Abstract
Phenotypic plasticity and the switching of vascular smooth muscle cells (SMCs) play a critical role in atherosclerosis. Although Runx2, a key osteogenic transcription factor, is expressed in atherosclerotic plaques, the molecular mechanisms by which Runx2 regulates SMC differentiation remain unclear. Here we demonstrated that Runx2 repressed SMC differentiation induced by myocardin, which acts as a coactivator for serum response factor (SRF). Myocardin-mediated induction of SMC gene expression was enhanced in mouse embryonic fibroblasts derived from Runx2 null mice compared to wild-type mice. Forced expression of Runx2 decreased the expression of SMC genes and promoted osteogenic gene expression, whereas the reduction of Runx2 expression by small interfering RNA enhanced SMC differentiation in human aortic SMCs. Runx2 interacted with SRF and interfered with the formation of the SRF/myocardin ternary complex. Thus, this study provides the first evidence that Runx2 inhibits SRF-dependent transcription, as a corepressor independent of its DNA binding. We propose that Runx2 plays a pivotal role in osteogenic conversion tightly coupled with repression of the SMC phenotype in atherosclerotic lesions.
Atherosclerosis, especially vascular calcification, is highly prevalent in patients with ischemic cardiovascular disease, cerebrovascular disorder, and renal failure. Previous studies have corroborated the observation that atherosclerosis not only is a passive invasive process but also involves the phenotypic conversion of vascular component cells (12, 18). Notably, smooth muscle cells (SMCs) in calcified atherosclerotic plaques expressed multiple markers of osteogenic differentiation, including alkaline phosphatase (ALP), osteopontin (OPN), Msx2, and Runx2 (40). Cultured SMCs treated with inorganic phosphate undergo a dramatic phenotypic transition characterized by the loss of SMC marker expression and upregulation of genes related to osteoblast/chondrocyte differentiation (36). Bone morphogenetic protein 2 (BMP2) also promotes the osteogenic conversion of vascular SMCs in vitro and in vivo (4, 35). These findings indicate that the osteogenic conversion of SMCs induced by atherogenic stimuli is important for the development of atherosclerosis.
The transcription factor Runx2, also called Cbfa1/Osf2/AML3/PEBP2αA, is one of the three mammalian members of the Runt-related transcription family (24). Runx2 is essential for skeletogenesis, as evidenced by the human autosomal dominant disease cleidocranial dysplasia, which is characterized by clavicular hypoplasia and craniofacial abnormalities caused by Runx2 mutations (22, 28). Runx2 null mice exhibit a complete lack of both intramembranous and endochondral ossification due to an absence of osteoblasts and consequently an unmineralized skeleton (21, 29). Runx2 is required for early commitment of mesenchymal stem cells into osteoprogenitors and functions later in osteoblast differentiation to regulate the formation of the extracellular matrix (8). Several lines of evidence indicate that Runx2 also plays an important role in adipogenesis and skeletal muscle differentiation. The accumulation of lipid droplets and increased adipogenic gene expression are observed in chondrocyte cells derived from Runx2 null mice (9). The Runx2 coactivator TAZ and CCAAT/enhancer binding protein β (C/EBPβ) promote osteogenesis, whereas they inhibit adipogenesis, in mesenchymal cells (15, 17). Runx2 prevents myogenesis and myotube formation via the suppression of MyoD and myogenin transcripts (11). Thus, Runx2 broadly modulates cellular fate, including cell traits.
Vascular SMCs do not terminally differentiate and undergo phenotypic change from a quiescent (contractile) to a proliferative (synthetic) phenotype in response to physiological and pathological stimuli. Serum response factor (SRF), a widely expressed MADS (MCM1, Agamous, Deficiens, SRF) box transcription factor, binds as a homodimer to a DNA consensus sequence known as a CArG box [CC(A/T)6GG], which is found in the promoter regions of SMC-specific genes (27). Myocardin is an SRF cofactor that is expressed specifically in smooth and cardiac muscle lineages throughout embryonic development and adulthood (42). Myocardin belongs to the SAP (SAF-A/B, Acinus, PIAS) domain family of transcription cofactors, which has been implicated in chromatin dynamics, and stimulates SRF-dependent transcription by interacting with the MADS domain of SRF and providing its strong transcriptional activation (3, 32). However, little is known about the role of myocardin in atherosclerosis.
Although an increasing number of studies have implicated the expression of Runx2 in the osteogenic differentiation of vascular SMCs, there is no direct evidence that Runx2 per se induces the osteogenic conversion and abrogates SMC phenotype. In this study, we demonstrate that Runx2 represses SMC gene expression and promotes osteogenic gene expression in human aortic SMCs (HASMCs). Runx2 physically interacts with SRF and disrupts the SRF/myocardin ternary complex. Dissociation of myocardin from chromatin provoked by Runx2 abolishes myocardin transcriptional activity. The results of this study suggest that Runx2 is crucial for SMC phenotypic conversion in the vascular remodeling observed in atherosclerosis.
MATERIALS AND METHODS
Plasmids.
The mouse Runx2 expression plasmids pCMV-Osf2 and pSG5-Runx2 were kindly provided by G. Karsenty (Baylor College of Medicine, Houston, TX) and Y. Ito (Institute of Molecular and Cell Biology, Singapore), respectively. SRF and myocardin expression plasmids (hemagglutinin [HA]-SRF and FLAG-myocardin) and SM22α and SM-MHC promoter constructs (SM22α-luc and SM-MHC-luc) were described previously (6, 7). The renilla luciferase plasmid pRL-CMV was obtained from Promega. SRE-tk-luc was generated by inserting two copies of c-fos CArG boxes into pGL3 (Promega) containing a thymidine kinase (tk) promoter. Runx2 with a FLAG tag or myocardin with an HA tag at the amino terminus was subcloned into pcDNA3.1(−) (Invitrogen) by PCR amplification (FLAG-Runx2 and HA-myocardin). FLAG-Runx2 ΔC was generated by removing the C-terminal sequence of FLAG-Runx2. FLAG-Runx2 ΔRunt, a Runt domain deletion mutant, was generated by PCR using FLAG-Runx2 as a template. Various deletion mutants (with deletions of amino acids 1 to 265, 133 to 265, 266 to 508, and 133 to 508) of the SRF expression plasmid were constructed by subcloning the PCR-amplified fragments of human SRF into HA-tagged pcDNA3.1(−). Glutathione S-transferase (GST)-SRF was generated by PCR amplification of full-length human SRF and subcloned into pGEX-4T-2 vector (Amersham). GST-Runx2 was a gift from G. Karsenty, and the various deletion mutants of GST-Runx2 (with deletions of amino acids 1 to 105, 106 to 241, 242 to 373, and 374 to 528) were constructed through PCR amplification and subcloning of these DNA fragments into pGEX-4T-2.
Cell culture, transfection, and luciferase assays.
C3H10T1/2 cells and 293T cells were obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS). HASMCs were obtained from Kurabo and cultured in HuMedia supplemented with 5% FBS, 2 ng/ml human fibroblast growth factor 2, 0.5 ng/ml human epidermal growth factor, and 5 μg/ml insulin. Primary cultures of adult rat aortic SMCs (RASMCs) were prepared as described previously (7). RASMCs were grown in M199 medium supplemented with 10% FBS. Mouse embryonic fibroblasts (MEFs) were isolated from 13.5-day-old embryos of pregnant heterozygous mice. Heads and livers were removed, and DNA for genotyping was isolated from these organs. The remaining tissues were minced, digested in 0.05% trypsin-EDTA, and incubated in Dulbecco's modified Eagle medium supplemented with 10% FBS overnight at 37°C. The next day, the medium and tissue clumps were removed and attached cells were cultured for experiments. All cells were cultured in a 5% CO2 atmosphere at 37°C.
C3H10T1/2 cells were transiently transfected with plasmid DNA using FuGENE 6 (Roche) or a modified calcium phosphate precipitation method as described previously (38). The total amount of DNA per well was kept constant by adding the corresponding amount of expression vector without a cDNA insert. Seventy-two hours later, cells were harvested and used for subsequent experiments, including RNA analyses and single or dual luciferase assays (Promega). Reporter gene luciferase activity was normalized to the activity of total protein or the control renilla luciferase activity. These assays were done in triplicate and repeated at least four times, and results from a representative experiment are shown with standard deviations.
RNA analysis.
