Abstract
Fibulin-2 is an extracellular matrix protein belonging to the five-member fibulin family, of which two members have been shown to play essential roles in elastic fiber formation during development. Fibulin-2 interacts with two major constituents of elastic fibers, tropoelastin and fibrillin-1, in vitro and localizes to elastic fibers in many tissues in vivo. The protein is prominently expressed during morphogenesis of the heart and aortic arch vessels and at early stages of cartilage development. To examine its role in vivo, we generated mice that do not express the fibulin-2 gene (Fbln2) through homologous recombination of embryonic stem cells. Unexpectedly, the fibulin-2-null mice were viable and fertile and did not display gross and anatomical abnormalities. Histological and ultrastructural analyses revealed that elastic fibers assembled normally in the absence of fibulin-2. No compensatory up-regulation of mRNAs for other fibulin members was detected in the aorta and skin tissue. However, in the fibulin-2 null aortae, fibulin-1 immunostaining was increased in the inner elastic lamina, where fibulin-2 preferentially localizes. The results demonstrate that fibulin-2 is not required for mouse development and elastic fiber formation and suggest possible functional redundancy between fibulin-1 and fibulin-2.
Fibulins constitute a small family of five extracellular matrix proteins that share a distinctive carboxyl-terminal globular domain and a tandem array of calcium-binding epidermal growth factor-like modules (1, 25). Fibulin-1 and fibulin-2 contain an additional domain of three anaphylatoxin modules preceding the calcium-binding epidermal growth factor-like repeats and therefore are larger than fibulin-3, -4, and -5, which have identical modular structures. Fibulin-2 possesses a large amino-terminal globular domain of approximately 400 amino acids that is not present in the other fibulins and hence is the largest member of the family (17). The protein is approximately 180 kDa in size and forms covalently linked dimers (22). The other four fibulins range between 50 and 100 kDa in size and are present mainly as monomers (8), though dimers of fibulin-5 have recently been described (31).
The fibulins not only share structural similarities but also have overlapping expression patterns (4). A notable common feature is that all fibulins are abundantly distributed in elastic tissues, and all except fibulin-3 have been localized to elastic fibers by immunoelectron microscopy (8, 18, 19, 27). However, the fine localization of the fibulins within the elastic fibers is not identical. While fibulin-2 and -4 are present at the interface between the central elastin core and its surrounding fibrillin microfibrils (8, 18), fibulin-1 is located within the elastin core and fibulin-5 is associated with fibrillin microfibrils (8, 19). Consistent with these observations, in vitro protein binding studies have shown that all fibulins are capable of binding to tropoelastin, albeit with different affinities (8, 20, 27), and that fibulin-2, -4, and -5 interact with the N-terminal region of fibrillin-1 (5, 6, 18).
The biological roles of most fibulins have been elucidated through studies of gene-targeted mouse models. Fibulin-1 null mice die perinatally, as a result of massive bleeding associated with abnormal endothelial lining of small blood vessels and severe defects in the basement membranes of many organs, including the kidneys and lungs (10). There is no apparent abnormality in elastic fiber formation. Mice deficient in fibulin-3 show early aging and develop multiple large hernias in a genetic background-dependent manner (12). A reduction in elastic fibers specifically in fascia connective tissues may explain the herniation phenotype. Mice lacking either fibulin-4 or fibulin-5 have highly disrupted and disorganized elastic fibers, leading to developmental defects in skin, arterial blood vessels, and lungs (13, 14, 27). Although the elastic fiber abnormalities are similar in these two mouse mutants, the fibulin-4 null mice are perinatally lethal, whereas the fibulin-5-deficient mice can survive until adulthood. The animal models demonstrate that fibulin-4 and fibulin-5 play essential yet nonredundant roles in elastic fiber formation during development.
