed] [Google Scholar]
- 10.Bloom, H. J., and W. W. Richardson. 1957. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br. J. Cancer 11359-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brigstock, D. R. 2003. The CCN family: a new stimulus package. J. Endocrinol. 178169-175. [DOI] [PubMed] [Google Scholar]
- 12.Brigstock, D. R. 1999. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr. Rev. 20189-206. [DOI] [PubMed] [Google Scholar]
- 13.Brigstock, D. R., R. Goldschmeding, K. I. Katsube, S. C. Lam, L. F. Lau, K. Lyons, C. Naus, B. Perbal, B. Riser, M. Takigawa, and H. Yeger. 2003. Proposal for a unified CCN nomenclature. Mol. Pathol. 56127-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cano, A., M. A. Perez-Moreno, I. Rodrigo, A. Locascio, M. J. Blanco, M. G. del Barrio, F. Portillo, and M. A. Nieto. 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 276-83. [DOI] [PubMed] [Google Scholar]
- 15.Dangles, V., V. Lazar, P. Validire, S. Richon, M. Wertheimer, V. Laville, J. L. Janneau, M. Barrois, C. Bovin, T. Poynard, G. Vallancien, and D. Bellet. 2002. Gene expression profiles of bladder cancers: evidence for a striking effect of in vitro cell models on gene patterns. Br. J. Cancer 861283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Craene, B., B. Gilbert, C. Stove, E. Bruyneel, F. van Roy, and G. Berx. 2005. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 656237-6244. [DOI] [PubMed] [Google Scholar]
- 17.Dhar, G., S. Mehta, S. Banerjee, A. Gardner, B. M. McCarty, S. C. Mathur, D. R. Campbell, S. Kambhampati, and S. K. Banerjee. 2007. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett. 25463-70. [DOI] [PubMed] [Google Scholar]
- 18.Easton, D. F. 1999. How many more breast cancer predisposition genes are there? Breast Cancer Res. 114-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritah, A., G. Redeuilh, and M. Sabbah. 2006. Molecular cloning and characterization of the human WISP-2/CCN5 gene promoter reveal its upregulation by oestrogens. J. Endocrinol. 191613-624. [DOI] [PubMed] [Google Scholar]
- 20.Fritah, A., C. Saucier, J. Mester, G. Redeuilh, and M. Sabbah. 2005. p21WAF1/CIP1 selectively controls the transcriptional activity of estrogen receptor alpha. Mol. Cell. Biol. 252419-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita, N., D. L. Jaye, M. Kajita, C. Geigerman, C. S. Moreno, and P. A. Wade. 2003. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113207-219. [DOI] [PubMed] [Google Scholar]
- 22.Gery, S., S. Tanosaki, S. Bose, N. Bose, J. Vadgama, and H. P. Koeffler. 2005. Down-regulation and growth inhibitory role of C/EBPalpha in breast cancer. Clin. Cancer Res. 113184-3190. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 10057-70. [DOI] [PubMed] [Google Scholar]
- 24.Hyder, S. M., C. Chiappetta, L. Murthy, and G. M. Stancel. 1997. Selective inhibition of estrogen-regulated gene expression in vivo by the pure antiestrogen ICI 182,780. Cancer Res. 572547-2549. [PubMed] [Google Scholar]
- 25.Inadera, H. 2003. Estrogen-induced genes, WISP-2 and pS2, respond divergently to protein kinase pathway. Biochem. Biophys. Res. Commun. 309272-278. [DOI] [PubMed] [Google Scholar]
- 26.Itahana, Y., J. Singh, T. Sumida, J. P. Coppe, S. Parrinello, J. L. Bennington, and P. Y. Desprez. 2003. Role of Id-2 in the maintenance of a differentiated and noninvasive phenotype in breast cancer cells. Cancer Res. 637098-7105. [PubMed] [Google Scholar]
- 27.Kowanetz, M., U. Valcourt, R. Bergstrom, C. H. Heldin, and A. Moustakas. 2004. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol. Cell. Biol. 244241-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lake, A. C., A. Bialik, K. Walsh, and J. J. Castellot, Jr. 2003. CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am. J. Pathol. 162219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapteva, N., A. G. Yang, D. E. Sanders, R. W. Strube, and S. Y. Chen. 2005. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 1284-89. [DOI] [PubMed] [Google Scholar]
- 30.Leask, A., and D. J. Abraham. 2006. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 1194803-4810. [DOI] [PubMed] [Google Scholar]
- 31.Malet, C., A. Gompel, H. Yaneva, H. Cren, N. Fidji, I. Mowszowicz, F. Kuttenn, and P. Mauvais-Jarvis. 1991. Estradiol and progesterone receptors in cultured normal human breast epithelial cells and fibroblasts: immunocytochemical studies. J. Clin. Endocrinol. Metab. 738-17. [DOI] [PubMed] [Google Scholar]