Total RNA was isolated from cells with TRIzol reagent (Invitrogen) according to the manufacturer's directions. Single-stranded cDNAs were synthesized from 1 μg of total RNA, and semiquantitative reverse transcription-PCR (RT-PCR) was performed using an RT-PCR kit (Takara) and Advantage 2 polymerase mix (Clontech). Real-time PCR was performed on a Mx3000 instrument (Stratagene). The reaction was carried out using SYBER green master mix (Toyobo) according to the protocol provided by the manufacturer. The relative quantities of transcripts were determined using a standard curve and normalized against GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA. All experiments were repeated at least three times, and results from a representative experiment are shown with standard deviations. The primers used for RT-PCR are listed in Table 1.
TABLE 1.
Primer sequences used for RT-PCR analyses and ChIP assays
| Test | Gene | Primer sequence
|
|
|---|---|---|---|
| Sense | Antisense | ||
| RT-PCR analyses | |||
| Mouse semiquantitative | SM22α | 5′-TCCAGTCCACAAACGACCAAGC-3′ | 5′-GAATTGAGCCACCTGTTCCATCTG-3′ |
| PCR | SM-MHC | 5′-AGGAAACACCAAGGTCAAGCA-3′ | 5′-CCCTGACATGGTGTCCAATC-3′ |
| Myocardin | 5′-CCAAACCAAAGGTGAAGAAGCTC-3′ | 5′-TGTCTTAACTCTGACACCTTGAG-3′ | |
| Runx2 | 5′-GAGGGCACAAGTTCTATCTG-3′ | 5′-CGCTCCGGCCCACAAATCTC-3′ | |
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ | |
| Mouse real-time PCRa | SM α-actin | 5′-AGTCGCTGTCAGGAACCCTGAGACG-3′ | 5′-ATCTTTTCCATGTCGTCCCAGTTG-3′ |
| Myocardin | 5′-CAAGGCTTAATACCGCCACTG-3′ | 5′-AATGTGCATAGTAACCAGGCTG-3′ | |
| GAPDH | 5′-AACGACCCCTTCATTGAC-3′ | 5′-TCCACGACATACTCAGCAC-3′ | |
| Human semiquantitative | SM22α | 5′-AACAGCCTGTACCCTGATGG-3′ | 5′-ATGACATGCTTTCCCTCCTG-3′ |
| PCRb | SM α-actin | 5′-ATGAGGGCTATGCCTTGCCC-3′ | 5′-CCCGATGAAGGATGGCTGGA-3′ |
| SM-MHC | 5′-GGAGGATGAGATCCTGGTCA-3′ | 5′-TGCTTTTCAGCCTTGTTCCT-3′ | |
| ALP | 5′-GGACATGCAGTACGAGCTGA-3′ | 5′-GCAGTGAAGGGCTTCTTGTC-3′ | |
| OPN | 5′-ATGAGAATTGCAGTGATTTGC-3′ | 5′-TTAATTGACCTCAGAAGATGCAC-3′ | |
| OSX | 5′-ACCTTTCAGCCCCCAAAACCAT-3′ | 5′-TTAAGGGGAGCAAAGTCAGAT-3′ | |
| Runx2 | 5′-CGCATTCCTCATCCCAGTAT-3′ | 5′-GACTGGCGGGGTGTAAGTAA-3′ | |
| ChIP assaysb | Human SM22α | 5′-CCCTCTCTCCAGCCAGACT-3′ | 5′-CCCCAGTGACTCCACACAG-3′ |
| Rat SM-MHC | 5′-GTACTGGGGTCCCCATAACG-3′ | 5′-TCGAGGTCTGAGCTGGTCCT-3′ | |
| Mouse SM-MHC | 5′-TCCAGCAGCACCCCGCTTTT-3′ | 5′-CCTCTTAGATCTGAGGGCGG-3′ | |
The primers for SM22α and SM-MHC are the same as those used in mouse semiquantitative PCR.
The primers for GAPDH are as same as the mouse GAPDH sequences used in semiquantitative PCR.
Immunoprecipitation and immunoblotting.
293T cells were transiently transfected with FLAG-tagged or nontagged Runx2, FLAG-myocardin, and HA-SRF, as indicated in the figure legends, by the calcium phosphate precipitation method. Cells were harvested in phosphate-buffered saline (PBS) at 72 h after transfection and were lysed in NETN buffer (20 mM Tris-HCl [pH 8], 100 mM NaCl, 1 mM EDTA, and 0.5% NP-40) supplemented with freshly prepared protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 2 μg/ml pepstatin A and aprotinin). Following a brief sonication and the removal of cellular debris by centrifugation, epitope-tagged proteins were precipitated with the indicated antibodies and protein A/G-Sepharose beads (Amersham). The bound proteins were washed four times in NETN buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. Membranes were immunoblotted with antibodies as indicated, and proteins were visualized with a chemiluminescence detection system (Millipore). Immunoprecipitation experiments were performed using C3H10T1/2 whole-cell extracts or in vitro-translated proteins prepared with a TNT T7-coupled reticulocyte lysate system (Promega). Mouse monoclonal antibodies against FLAG (M2), and α-tubulin (DM1A) were purchased from Sigma, and rabbit antibodies against HA (Y-11), PEBP2αA (M-70), and SRF (G-20) were purchased from Santa Cruz.
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay was performed as described previously (6). Cultured cells were cross-linked with 1% formaldehyde for 10 min at room temperature. After the cells were collected in PBS, the pellets were resuspended in SDS lysis buffer (1% SDS, 5 mM EDTA, 50 mM Tris-HCl [pH 8], and protease inhibitors). The cross-linked chromatin was sonicated to shear genomic DNA. The cross-linked DNA/protein extracts were diluted with dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, 16.7 mM Tris-HCl [pH 8], and protease inhibitors) and immunoprecipitated with 5 μg of normal rabbit immunoglobulin G (IgG) (Santa Cruz), anti-SRF, or anti-HA antibodies overnight at 4°C. Protein A-Sepharose beads were added to the supernatant, and the mixture was incubated for 1 h. The beads were washed sequentially with TSE I buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, and 20 mM Tris-HCl [pH 8]), TSE II buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 500 mM NaCl, and 20 mM Tris-HCl [pH 8]), TSE III buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, and 10 mM Tris-HCl [pH 8]), and TE buffer (10 mM Tris-HCl [pH 8] and 1 mM EDTA) twice. The precipitates were eluted with 150 μl of elution buffer (1% SDS, 0.1 M NaHCO3), and incubated with 6 μl of 5 M NaCl at 65°C for 4 h to reverse cross-linking. After treatment with proteinase K (Roche) and RNase A (Sigma), DNA fragments were purified with a PCR purification kit (Qiagen). Input DNA and DNA isolated from precipitated chromatin were subjected to conventional PCR. The primers used for ChIP assays are listed in Table 1.
GST pull-down assay.
GST fusion proteins were prepared in BL21-CodonPlus competent cells (Stratagene) as described previously (38). In vitro-translated [35S]methionine-labeled proteins were prepared using a TNT T7-coupled reticulocyte lysate system. Glutathione beads conjugated with protein were incubated with the TNT product in NETN buffer supplemented with freshly prepared protease inhibitors for 1 h at 4°C. The beads were washed four times in NETN buffer. Proteins eluted in SDS loading buffer were separated by SDS-PAGE and analyzed by autoradiography.
EMSA.
35S-labeled or unlabeled in vitro-translated HA-SRF and FLAG-Runx2 proteins were prepared in parallel using the TNT T7-coupled reticulocyte lysate system. A probe containing the c-fos CArG box sequence (underlined; 5′-CTTTACACAGGATGTCCATATTAGGACATCTGCGTCAGGT-3′) was prepared by Klenow fill-in with a [32P]dCTP. Unlabeled TNT products were incubated with 32P-labeled c-fos CArG box probe, and electrophoretic mobility shift assay (EMSA) was performed as previously described (37). 35S-labeled TNT products were separated by SDS-PAGE and analyzed by autoradiography.
Immunohistochemical analysis.
Human coronary artery tissues with atherosclerosis were obtained by directional coronary atherectomy (DCA) from symptomatic patients diagnosed with ischemic heart disease at Gunma University Hospital. This protocol was approved by the Institutional Review Board at Gunma University Hospital, and informed consent was obtained from the patients. Tissue samples were fixed in 10% buffered formalin and embedded in paraffin, and serial sections were prepared. Double-immunofluorescence staining was performed as follows. After deparaffinization and blocking, samples were incubated with antibodies against PEBP2αA (M-70), SM α-actin (Enzo; CGA7), and SRF (A-11) overnight at 4°C, and then with Cy3- and fluorescein isothiocyanate-conjugated secondary antibodies (Sigma) for 1 h at room temperature. Nuclear staining was preformed with DAPI (4′,6′-diamidino-2-phenylindole). Von Kossa staining was performed as follows. After treatment with a 5% silver nitrate (Wako) solution for 1 h, the samples were exposed to strong light to visualize calcium deposits, and then a 5% sodium thiosulfate solution and Kernechtrot (Muto Pure Chemical) were added. The serial sections were also stained using hematoxylin-eosin. The samples were examined using a fluorescence microscope (Olympus; DP71). Immunohistochemical staining of mouse embryo sections was performed with the CSA kit (Dako) according to the manufacturer's protocol.