The in vivo function of fibulin-2 remains poorly understood. Here we report the generation and characterization of mice deficient in fibulin-2. We show that the fibulin-2 null mice develop normally and are phenotypically indistinguishable from their wild-type littermates. Our study indicates that fibulin-2 is dispensable for mouse development and elastic fiber formation, possibly due to functional redundancy with fibulin-1.
MATERIALS AND METHODS
Construction of the targeting vector.
A cosmid clone, T3b, containing the 5′ portion of the mouse fibulin-2 gene was isolated from a 129/Sv genomic library by screening with a mouse fibulin-2 cDNA clone as described previously (7). A gene targeting vector was prepared using a 9.0-kb BamHI fragment isolated from the cosmid, which contains the first coding exon (exon 2 of 1,288 bp) and its flaking introns. A neomycin resistance gene driven by the phosphoglycerate kinase promoter (PGK-Neo) was inserted by blunt-end ligation into the SpeI site in exon 2 (see Fig. 1A). The PGK-Neo gene was in the same transcription orientation as the fibulin-2 gene.
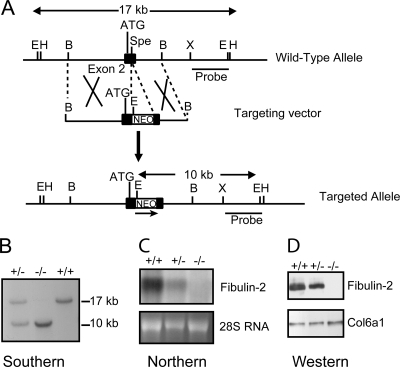
FIG. 1.
Generation of the fibulin-2 null mice. (A) Schematic diagram of the mouse fibulin-2 gene, the targeting vector, and the mutant allele after homologous recombination. The targeting vector contains a PGK-Neo gene (NEO) flanked by LoxP sequences inserted into the SpeI site of exon 2 (black box) by blunt-end ligation, resulting in the deletion of the SpeI site and addition of an EcoRI site located in the PGK-Neo fragment. Restriction sites shown are BamHI (B), EcoRI (E), XhoI (X), HindIII (H), and SpeI (Spe). The translation start site (ATG) and the probe used for Southern blotting are indicated. (B) Southern blot analysis of mouse tail DNA digested with EcoRI. (C) Northern blot analysis of total RNA isolated from embryonic fibroblasts of the Fbln2+/+, Fbln2+/−, and Fbln2−/− mice using full-length mouse fibulin-2 cDNA as a probe (top panel). Ethidium bromide staining of 28S RNA is shown in the bottom panel. (D) Western blot analysis of culture medium from mouse fibroblasts of the three genotypes, using antibodies specific for fibulin-2 and the α1(VI) collagen (Col6a1).
Generation of fibulin-2-deficient mice.
The targeting vector, containing 5.0-kb and 2.5-kb genomic sequences flanking the PGK-Neo cassette, was linearized with NotI and electroporated into 5 × 107 mouse 129/Sv embryonic stem (ES) cells (W9.5) under standard conditions (9). ES clones were selected with 250 μg/ml G418 and analyzed by Southern blotting. Correctly targeted ES clones were injected into blastocysts isolated from C57BL/6 mice and implanted into pseudopregnant B6CBA F1 females to obtain chimeric mice. The chimeric founders were mated with C57BL/6 mice to generate heterozygous Fbln2+/− mice, which were intercrossed to produce homozygous Fbln2−/− mice. Initial phenotype analyses were performed with littermates on a mixed 129/Sv and C57BL/6 background. The Fbln2 null allele was introduced into two different genetic backgrounds, 129S1/SvImJ and C57BL/6J, by backcrossing for 10 generations. All animal procedures described in this study were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and all experimental protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Genotyping of ES cells and mice.