- 32.Mason, H. R., A. C. Lake, J. E. Wubben, R. A. Nowak, and J. J. Castellot, Jr. 2004. The growth arrest-specific gene CCN5 is deficient in human leiomyomas and inhibits the proliferation and motility of cultured human uterine smooth muscle cells. Mol. Hum. Reprod. 10181-187. [DOI] [PubMed] [Google Scholar]
- 33.Muller, A., B. Homey, H. Soto, N. Ge, D. Catron, M. E. Buchanan, T. McClanahan, E. Murphy, W. Yuan, S. N. Wagner, J. L. Barrera, A. Mohar, E. Verastegui, and A. Zlotnik. 2001. Involvement of chemokine receptors in breast cancer metastasis. Nature 41050-56. [DOI] [PubMed] [Google Scholar]
- 34.Parrinello, S., C. Q. Lin, K. Murata, Y. Itahana, J. Singh, A. Krtolica, J. Campisi, and P. Y. Desprez. 2001. Id-1, ITF-2, and Id-2 comprise a network of helix-loop-helix proteins that regulate mammary epithelial cell proliferation, differentiation, and apoptosis. J. Biol. Chem. 27639213-39219. [DOI] [PubMed] [Google Scholar]
- 35.Pennica, D., T. A. Swanson, J. W. Welsh, M. A. Roy, D. A. Lawrence, J. Lee, J. Brush, L. A. Taneyhill, B. Deuel, M. Lew, C. Watanabe, R. L. Cohen, M. F. Melhem, G. G. Finley, P. Quirke, A. D. Goddard, K. J. Hillan, A. L. Gurney, D. Botstein, and A. J. Levine. 1998. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc. Natl. Acad. Sci. USA 9514717-14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planque, N., and B. Perbal. 2003. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int. 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rachfal, A. W., and D. R. Brigstock. 2005. Structural and functional properties of CCN proteins. Vitam. Horm. 7069-103. [DOI] [PubMed] [Google Scholar]
- 38.Redeuilh, G., A. Attia, J. Mester, and M. Sabbah. 2002. Transcriptional activation by the oestrogen receptor alpha is modulated through inhibition of cyclin-dependent kinases. Oncogene 215773-5782. [DOI] [PubMed] [Google Scholar]
- 39.Saxena, N., S. Banerjee, K. Sengupta, M. N. Zoubine, and S. K. Banerjee. 2001. Differential expression of WISP-1 and WISP-2 genes in normal and transformed human breast cell lines. Mol. Cell. Biochem. 22899-104. [DOI] [PubMed] [Google Scholar]
- 40.Schwalbe, M., J. Sanger, R. Eggers, A. Naumann, A. Schmidt, K. Hoffken, and J. H. Clement. 2003. Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int. J. Oncol. 2389-95. [PubMed] [Google Scholar]
- 41.Sengupta, K., S. Banerjee, K. Dhar, N. K. Saxena, S. Mehta, D. R. Campbell, and S. K. Banerjee. 2006. WISP-2/CCN5 is involved as a novel signaling intermediate in phorbol ester-protein kinase Calpha-mediated breast tumor cell proliferation. Biochemistry 4510698-10709. [DOI] [PubMed] [Google Scholar]
- 42.Shipitsin, M., L. L. Campbell, P. Argani, S. Weremowicz, N. Bloushtain-Qimron, J. Yao, T. Nikolskaya, T. Serebryiskaya, R. Beroukhim, M. Hu, M. K. Halushka, S. Sukumar, L. M. Parker, K. S. Anderson, L. N. Harris, J. E. Garber, A. L. Richardson, S. J. Schnitt, Y. Nikolsky, R. S. Gelman, and K. Polyak. 2007. Molecular definition of breast tumor heterogeneity. Cancer Cell 11259-273. [DOI] [PubMed] [Google Scholar]
- 43.Thiery, J. P. 2002. Epithelial-mesenchymal transitions in tumour progression. Nat Rev. Cancer 2442-454. [DOI] [PubMed] [Google Scholar]
- 44.Thiery, J. P., and J. P. Sleeman. 2006. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7131-142. [DOI] [PubMed] [Google Scholar]
- 45.Visvader, J. E., and G. J. Lindeman. 2003. Transcriptional regulators in mammary gland development and cancer. Int. J. Biochem. Cell. Biol. 351034-1051. [DOI] [PubMed] [Google Scholar]
- 46.Welsh, J. B., L. M. Sapinoso, A. I. Su, S. G. Kern, J. Wang-Rodriguez, C. A. Moskaluk, H. F. Frierson, Jr., and G. M. Hampton. 2001. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 615974-5978. [PubMed] [Google Scholar]
- 47.Zhang, R., L. Averboukh, W. Zhu, H. Zhang, H. Jo, P. J. Dempsey, R. J. Coffey, A. B. Pardee, and P. Liang. 1998. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol. Cell. Biol. 186131-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zoubine, M. N., S. Banerjee, N. K. Saxena, D. R. Campbell, and S. K. Banerjee. 2001. WISP-2: a serum-inducible gene differentially expressed in human normal breast epithelial cells and in MCF-7 breast tumor cells. Biochem. Biophys. Res. Commun. 282421-425. [DOI] [PubMed] [Google Scholar]