Adenovirus.
Adenoviruses encoding HA-myocardin (Ad-myocardin), FLAG-Runx2 (Ad-Runx2), and LacZ (Ad-LacZ) were produced using the Gateway system (Invitrogen). Protein expression was confirmed by Western blotting (data not shown).
siRNA.
The small interfering RNA (siRNA) sequences specific for Runx2 (siRunx2) sequences were 5′-GCUUGAUGACUCUAAACCUTT-3′ and 5′-AGGUUUAGAGUCAUCAAGCTT-3′. GFP siRNAs (siGFP) (5′-GUUCAGCGUGUCCGGCGAGTT-3′ and 5′-CUCGCCGGACACGCUGAACTT-3′) were used as a control. Duplex siRNAs were introduced into HASMCs using Lipofectamine 2000 (Invitrogen).
ALP activity.
Ad-LacZ or Ad-Runx2-transfected HASMCs were treated every 3 days with bone differentiation medium (BM) (50 ng/ml human recombinant BMP2 [obtained from Astellas Pharma Inc.], 10 mM β-glycerol phosphate [Sigma], and 100 μg/ml ascorbic acid [Sigma]). After 10 days, the cultured cells were rinsed with PBS and fixed in 10% buffered formalin, and ALP activity was histochemically detected using nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Sigma). HASMCs were transfected with either siRunx2 or siGFP and were harvested 7 days after BM treatment. The ALP activity was measured with a microplate reader with a 405-nm-wavelength filter using LabAssay ALP (Wako) and was normalized to the protein amount.
RESULTS
Runx2 is colocalized with SM α-actin and SRF in noncalcified human atherosclerotic plaques.
Previous studies have shown that SMCs undergo a phenotypic change characterized by loss of SMC-specific gene expression and upregulation of a set of osteoblastic genes, including ALP, OPN, and Runx2, concomitant with vascular mineralization (40). We performed a series of experiments using anti-Runx2 and anti-SM α-actin antibodies and von Kossa staining to evaluate the state of mineralization in DCA specimens from patients with ischemic heart disease. Runx2 expression in SM α-actin-negative and von Kossa-positive lesions was observed as previously reported (data not shown). We also found that subsets of SM α-actin-positive cells in noncalcified atherosclerotic plaques were stained by anti-Runx2 antibody (Fig. 1Ac). In addition, Runx2 staining was partially colocalized with SRF staining in human DCA samples (Fig. 1Bc). Given the colocalization of Runx2 with SM α-actin and SRF in atherosclerotic lesions, we hypothesized that Runx2 regulates SMC differentiation in the process of mineralization.
FIG. 1.
Colocalization of Runx2 with SM α-actin and SRF in noncalcified plaques of human coronary artery. The expression of Runx2 (a), SM α-actin (Ab), and SRF (Bb) was detected by immunofluorescence analysis in DCA specimens from patients with ischemic heart disease. The colocalized expression of Runx2 and SM α-actin or SRF is yellow (c). Nuclear staining was performed with DAPI (d). The sample was subjected to hematoxylin-eosin staining (e).
Runx2 represses myocardin-induced SMC gene expression.
Myocardin is a potent transcriptional activator that induces SMC genes by tethering to CArG boxes with SRF (32). To examine whether Runx2 modulates SMC differentiation, we utilized C3H10T1/2 mesenchymal cells, which differentiate and express a set of SMC contractile genes in response to myocardin overexpression. Consistent with previous reports, cells transfected with myocardin exhibited a dramatic increase in SM22α and SM-MHC gene expression in RT-PCR analyses. When the cells were cotransfected with the Runx2 expression vector, myocardin-induced SMC gene expression was completely abrogated (Fig. 2A).
FIG. 2.
Runx2 represses myocardin-induced SMC gene expression. (A) C3H10T1/2 cells were transiently transfected with HA-myocardin in the absence or presence of FLAG-Runx2 as indicated. RNA was isolated, and SMC gene transcripts, including SM22α and SM-MHC, were detected by semiquantitative RT-PCR analyses. Note that the exogenous gene expression of myocardin and Runx2 was almost equal. GAPDH was used as an internal control for RNA loading. (B) C3H10T1/2 cells were transiently transfected with HA-myocardin, FLAG-Runx2, or both, along with the luciferase reporter controlled by the SM22α promoter (SM22α-luc) or the SM-MHC promoter (SM-MHC-luc). The total amount of DNA in each sample was adjusted with an empty expression plasmid. Values are presented as relative luciferase activity compared with the pcDNA3.1 vector alone. (C) C3H10T1/2 cells were transiently transfected with SRE-tk-luc and indicated amounts (ng) of HA-myocardin and FLAG-Runx2. (D) C3H10T1/2 cells were transiently transfected with HA-myocardin (600 ng), FLAG-Runx2 (600 ng), or both in the presence or absence of HA-SRF (600 ng), along with SRE-tk-luc. Error bars indicate standard deviations.
We next evaluated the Runx2-mediated repressive effect on SMC gene promoter activity by cotransfecting C3H10T1/2 cells with expression plasmids carrying myocardin and Runx2. Forced expression of Runx2 repressed myocardin-induced activation of the SM22α and SM-MHC promoter (Fig. 2B). To confirm the SRF/CArG dependency in the transcriptional repression function of Runx2, we performed luciferase reporter assays using SRE-tk-luc, which contains two copies of c-fos CArG box sequences. As shown in Fig. 2C, myocardin-induced activation of SRE-tk-luc was effectively inhibited by Runx2 in a dose-dependent manner, implying that SRF plays a critical role in the Runx2-mediated repression. It has been reported that myocardin-induced transcriptional activation is sensitive to the level of SRF (42), and Runx2 repressed myocardin-induced CArG-dependent promoter activity regardless of the presence or absence of SRF (Fig. 2D). Taken together, these results demonstrate that Runx2 represses myocardin-induced SMC gene activation in a manner dependent on SRF transcription.
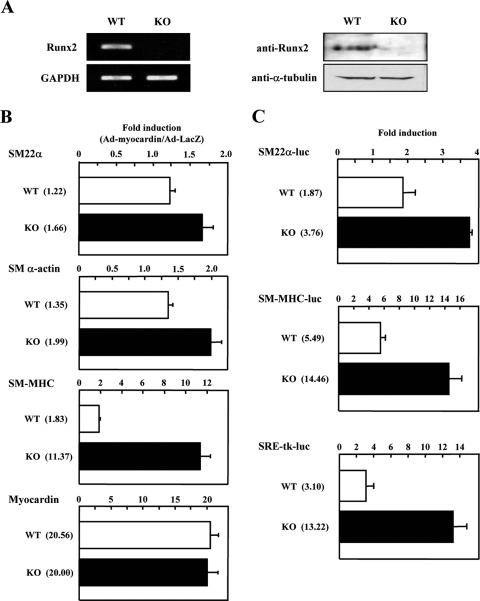
Enhancement of myocardin-induced SMC gene expression in Runx2 null MEFs.
To confirm the role of Runx2 in myocardin-induced SMC gene expression, we used MEFs derived from Runx2-knockout (KO) mice and wild-type (WT) mice (Fig. 3A). As shown in Fig. 3B, the levels of myocardin gene expression achieved with the adenovirus were comparable in KO and WT MEFs as evaluated by real-time PCR analyses. The induction of SM-MHC gene expression by myocardin was greatly enhanced in the KO MEFs compared with the WT MEFs (KO versus WT SM-MHC, 11.37-fold versus 1.83-fold). Consistent with this, myocardin-induced SM22α and SM α-actin gene expression was observed to a lesser extent in MEFs from KO mice (KO versus WT SM22α; 1.66-fold versus 1.22-fold; KO versus WT SM α-actin, 1.99-fold versus 1.35-fold). The less remarkable induction of SM22α and SM α-actin expression was probably due to the high basal expression levels of these genes in KO MEFs.
FIG. 3.