Genomic DNA was isolated from ES cells and mouse tails, digested with EcoRI, separated on 0.7% agarose gels, transferred to Hybond nylon membranes (GE Healthcare, Piscataway, NJ), and hybridized with a 3.5-kb EcoRI-XhoI external probe (see Fig. 1A) labeled with [32P]dCTP using a random prime labeling system (Rediprime II; GE Healthcare). Genotyping of mice was also carried out by PCR amplification of the tail DNA. A 480-bp PCR product from the wild-type allele was detected using primers 5′-CTACGGCCATTGTGAACGAG-3′ and 5′-GTGATCGCTGGGCTTTACTG-3′, located in exon 2. The targeted allele yielded a 331-bp product with primers 5′-GCCAAAGCCAGGAGAGTGAC-3′ and 5′-ACCGGTGGATGTGGAATGTG-3′, located in exon 2 and the PGK-Neo gene, respectively.
Cell cultures.
Embryonic fibroblasts were prepared from carcasses of embryos at 16 days postcoitum using littermates from intercrossing heterozygous animals. Cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). For immunofluorescence analysis, cells were grown in eight-chamber slides (Nalge Nunc, Rochester, NY) for 7 days postconfluency.
Northern blot analysis.
Total RNA from mouse embryonic fibroblasts and adult mouse tissues were isolated using the Totally RNA kit (Ambion, Austin, TX). Ten micrograms of the RNA samples were electrophoresed on a 1% agarose gel containing 6% formaldehyde, transferred to nylon membranes (Stratagene, La Jolla, CA), and hybridized to cDNA probes radiolabeled with [32P]dCTP by the Rediprime II labeling system (GE Healthcare). The cDNA fragments used for probing the Northern blots were amplified from mouse embryonic fibroblast RNA by reverse transcription-PCR and then cloned into the pCRII plasmid (Invitrogen). Hybridization signals were detected by a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Western blot analysis.
Confluent fibroblasts were grown in Dulbecco's modified Eagle's medium (Invitrogen) in the absence of serum for 24 h. One hundred microliters serum-free medium was precipitated with 900 μl of 100% ethyl alcohol, the protein pellet resuspended in Laemmli sample buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 25% glycerol, and 0.01% bromophenol blue, electrophoresed through 4 to 12% sodium dodecyl sulfate-polyacrylamide gradient gels, and transferred to polyvinylidene difluoride membranes (GE Healthcare). The membranes were incubated with polyclonal antibodies against fibulin-2 (17) and the α1(VI) collagen chain (24). Positive signals were detected by the ECL Plus chemiluminescent reagent (GE Healthcare).
Histology and immunohistochemistry.
For histological analyses, mouse tissues were fixed with 10% buffered formalin for 1 to 3 days, dehydrated through a graded ethanol series, and embedded in paraffin. Sections 5 μm thick were stained with hematoxylin-eosin, Masson's trichrome collagen stain, or Verhoeff's van Gieson elastin stain. Immunohistochemistry was performed using 6- to 8-μm-thick cryosections of mouse tissues or fibroblasts grown on chamber slides by the method described elsewhere (26). Primary antibodies included those specific for each of the five fibulins (1:1,000 dilution) (8, 17), fibrillin-1 (a generous gift of Lynn Sakai), elastin (PR387; Elastin Products Company, Owensville, MO), and fibronectin (Sigma, St. Louis, MO). Cy3-conjugated anti-rabbit immunoglobulin G (1: 800 dilution; Jackson ImmunoResearch Laboratory, West Grove, PA) was used as the secondary antibody. Nuclei were counterstained with 4′, 6′-diamidino-2-phenylindole hydrochloride. Images were captured using a Zeiss Axioskop epifluorescence microscope with a Toshiba 3CCD camera and ImagePro software (Media Cybernetics, Silver Spring, MD).
Elastin content measurement.
Descending aortae from adult mice were dissected, and the desmosine/isodesmosine contents were determined using a Beckman 6300 amino acid analyzer as previously described (3).
Electron microscopy.