Myocardin-induced SMC gene expression is enhanced in Runx2 KO MEFs. (A) Runx2 mRNA and protein levels in MEFs derived from WT and Runx2 KO mice were determined by RT-PCR (left panel) and immunoblotting (right panel). GAPDH and α-tubulin were used as internal controls for even loading. (B) MEFs were infected with either Ad-myocardin or Ad-LacZ, followed by RNA extraction and RT reaction. Expression of SM22α, SM α-actin, SM-MHC, and exogenous myocardin transcripts was quantified by real-time PCR analyses. Induction was calculated by the ratio of myocardin- to LacZ-infected transcripts in the MEFs. The n-fold induction is given in parentheses. (C) MEFs were transiently transfected with luciferase reporter plasmid pRL-CMV; either SM22α-luc, SM-MHC-luc, or SRE-tk-luc; and expression vector HA-myocardin or pcDNA3.1. Dual luciferase reporter assay was employed to measure SRF-dependent promoter activity. Induction was calculated by the ratio of myocardin- to pcDNA3.1-transfected luciferase activity in the MEFs. The n-fold induction is given in parentheses. Error bars indicate standard deviations.
Based on the observation that overexpression of Runx2 repressed myocardin-induced SM22α-luc, SM-MHC-luc, and SRE-tk-luc activation (Fig. 2B, C, and D), we next tested whether these CArG-dependent promoter activities were enhanced in Runx2-deficient cells. As shown in Fig. 3C, cotransfection of myocardin augmented the SM22α-luc, SM-MHC-luc, and SRE-tk-luc activities in KO MEFs compared with WT MEFs. These results support our hypothesis that Runx2 is a negative regulator of myocardin-induced SMC gene expression.
Runx2 directly interacts with SRF.
The preceding results suggest that Runx2 and SRF physically interact with each other. To assess this possibility, we cotransfected 293T cells with HA-SRF and FLAG-Runx2 and performed coimmunoprecipitation assays. HA-SRF and FLAG-Runx2 proteins were immunoprecipitated with anti-HA or with anti-FLAG antibodies from whole-cell extracts. The results showed that Runx2 specifically interacted with SRF, and this interaction was confirmed by reciprocal precipitation (Fig. 4A).
FIG. 4.
Interaction of Runx2 with SRF. (A) 293T cells were transiently transfected with HA-SRF or FLAG-Runx2 alone or together. Cell lysates were incubated with anti-HA or anti-FLAG antibodies or normal mouse IgG, and immunoprecipitates (IP) were subjected to SDS-PAGE and immunoblotted with anti-FLAG or anti-HA antibodies as indicated. Whole-cell extracts were loaded for input. (B) GST pull-down assay shows that Runx2 directly interacts with SRF. In vitro-translated 35S-labeled SRF was incubated with either GST alone or GST-Runx2. After vigorous washing, bound protein and 10% input were detected by autoradiography after SDS-PAGE. A reciprocal experiment was performed using in vitro-translated 35S-labeled Runx2 and GST-SRF. (C) C3H10T1/2 whole-cell extract was immunoprecipitated with anti-SRF antibody or normal rabbit IgG, and immunoprecipitates were subjected to SDS-PAGE and immunoblotted with anti-Runx2 and anti-SRF antibodies. (D) In vitro-translated 35S-labeled myocardin or HERP1 was incubated with either GST alone or GST-Runx2. Interacting proteins were visualized by autoradiography after SDS-PAGE. (E and F) Upper panels show schematic representations of deletion constructs of GST-Runx2 (1 to 4) and HA-SRF (1 to 4) used in the respective lower panels. Lower panels show the mapping of the interactive domain of Runx2 and SRF by a GST pull-down experiment. (E) In vitro-translated 35S-labeled full-length SRF and deletion mutants of GST-Runx2 were incubated. (F) A reciprocal experiment was performed using in vitro-translated 35S-labeled HA-SRF fragments and GST-Runx2. After vigorous washing, bound protein and 10% input were subjected to SDS-PAGE and Coomassie brilliant blue (CBB) staining and then detected by autoradiography.
We further confirmed this interaction by conducting GST pull-down assays. As shown in Fig. 4B, in vitro-translated SRF and Runx2 proteins could associate with GST-Runx2 and GST-SRF fusion protein, respectively, whereas no interaction of the 35S-labeled proteins with GST alone was observed. These experiments revealed the direct interaction between Runx2 and SRF. We further found that endogenous SRF interacted with Runx2 in C3H10T1/2 cell extracts, indicating that Runx2 forms a protein complex with SRF in cells (Fig. 4C). In contrast, in vitro-translated 35S-labeled myocardin did not interact with GST-Runx2. HES-related repressor protein 1 (HERP1) was used as a positive control for Runx2 binding (10) (Fig. 4D).
To map the Runx2-binding region within SRF, we carried out GST pull-down experiments using GST-Runx2 deletion mutant proteins and SRF translated in vitro. As shown in Fig. 4E, the Runt domain of Runx2 (GST-Runx2 2) efficiently interacted with SRF, whereas other mutants did not. Using a series of 35S-labeled in vitro-translated SRF deletion mutants, we confirmed that the MADS box of SRF was required for the interaction with GST-Runx2 (Fig. 4F).
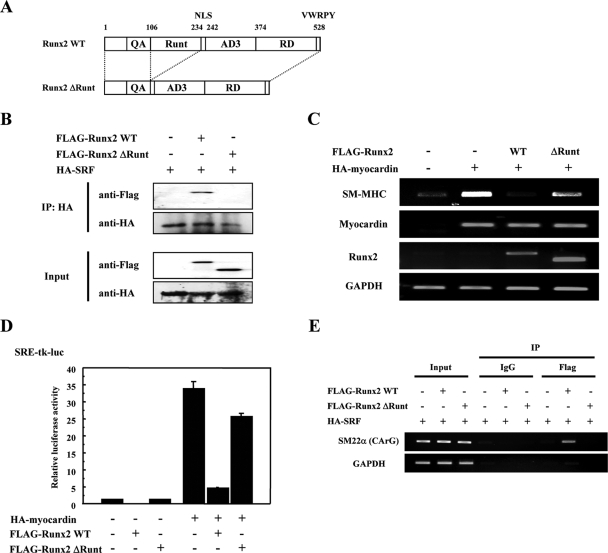
The Runx2 Runt domain is necessary and sufficient for SRF interaction and Runx2-mediated repression of SMC gene expression.
To confirm the functional significance of the Runt domain, we generated the Runx2 ΔRunt plasmid which is a deletion mutant of the Runt domain (Fig. 5A). As shown in Fig. 5B, Runx2 ΔRunt could not associate with SRF. Furthermore, Runx2 ΔRunt did not repress the myocardin-induced SM-MHC mRNA expression and SRE-tk-luc transcriptional activity (Fig. 5C and D). In addition, Runx2 ΔRunt did not bind the CArG box sequence of the SM22α promoter, which was immunoprecipitated with anti-FLAG antibody in 293T cells expressing HA-SRF and FLAG-Runx2 WT or ΔRunt (Fig. 5E). Thus, the Runt domain of Runx2 is critical for the interaction with SRF and the Runx2-mediated transcriptional repression.
FIG. 5.
The Runx2 Runt domain is critical to SRF interaction and Runx2-mediated repression of SMC gene expression. (A) Schematic representation of Runx2 WT and ΔRunt. Runx2 ΔRunt is a Runt domain deletion mutant. (B) In vitro-translated HA-SRF, FLAG-Runx2 WT, and ΔRunt proteins were prepared. Proteins were incubated with anti-SRF antibodies, and immunoprecipitates (IP) and 10% input were subjected to SDS-PAGE and immunoblotted with anti-FLAG or anti-HA antibodies as indicated. (C) C3H10T1/2 cells were transiently transfected with HA-myocardin in presence of FLAG-Runx2 WT or ΔRunt as indicated. The mRNA expression of SM-MHC and the exogenous myocardin and Runx2 was detected by semiquantitative RT-PCR analyses. GAPDH was used as an internal control for RNA loading. (D) C3H10T1/2 cells were transiently transfected with HA-myocardin and either FLAG-Runx2 WT or ΔRunt, along with SRE-tk-luc. The total amount of DNA in each sample was adjusted with an empty expression plasmid. Values are presented as relative luciferase activity compared with the pcDNA3.1 vector alone. Error bars indicate standard deviations. (E) Runx2 ΔRunt does not interact with chromatin DNA. 293T cells were transiently transfected with HA-SRF and either FLAG-Runx2 WT or ΔRunt. ChIP assay was performed as described in Materials and Methods. The cross-linked chromatin was immunoprecipitated with normal mouse IgG or anti-FLAG antibody, followed by PCR amplification using primers containing the CArG box of human SM22α. Input represents the amount of DNA prior to immunoprecipitation in each sample.
Repressor proteins do not contribute to Runx2-mediated SMC gene repression.
To determine the mechanism by which Runx2 repressed SRF-dependent gene transcription, we tested the following possibilities: (i) recruitment of repressor proteins, (ii) inhibition of SRF/DNA binding, and (iii) disruption of the SRF/myocardin complex.
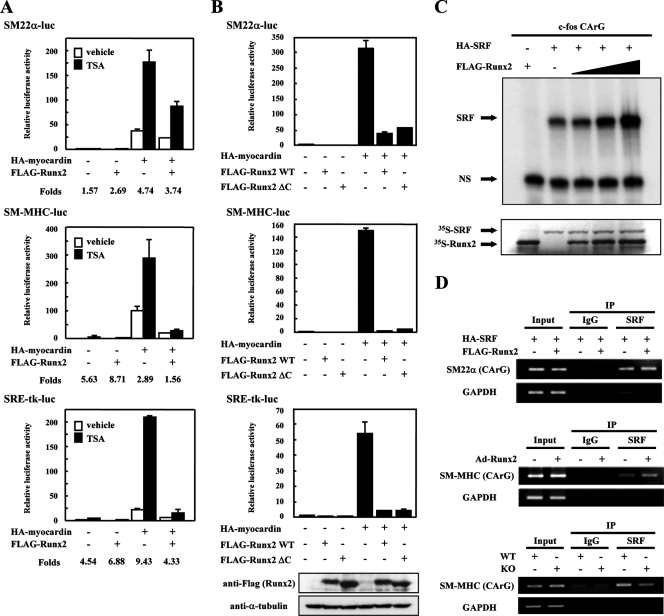
Previous studies have identified several corepressor proteins, including histone deacetylases (HDACs) and transducin-like enhancer of split (TLE), which interact with Runx2 and regulate gene expression. Association with histone acetyltransferase p300 enhances and interaction with HDACs represses myocardin activity in SRF target gene expression (3). Given the emerging role of HDACs in regulating Runx2, we tested whether HDAC activity was responsible for Runx2-mediated repression. The HDAC inhibitor trichostatin A (TSA) enhanced the transcriptional activity of myocardin in the CArG box-containing promoter constructs, including SM22α-luc, SM-MHC-luc, and SRE-tk-luc, as previously reported (3). In the absence or presence of TSA, Runx2 significantly reduced myocardin-induced SRF-dependent transcription at similar levels (Fig. 6A).
FIG. 6.
Runx2 does not recruit repressor proteins and inhibit SRF/DNA binding. (A) HDAC inhibitor does not abrogate Runx2-mediated repression in myocardin-induced SMC gene activation. C3H10T1/2 cells were transiently transfected with luciferase reporter plasmid; SM22α-luc, SM-MHC-luc, or SRE-tk-luc; and expression vectors FLAG-Runx2 and HA-myocardin. Cells were treated with 300 nM TSA for 24 h prior to harvest. Relative luciferase activity compared with pcDNA3.1 vector alone is presented. The induction was calculated as the ratio of TSA-treated (filled bars) to vehicle-treated (open bars) luciferase activity. Error bars indicate standard deviations. (B) TLE is not related to the Runx2 repression for myocardin-induced SMC gene activation. C3H10T1/2 cells were transiently transfected with luciferase reporter plasmid; SM22α-luc, SM-MHC-luc, or SRE-tk-luc; and expression vectors FLAG-Runx2 WT or C-terminal deletion mutant (ΔC) in the absence or presence of HA-myocardin. Identical samples for the SRE-tk-luc experiment were subjected to SDS-PAGE and immunoblotted with anti-FLAG and anti-α-tubulin antibodies. (C) Runx2 does not interfere with SRF/DNA binding. In vitro-translated HA-SRF and FLAG-Runx2 proteins were incubated with 32P-labeled c-fos CArG box probe, and EMSA was performed. DNA/protein complexes were detected by autoradiography. Parallel preparations of 35S-labeled proteins were separated by SDS-PAGE and analyzed by autoradiography. The arrows show SRF/DNA complexes (SRF) and nonspecific bands (NS). (D) Runx2 does not interfere with DNA binding of SRF in intact chromatin. Upper panel, 293T cells were transiently transfected with HA-SRF in the absence or presence of FLAG-Runx2. Middle panel, RASMCs were infected with Ad-Runx2 or Ad-LacZ. Lower panel, Runx2 KO MEFs and WT MEFs. ChIP assays were performed as described in Materials and Methods. The cross-linked chromatin was immunoprecipitated with normal rabbit IgG or anti-SRF antibody, followed by PCR amplification using primers containing the CArG box of human SM22α, rat SM-MHC, or mouse SM-MHC. Input represents the amount of DNA prior to immunoprecipitation in each sample.
We next examined the participation of TLE in the transcriptional suppression by Runx2. Deletion of the last pentapeptide, VWPRY, which is conserved in all Runx proteins and is sufficient to bind to Groucho/TLE proteins (1), led to a significant increase in Runx2 transcriptional activity (39). As shown in Fig. 6B, Runx2 ΔC, which lacks the C-terminal pentapeptide of Runx2, and WT Runx2 exerted comparable effects on the myocardin-induced activation of SM22α-luc, SM-MHC-luc, and SRE-tk-luc. These results suggest that an HDAC- and TLE-independent mechanism contributes to the Runx2-mediated repression of myocardin-induced SMC gene expression.
Runx2 does not inhibit SRF/DNA binding.
We reported that HERP1, which is a direct target of Notch signal and acts as a transcriptional repressor, inhibits the myocardin-induced SMC marker gene expression in C3H10T1/2 cells by interfering with the binding of SRF to the CArG box of SMC gene promoters (7). To test whether Runx2 inhibits the binding of SRF to the CArG box, we performed EMSA using SRF and Runx2 proteins translated in vitro and a radiolabeled CArG box derived from the c-fos gene as a probe. As shown in Fig. 6C, Runx2 alone showed no detectable DNA binding to the CArG box. Runx2 did not inhibit but rather increased the binding of SRF to the probe in a dose-dependent manner. In addition, the chromatin was immunoprecipitated with anti-SRF antibody in 293T cells expressing SRF in the absence or presence of Runx2, and expected PCR products containing the CArG box sequence of the SM22α promoter were observed, with a slight increase (Fig. 6D, top). The CArG element of the SM-MHC promoter immunoprecipitated with anti-SRF antibody in RASMCs was enhanced by Runx2 (Fig. 6D, middle). In contrast, a slight decrease of the SM-MHC promoter CArG box sequence immunoprecipitated by anti-SRF antibody was observed in KO MEFs compared to WT MEFs (Fig. 6D, bottom). Since HERP1 inhibits SRF/DNA binding, the Runx2-mediated repression is not dependent on HERP1 function. Taken together, these results indicate that Runx2 does not interfere with, but rather enhances, the binding of SRF to the CArG box of SMC gene promoters.
Runx2 disrupts the SRF/myocardin ternary complex.
Because Runx2 interacted with the MADS domain of SRF, which also serves as a myocardin-binding domain, we tested whether Runx2 competes with myocardin to bind SRF. We carried out the coimmunoprecipitation of SRF and myocardin with or without Runx2 in 293T cells. SRF interacted with myocardin, and this interaction was diminished in the presence of Runx2 (Fig. 7A, lanes 7 and 8).
FIG. 7.
Runx2 disrupts the SRF/myocardin ternary complex. (A) 293T cells were transiently transfected with HA-SRF and FLAG-myocardin in the absence or presence of pSG5-Runx2. HA-SRF protein was immunoprecipitated (IP) with anti-HA antibody, and input (whole-cell extract) and bound proteins were separated by SDS-PAGE. FLAG-myocardin, HA-SRF, and Runx2 proteins were detected by anti-FLAG, anti-HA, and anti-Runx2 antibodies. (B) C3H10T1/2 cells were infected with adenovirus encoding HA-myocardin (Ad-myocardin) and either Ad-Runx2 or Ad-LacZ. The cross-linked chromatin DNA was immunoprecipitated with normal rabbit IgG or anti-HA antibody, followed by PCR amplification using mouse SM-MHC CArG box primers. Input represents the amount of DNA prior to immunoprecipitation in each sample. (C) In vitro-translated HA-SRF, HA-myocardin, and increasing amounts of FLAG-Runx2 proteins were prepared. Proteins were incubated with anti-SRF antibodies, and immunoprecipitates were subjected to SDS-PAGE and immunoblotted with anti-FLAG or anti-HA antibodies as indicated.