Descending aortae and back skin were dissected from adult wild-type and fibulin-2 null mice, and samples for transmission electron microscopy were prepared as described previously (2). Briefly, tissues were fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M sodium cacodylate (pH 7.4) with 8.0 mM CaCl2 and then post-fixed with 1% osmium tetroxide (containing 2% tannic acid for aorta samples only). After dehydration in an ethanol series, followed by propylene oxide, the samples were infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA). Thin sections were cut using a Reichert UCT ultramicrotome and post-stained with aqueous uranyl acetate followed by phosphotungstic acid or lead citrate. Sections were examined at 80 kV using a Tecnai12 transmission electron microscope equipped with a Gatan Ultrascan US1000 2 K digital camera.
RESULTS
Generation of fibulin-2-deficient mice.
The gene targeting vector was designed to disrupt the fibulin-2 gene by inserting the PGK-Neo cassette after codon 282. Approximately 400 G418-resistant ES clones were screened by Southern blotting with the 3′ external probe, which detected a 17-kb EcoRI band from the wild-type allele and a 10-kb EcoRI band from the targeted allele (Fig. 1A and B). The screening revealed that approximately 10% of the clones were correctly targeted. Four of the targeted ES clones were microinjected into the C57BL/6 blastocytes, and Fbln2+/− mice were obtained from chimeric founders from each ES cell clone. Intercrossing of the heterozygous (Fbln2+/−) F1 mice from each ES cell clone yielded offspring of the three genotypes (Fbln2+/+, Fbln2+/−, and Fbln2−/−) at the expected Mendelian frequencies. Embryonic fibroblasts originating from the Fbln2+/+, Fbln2+/−, and Fbln2−/− littermates were analyzed by Northern and Western blotting. The results confirmed the absence of fibulin-2 mRNA and protein in the Fbln2 null animals and indicated that the heterozygous animals expressed approximately half the amounts of mRNA and protein expressed in the wild-type mice (Fig. 1C and D).
Fibulin-2-deficient mice are fertile and have no gross abnormalities.
The Fbln2−/− mice on either a 129S1/SvImJ or a C57BL/6J background did not display any apparent, abnormal phenotype. No statistically significant differences in body weights were observed between the Fbln2+/+, Fbln2+/−, and Fbln2−/− mice, indicating normal growth. Since fibulin-2 expression is specifically associated with cardiovascular morphogenesis (26, 28), anatomical analyses were performed on mice at days 11 and 13 of embryonic development and adult stages (6 to 8 weeks). There were no noticeable differences in the morphology of cardiac valves and major blood vessels between the Fbln2−/− and Fbln2+/+ animals. Inbreeding of Fbln2−/− mice resulted in litters with numbers of pups per litter and pups of normal weight and appearance equal to those of Fbln2+/+ litters. The number of litters produced by the male and female Fbln2−/− animals was indistinguishable from that of their wild-type counterparts. The Fbln2−/− mice had a normal life span, surviving beyond 2 years of age.
Loss of fibulin-2 does not affect elastogenesis.