To confirm the disruption of SRF/myocardin interaction in vivo, we performed a ChIP assay. Chromatin fragments from lysates of C3H10T1/2 cells infected with adenovirus expressing HA-myocardin and FLAG-Runx2 were precipitated with anti-HA antibody or control IgG and subjected to PCR analyses using primers specific to the CArG box of the SM-MHC promoter. The results showed that myocardin specifically bound the SRF/CArG box in the SM-MHC promoter in the absence of Runx2, and the chromatin fragments precipitated with anti-HA antibody were obviously decreased in cells expressing both HA-myocardin and FLAG-Runx2 (Fig. 7B).
To test whether Runx2 disrupts the SRF/myocardin complex, we prepared in vitro-translated FLAG-Runx2, HA-SRF, and HA-myocardin proteins. As shown in Fig. 7C, Runx2 dose-dependently competed with myocardin for binding to SRF. Taken together, our data indicate that Runx2 represses myocardin-induced SMC gene expression by disrupting the SRF/myocardin ternary complex.
Runx2 facilitates osteogenic transdifferentiation of HASMCs.
BM treatment increased the expression of Runx2 mRNA and protein in HASMCs (Fig. 8A). We then tested whether forced expression of Runx2 enhances the osteogenic transdifferentiation of HASMCs. BMP2 stimulation as well as Ad-Runx2 infection increased ALP activity, and furthermore, Runx2 drastically enhanced BMP2-induced ALP activity in HASMCs (Fig. 8B). Consistent with this, BMP2-induced ALP activity was decreased in HASMCs transfected with siRunx2 (Fig. 8C). RT-PCR analyses showed that osteogenic marker genes, including ALP, OPN, and osterix (OSX), were upregulated in Runx2-expressing HASMCs. In contrast, the expression levels of SMC genes, including SM22α and SM α-actin, were markedly decreased (Fig. 8D). As shown in Fig. 8E, in reciprocal experiments with siRunx2, the expression levels of SM22α, SM α-actin, and SM-MHC were increased. These observations show that Runx2 plays a critical role in osteogenic transdifferentiation and modulates the SMC phenotype in HASMCs.
FIG. 8.
Runx2 facilitates osteogenic conversion in HASMCs. (A) Runx2 mRNA and protein expression in HASMCs treated with BM were determined by RT-PCR (upper panel) and immunoblotting (lower panel). GAPDH and α-tubulin were used as internal controls for even loading. (B) HASMCs infected with either Ad-Runx2 or Ad-LacZ at a multiplicity of infection of 10 were cultured for 10 days in the absence or presence of BM. After cells were fixed, detection of ALP activity was achieved by incubation with nitroblue tetrazolium-BCIP. Macroscopic (upper panel) and microscopic (lower panel) images were taken after staining. (C) HASMCs were transfected with either siRunx2 or siGFP and were harvested 7 days after vehicle or BM treatment. The ALP activity was measured with a microplate reader using p-nitrophenylphosphate as a substrate and normalized to the protein amount. Error bars indicate standard deviations. (D) HASMCs were infected with either Ad-Runx2 or Ad-LacZ at a multiplicity of infection of 10 and were cultured for 3 days. Expression of SM22α, SM α-actin, ALP, OPN, and OSX transcripts was detected by RT-PCR analyses. Exogenous Runx2 mRNA was detected using mouse Runx2 primers. GAPDH was used as an internal control for RNA loading. (E) HASMCs were transfected with either siRunx2 or siGFP and were harvested at 3 days after transfection. Expression of SMC genes, including SM22α, SM α-actin, and SM-MHC, was evaluated by RT-PCR. The knockdown level of Runx2 expression is represented. GAPDH was used as an internal control for RNA loading. (F) Vascular SMC phenotypes in Runx2 KO and WT embryos. Shown are the dorsal aortas of WT (a and b) and Runx2 null (c and d) embryos at embryonic day 17.5. Histological sections of embryos were stained for SM α-actin (a and c) and with hematoxylin-eosin (b and d). (G) A model for Runx2-mediated SMC dedifferentiation and osteogenic transdifferentiation in HASMCs. Ectopic expression of Runx2, induced by osteogenic stimuli such as BMP2 in atherosclerotic lesions, represses SMC gene expression by disruption of the SRF/myocardin ternary complex, whereas it promotes osteogenic conversion of SMCs via its target gene induction.
Runx2 null mouse embryos do not display overt abnormalities of vascular SMCs.
To investigate the role of Runx2 in the vascular SMC phenotype in vivo, we examined the expression of SM α-actin in dorsal aorta of Runx2 KO embryos. As shown in Fig. 8F, no overt SMC phenotype of aorta was observed in KO embryos compared to WT. This result suggests that Runx2 does not play an indispensable role during vascular development.
DISCUSSION
There has been considerable progress in identifying the molecular process causing the phenotypic modulation of vascular SMCs observed in atherosclerotic lesions. In addition, vascular calcification is recognized, at least in part, as a process similar to bone formation. Runx2 is a transcription factor critical for osteogenesis and bone formation and is expressed during ectopic vascular calcification. However, there is no direct evidence that Runx2 functions in the phenotypic modulation of SMCs. The results of this study demonstrate that Runx2 represses myocardin-induced differentiation and concomitantly promotes the osteogenic conversion of SMCs. The following observations support this conclusion. (i) Runx2 colocalizes with SM α-actin-positive cells in early and noncalcified human atherosclerotic lesions. (ii) Myocardin-induced SMC gene expression is drastically repressed by Runx2, whereas it is enhanced in Runx2 null cells. (iii) Runx2 disrupts the formation of the SRF/myocardin ternary complex. (iv) Forced expression of Runx2 promotes dedifferentiation and osteogenic conversion, and reduction of Runx2 elicits SMC differentiation in HASMCs. Based on these observations, we propose the working model of Runx2-mediated SMC phenotypic modulation shown in Fig. 8G, illustrating that Runx2 plays a critical role in SMC phenotypic modulation.
Runx2 acts as a corepressor of SRF independent of DNA binding.
It is worth stressing that Runx2 acts as a repressor independent of its DNA binding, because DNA binding has been considered to be fundamentally required for Runx2-dependent repression of gene expression and for maintenance of repression (43). There is a report that Runt, the Drosophila homologue of Runx proteins, represses the Drosophila melanogaster segmentation gene engrailed (en) by a mechanism that does not involve DNA binding. The proposed mechanism in Runt-mediated en repression is that Runt tethers to the DNA by interacting with the zinc finger DNA-binding protein Tramtrack (Ttk). In addition, Runx1, which is a mammalian homologue of Runx2 and is essential for hematopoiesis, inhibits the transcriptional elongation by polymerase II (19). Such a mechanism is reminiscent of the transrepression of AP-1-dependent genes by the glucocorticoid receptor (5).
Previous studies demonstrated that HDACs 3, 4, and 6 repress the transcriptional activity of Runx2 via physical interaction (34, 41, 44). In contrast to those studies, we demonstrate that TSA, an inhibitor of HDACs, has no significant effect on the Runx2-mediated repression of myocardin-induced SM22α-luc and SM-MHC-luc activities, suggesting that HDACs do not play a major role in the Runx2 repression of SRF-dependent gene transcription. In addition, by using Runx2 ΔC, which has a C-terminal deletion of the pentapeptide VWRPY, we excluded the possibility that Groucho/TLE are involved in the repression of SRF-dependent gene expression by Runx2. The VWRPY motif is phylogenetically conserved among all Runx family members and is required for the binding of Groucho/TLE proteins to repress gene expression. Although it remains unclear whether Runx2 recruits repressor proteins other than HDACs and TLE, Runx2 clearly represses myocardin-induced activity of SRE-tk-luc, which contains tandem SRF-binding elements and has no binding site for Runx2 (Fig. 2C and D and 3C). In addition, Runx2 ΔRunt, which lacks the SRF-binding domain, does not interact with chromatin DNA and fails to repress SRF-mediated transcription (Fig. 5C, D, and E). We conclude that Runx2 acts as a corepressor in SMC gene expression through a mechanism independent of DNA binding.
Mechanism of Runx2-mediated vascular SMC gene repression.