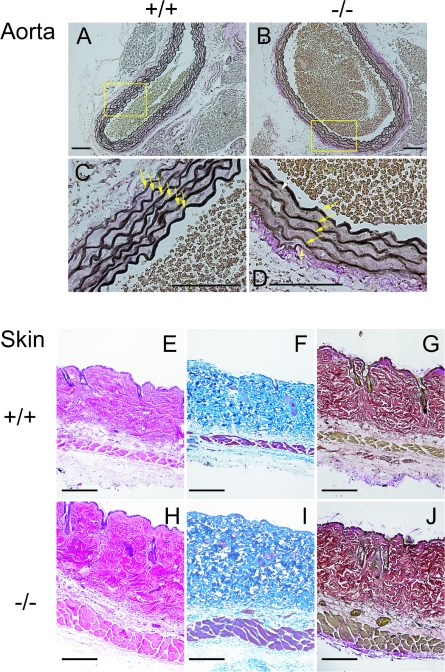
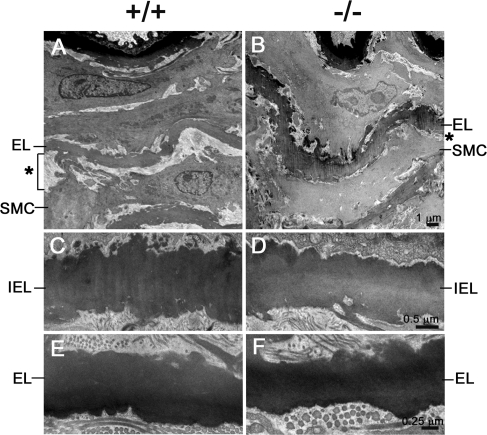
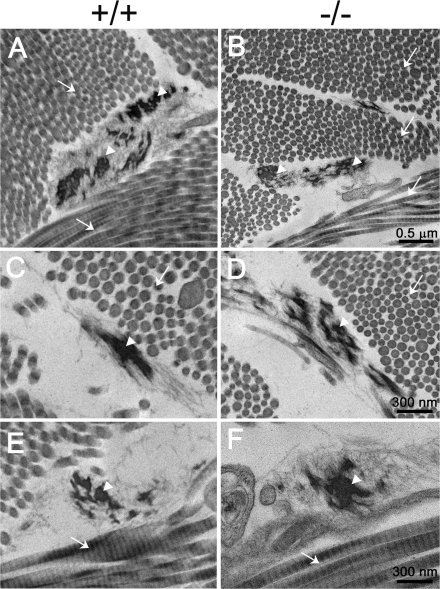
Histological and ultrastructural analyses were carried out with two elastic fiber-enriched organs, aorta and skin, from the adult mice. As shown in Fig. 2, the elastic laminae of the ascending aorta formed normally in the Fbln2−/− mice. Analysis of C57BL/6 Fbln2−/− congenic mice revealed that the number of aortic laminar units in the Fbln2−/− mice was not significantly different from that in the Fbln2+/+ mice. The amounts of cross-linked elastin (pmole desmosine/mg protein), determined by the desmosine contents of the aortae, were comparable between the Fbln2−/− and Fbln2+/+ mice (for Fbln2−/− mice, 47.6 ± 14.4 [n = 5]; for Fbln2+/+ mice, 41.7 ± 10.9 [n = 6]). Ultrastructural examination of the Fbln2−/− aortae by transmission electron microscopy confirmed that the elastic laminae were not disrupted (Fig. 3). The connective tissue layer, consisting mainly of collagen fibrils, between smooth muscle cells and the elastic laminae appeared to be decreased in the Fbln2−/− mice compared to that in the Fbln2+/+ animals. Skin from the Fbln2−/− mice appeared normal by histological staining with hematoxylin/eosin, Masson's trichrome collagen stain, and Verhoeff's Van Gieson elastin stain (Fig. 2). Ultrastructural analyses of the skin did not reveal apparent differences in either collagen fibrils or elastic fibers between Fbln2−/− and Fbln2+/+ animals (Fig. 4).
FIG. 2.
Histological analysis of ascending aortae and skin from Fbln2+/+ and Fbln2−/− mice. (A to D) Paraffin-embedded aortic sections were stained with Verhoeff's solution, in which elastin appears dark brown or black. Images were taken at two different magnifications. Arrows indicate elastin laminae. Magnification bar = 100 μm. (E to J) Skin sections were subjected to hematoxylin-eosin (E and H), Masson's trichrome collagen (F and I), and Verhoeff's van Gieson elastin (G and J) stains. Bars = 100 μm.
FIG. 3.