Phenotypic modulation of the vascular SMCs has been implicated in the development of atherosclerosis. A number of positive and negative regulatory factors of vascular SMC differentiation have been identified (30). SRF plays a key role in the control of SMC phenotype as a consequence of its association with differentiation-promoting cofactors such as myocardin and its responsiveness to growth factors. Negative regulators of myocardin such as KLF4, Foxo4, and HERP1, which are induced in neointima after vascular injury, have been described (7, 25, 26, 33). In the present study, we demonstrate that Runx2 is a novel negative regulator of myocardin. Because the domains necessary for Runx2 and SRF interaction are mapped to the Runt domain of Runx2 and the MADS domain of SRF, each of which has also been known to contribute to DNA binding (Fig. 4E and F), we postulated that Runx2 interferes with the binding of SRF to DNA. Interestingly, unlike HERP1, which dissociates SRF from DNA and represses SRF-dependent transcription (7), Runx2 does not interfere with but rather augments the binding of SRF to naked and chromatin DNA (Fig. 6C and D). We speculate that Runx2 increases nonfunctional binding of SRF to DNA. Since myocardin also interacts with the MADS domain of SRF (42), Runx2 might compete with myocardin for binding to SRF and repress SMC gene expression. In fact, the interaction of SRF and myocardin is disrupted in the presence of Runx2 (Fig. 7A, B, and C), suggesting that Runx2 forms a ternary complex with SRF and thereby inhibits the transcriptional activity of myocardin.
Regulation of vascular SMC phenotype by osteogenic transcription factors.
We demonstrate that overexpression of Runx2 leads to a notable decrease in SM22α and SM α-actin gene expression along with the induction of osteoblast marker gene expression. Conversely, the inhibition of endogenous Runx2 by siRNA promotes SMC differentiation associated with an increase in differentiation markers, including SM-MHC gene expression (Fig. 8D and E). This is the first report to identify Runx2 as a regulator for the phenotypic switching of vascular SMCs from the SMC to the osteogenic phenotype. Moreover, our study supports the hypothesis that expression levels or activity of Runx2 protein is critical for the osteoblastic differentiation of vascular SMCs.
There are reports indicating that osteogenic factors regulate SMC phenotype. Brunelli et al. showed that Msx2 functions as a positive regulator for SMC gene expression during the differentiation of mesoangioblasts (2). Conversely, Hayashi et al. reported that Msx1 and Msx2, which are induced in vascular SMCs by BMPs (BMP2, BMP4, and BMP6), repress myocardin-induced SMC gene expression (16). They demonstrated that Msx1 interacts with either SRF or myocardin and inhibits the binding of the SRF/myocardin complex to the CArG box. We showed that Runx2 disrupts the SRF/myocardin complex but does not interfere with the binding of SRF to the CArG box. Thus, Runx2 and Msx proteins inhibit myocardin-induced SMC differentiation by distinct mechanisms.
In addition, the transcriptional cofactor four and a half LIM domains 2 (FHL2), which has been identified as an osteogenic factor binding to Runx2 (14), inhibits SMC gene expression by competing with the coactivator MAL/MRTF-A, which is a homologue of myocardin, for binding to SRF (31). Analogous to our study, they showed that FHL2 did not inhibit the binding of SRF to the CArG box. Given that FHL2 binds to Runx2, our results support the hypothesis that Runx2 and FHL2 cooperatively modulate SMC phenotype, including dedifferentiation and osteogenic transdifferentiation in atherosclerosis.
Implications for vascular disease.
Previous studies demonstrated that Runx2 expression was increased in calcifying human atherosclerotic plaques (40). In this study, double immunostaining revealed the expression of Runx2 in SM α-actin-positive cells in atherosclerotic lesions which show no apparent calcification. These findings suggest the relevance of Runx2 not only to vascular calcification but also to phenotypic control of SMCs during human atherosclerosis. Although we did not address the mechanism by which Runx2 transcriptional activity is induced, fibroblast growth factor 2 as well as BMP2 has been characterized as a potent growth factor capable of inducing Runx2 expression and its transactivating function (13, 20, 23, 45, 46). Further studies are necessary to determine the signaling pathway which induces Runx2 activity in atherosclerotic lesions.
We found no overt abnormality of the vascular phenotype in Runx2 null mouse embryos (Fig. 8F), suggesting that Runx2 does not profoundly participate in vascular formation during the embryonic period. Runx2 might regulate the phenotype of vascular SMCs in a pathophysiological process. However, because Runx2 KO mice die soon after birth due to respiratory failure (21, 29), vascular SMC-targeted conditional Runx2 KO mice will be required to investigate the role of Runx2 in the development of atherosclerosis. In summary, this study demonstrates a novel mechanism for the repression of SRF function by Runx2 in vascular SMCs, involving direct physical interaction between Runx2 and SRF through the Runt and MADS domains, respectively. Stimulation of osteogenic gene expression by Runx2 is concomitant with the repression of the SMC phenotype in vascular SMCs. These findings bear important implications for the understanding of the plasticity of vascular SMCs during the development of human atherosclerotic plaques. More work is required to clarify the mechanisms by which Runx2 is induced in vascular SMCs of atherosclerotic plaques.
Acknowledgments
We are grateful to Gerard Karsenty and Yoshiaki Ito for critical reagents. We thank Yoshiko Nonaka, Miyuki Nomura, and Yukiyo Tosaka for technical assistance.
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, Sport and Culture of Japan; a grant from the Japan Cardiovascular Foundation (to M.K.) and Kowa Pharmaceutical; and a Japan Heart Foundation/Novartis Grant for Research Award on Molecular and Cellular Cardiology, 2007 (to T.T.).
Footnotes
Published ahead of print on 26 November 2007.
REFERENCES
- 1.Aronson, B. D., A. L. Fisher, K. Blechman, M. Caudy, and J. P. Gergen. 1997. Groucho-dependent and -independent repression activities of Runt domain proteins. Mol. Cell. Biol. 175581-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunelli, S., E. Tagliafico, F. G. De Angelis, R. Tonlorenzi, S. Baesso, S. Ferrari, M. Niinobe, K. Yoshikawa, R. J. Schwartz, I. Bozzoni, S. Ferrari, and G. Cossu. 2004. Msx2 and necdin combined activities are required for smooth muscle differentiation in mesoangioblast stem cells. Circ. Res. 941571-1578. [DOI] [PubMed] [Google Scholar]
- 3.Cao, D., Z. Wang, C. L. Zhang, J. Oh, W. Xing, S. Li, J. A. Richardson, D. Z. Wang, and E. N. Olson. 2005. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol. Cell. Biol. 25364-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, S. L., J. S. Shao, N. Charlton-Kachigian, A. P. Loewy, and D. A. Towler. 2003. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J. Biol. Chem. 27845969-45977. [DOI] [PubMed] [Google Scholar]
- 5.Diamond, M. I., J. N. Miner, S. K. Yoshinaga, and K. R. Yamamoto. 1990. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science 2491266-1272. [DOI] [PubMed] [Google Scholar]
- 6.Doi, H., T. Iso, H. Sato, M. Yamazaki, H. Matsui, T. Tanaka, I. Manabe, M. Arai, R. Nagai, and M. Kurabayashi. 2006. Jagged1-selective Notch signaling induces smooth muscle differentiation via a RBP-Jκ-dependent pathway. J. Biol. Chem. 28128555-28564. [DOI] [PubMed] [Google Scholar]
- 7.Doi, H., T. Iso, M. Yamazaki, H. Akiyama, H. Kanai, H. Sato, K. Kawai-Kowase, T. Tanaka, T. Maeno, E. Okamoto, M. Arai, L. Kedes, and M. Kurabayashi. 2005. HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box. Arterioscler. Thromb. Vasc. Biol. 252328-2334. [DOI] [PubMed] [Google Scholar]
- 8.Ducy, P., R. Zhang, V. Geoffroy, A. L. Ridall, and G. Karsenty. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89747-754. [DOI] [PubMed] [Google Scholar]
- 9.Enomoto, H., T. Furuichi, A. Zanma, K. Yamana, C. Yoshida, S. Sumitani, H. Yamamoto, M. Enomoto-Iwamoto, M. Iwamoto, and T. Komori. 2004. Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J. Cell Sci. 117417-425. [DOI] [PubMed] [Google Scholar]
- 10.Garg, V., A. N. Muth, J. F. Ransom, M. K. Schluterman, R. Barnes, I. N. King, P. D. Grossfeld, and D. Srivastava. 2005. Mutations in NOTCH1 cause aortic valve disease. Nature 437270-274. [DOI] [PubMed] [Google Scholar]
- 11.Gersbach, C. A., B. A. Byers, G. K. Pavlath, and A. J. Garcia. 2004. Runx2/Cbfa1 stimulates transdifferentiation of primary skeletal myoblasts into a mineralizing osteoblastic phenotype. Exp. Cell Res. 300406-417. [DOI] [PubMed] [Google Scholar]
- 12.Giachelli, C. M. 2004. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 152959-2964. [DOI] [PubMed] [Google Scholar]
- 13.Gori, F., T. Thomas, K. C. Hicok, T. C. Spelsberg, and B. L. Riggs. 1999. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J. Bone Miner. Res. 141522-1535. [DOI] [PubMed] [Google Scholar]
- 14.Gunther, T., C. Poli, J. M. Muller, P. Catala-Lehnen, T. Schinke, N. Yin, S. Vomstein, M. Amling, and R. Schule. 2005. Fhl2 deficiency results in osteopenia due to decreased activity of osteoblasts. EMBO J. 243049-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata, K., R. Nishimura, M. Ueda, F. Ikeda, T. Matsubara, F. Ichida, K. Hisada, T. Nokubi, A. Yamaguchi, and T. Yoneda. 2005. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol. Cell. Biol. 251971-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi, K., S. Nakamura, W. Nishida, and K. Sobue. 2006. Bone morphogenetic protein-induced Msx1 and Msx2 inhibit myocardin-dependent smooth muscle gene transcription. Mol. Cell. Biol. 269456-9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong, J. H., E. S. Hwang, M. T. McManus, A. Amsterdam, Y. Tian, R. Kalmukova, E. Mueller, T. Benjamin, B. M. Spiegelman, P. A. Sharp, N. Hopkins, and M. B. Yaffe. 2005. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 3091074-1078. [DOI] [PubMed] [Google Scholar]
- 18.Hruska, K. A., S. Mathew, and G. Saab. 2005. Bone morphogenetic proteins in vascular calcification. Circ. Res. 97105-114. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, H., F. Zhang, T. Kurosu, and B. M. Peterlin. 2005. Runx1 binds positive transcription elongation factor b and represses transcriptional elongation by RNA polymerase II: possible mechanism of CD4 silencing. Mol. Cell. Biol. 2510675-10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, B. G., H. J. Kim, H. J. Park, Y. J. Kim, W. J. Yoon, S. J. Lee, H. M. Ryoo, and J. Y. Cho. 2006. Runx2 phosphorylation induced by fibroblast growth factor-2/protein kinase C pathways. Proteomics 61166-1174. [DOI] [PubMed] [Google Scholar]
- 21.Komori, T., H. Yagi, S. Nomura, A. Yamaguchi, K. Sasaki, K. Deguchi, Y. Shimizu, R. T. Bronson, Y. H. Gao, M. Inada, M. Sato, R. Okamoto, Y. Kitamura, S. Yoshiki, and T. Kishimoto. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89755-764. [DOI] [PubMed] [Google Scholar]
- 22.Lee, B., K. Thirunavukkarasu, L. Zhou, L. Pastore, A. Baldini, J. Hecht, V. Geoffroy, P. Ducy, and G. Karsenty. 1997. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat. Genet. 16307-310. [DOI] [PubMed] [Google Scholar]
- 23.Lee, K. S., H. J. Kim, Q. L. Li, X. Z. Chi, C. Ueta, T. Komori, J. M. Wozney, E. G. Kim, J. Y. Choi, H. M. Ryoo, and S. C. Bae. 2000. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 208783-8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levanon, D., V. Negreanu, Y. Bernstein, I. Bar-Am., L. Avivi, and Y. Groner. 1994. AML1, AML2, and AML3, the human members of the runt domain gene-family: cDNA structure, expression, and chromosomal localization. Genomics 23425-432. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., S. Sinha, O. G. McDonald, Y. Shang, M. H. Hoofnagle, and G. K. Owens. 2005. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 2809719-9727. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Z. P., Z. Wang, H. Yanagisawa, and E. N. Olson. 2005. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev. Cell 9261-270. [DOI] [PubMed] [Google Scholar]
- 27.Miano, J. M. 2003. Serum response factor: toggling between disparate programs of gene expression. J. Mol. Cell Cardiol. 35577-593. [DOI] [PubMed] [Google Scholar]
- 28.Mundlos, S., F. Otto, C. Mundlos, J. B. Mulliken, A. S. Aylsworth, S. Albright, D. Lindhout, W. G. Cole, W. Henn, J. H. Knoll, M. J. Owen, R. Mertelsmann, B. U. Zabel, and B. R. Olsen. 1997. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89773-779. [DOI] [PubMed] [Google Scholar]
- 29.Otto, F., A. P. Thornell, T. Crompton, A. Denzel, K. C. Gilmour, I. R. Rosewell, G. W. Stamp, R. S. Beddington, S. Mundlos, B. R. Olsen, P. B. Selby, and M. J. Owen. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89765-771. [DOI] [PubMed] [Google Scholar]
- 30.Owens, G. K., M. S. Kumar, and B. R. Wamhoff. 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84767-801. [DOI] [PubMed] [Google Scholar]
- 31.Philippar, U., G. Schratt, C. Dieterich, J. M. Muller, P. Galgoczy, F. B. Engel, M. T. Keating, F. Gertler, R. Schule, M. Vingron, and A. Nordheim. 2004. The SRF target gene Fhl2 antagonizes RhoA/MAL-dependent activation of SRF. Mol. Cell 16867-880. [DOI] [PubMed] [Google Scholar]
- 32.Pipes, G. C., E. E. Creemers, and E. N. Olson. 2006. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 201545-1556. [DOI] [PubMed] [Google Scholar]
- 33.Sakata, Y., F. Xiang, Z. Chen, Y. Kiriyama, C. N. Kamei, D. I. Simon, and M. T. Chin. 2004. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler. Thromb. Vasc. Biol. 242069-2074. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder, T. M., R. A. Kahler, X. Li, and J. J. Westendorf. 2004. Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J. Biol. Chem. 27941998-42007. [DOI] [PubMed] [Google Scholar]
- 35.Shao, J. S., J. Cai, and D. A. Towler. 2006. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 261423-1430. [DOI] [PubMed] [Google Scholar]
- 36.Steitz, S. A., M. Y. Speer, G. Curinga, H. Y. Yang, P. Haynes, R. Aebersold, T. Schinke, G. Karsenty, and C. M. Giachelli. 2001. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 891147-1154. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka, T., H. Kanai, K. Sekiguchi, Y. Aihara, T. Yokoyama, M. Arai, T. Kanda, R. Nagai, and M. Kurabayashi. 2000. Induction of VEGF gene transcription by IL-1 beta is mediated through stress-activated MAP kinases and Sp1 sites in cardiac myocytes. J. Mol. Cell Cardiol. 321955-1967. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka, T., D. Nishimura, R. C. Wu, M. Amano, T. Iso, L. Kedes, H. Nishida, K. Kaibuchi, and Y. Hamamori. 2006. Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J. Biol. Chem. 28115320-15329. [DOI] [PubMed] [Google Scholar]
- 39.Thirunavukkarasu, K., D. L. Halladay, R. R. Miles, X. Yang, R. J. Galvin, S. Chandrasekhar, T. J. Martin, and J. E. Onyia. 2000. The osteoblast-specific transcription factor Cbfa1 contributes to the expression of osteoprotegerin, a potent inhibitor of osteoclast differentiation and function. J. Biol. Chem. 27525163-25172. [DOI] [PubMed] [Google Scholar]
- 40.Tyson, K. L., J. L. Reynolds, R. McNair, Q. Zhang, P. L. Weissberg, and C. M. Shanahan. 2003. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler. Thromb. Vasc. Biol. 23489-494. [DOI] [PubMed] [Google Scholar]
- 41.Vega, R. B., K. Matsuda, J. Oh, A. C. Barbosa, X. Yang, E. Meadows, J. McAnally, C. Pomajzl, J. M. Shelton, J. A. Richardson, G. Karsenty, and E. N. Olson. 2004. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119555-566. [DOI] [PubMed] [Google Scholar]
- 42.Wang, D., P. S. Chang, Z. Wang, L. Sutherland, J. A. Richardson, E. Small, P. A. Krieg, and E. N. Olson. 2001. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105851-862. [DOI] [PubMed] [Google Scholar]
- 43.Westendorf, J. J. 2006. Transcriptional co-repressors of Runx2. J. Cell Biochem. 9854-64. [DOI] [PubMed] [Google Scholar]
- 44.Westendorf, J. J., S. K. Zaidi, J. E. Cascino, R. Kahler, A. J. van Wijnen, J. B. Lian, M. Yoshida, G. S. Stein, and X. Li. 2002. Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol. Cell. Biol. 227982-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao, G., D. Jiang, R. Gopalakrishnan, and R. T. Franceschi. 2002. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J. Biol. Chem. 27736181-36187. [DOI] [PubMed] [Google Scholar]
- 46.Zhou, Y. X., X. Xu, L. Chen, C. Li, S. G. Brodie, and C. X. Deng. 2000. A Pro250Arg substitution in mouse Fgfr1 causes increased expression of Cbfa1 and premature fusion of calvarial sutures. Hum. Mol. Genet. 92001-2008. [DOI] [PubMed] [Google Scholar]