Transmission electron micrographs of descending aortae from Fbln2+/+ and Fbln2−/− mice. (A and B) Low-magnification micrographs with the luminal side of the aorta on top. Note that the elastic laminae (EL) in the fibulin-2-deficient mice form normally, but the space (*) between the smooth muscle cell (SMC) and elastic lamina appears to be narrower than that for the wild-type mice. (C to F) High-magnification micrographs showing the internal elastic lamina (IEL, panels C and D) and the elastic lamina (EL) in the medial layer (E and F) of the aorta are comparable between the fibulin-2 null and wild-type mice.
FIG. 4.
Transmission electron micrographs of the dermis from Fbln2+/+ and Fbln2−/− mice. (A and B) Low-magnification micrographs show that the elastic fibers (arrowheads) and collagen fibrils (arrows) are comparable in the fibulin-2-deficient and wild-type mice. (C to F) High-magnification micrographs showing elastic fibers and collagen fibrils in cross sections (C and D) and longitudinal sections (E and F).
Altered distribution of fibulin-1 in the fibulin-2-deficient aorta.
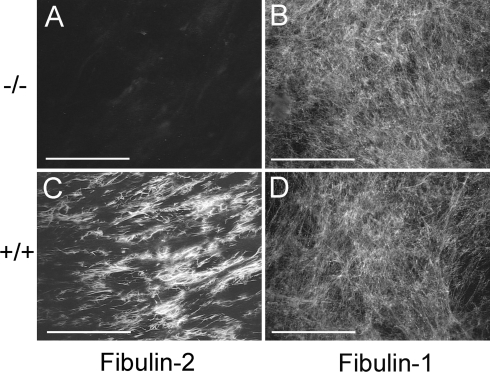
Cryosections of the skin and descending aortae were immunostained with antibodies specific for elastin, fibrillin-1, and each of the five fibulins. There was no significant difference in the expression of these proteins in the skin of the Fbln2−/− mice and Fbln2+/+ mice. However, immunostaining of aorta sections showed that fibulin-1 expression was significantly increased in the internal elastic lamina of the Fbln2−/− null mice compared to levels for wild-type mice (Fig. 5). In the wild-type aorta, fibulin-2 expression was most prominent in the inner elastic lamina along the endothelial basement membrane and fibulin-1 was evenly distributed in all elastic laminae. The distribution of fibulin-3, -4, and -5 protein in the Fbln2−/− aortae was unchanged (data not shown). To determine whether fibulin-2-deficient cells deposited more fibulin-1 protein, Fbln2−/− and Fbln2+/+ embryonic fibroblasts were cultured for 7 days postconfluency and immunostained with antibodies against fibulin-1 and fibulin-2. The fibulin-1 fibrils deposited by fibroblasts from these two genotypes were comparable (Fig. 6).
FIG. 5.
Immunofluorescence staining of ascending aortae from Fbln2+/+ and Fbln2−/− mice. Frozen sections were stained with polyclonal antibodies against fibulin-2 (A and C) or fibulin-1 (B and D). Arrows indicate the internal elastic laminae. Bars = 100 μm.
FIG. 6.
Immunofluorescence staining of embryonic fibroblasts from Fbln2+/+ and Fbln2−/− mice with antibodies against mouse fibulin-2 (A and C) and fibulin-1 (B and D). Bars = 100 μm.
No compensatory up-regulation of mRNA expression for other fibulin members.
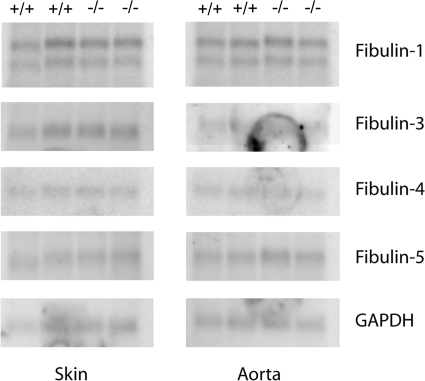
To determine whether loss of fibulin-2 results in a change in gene expression of the other fibulins, total RNA extracted from the aorta and skin of the newborn mice was evaluated by Northern blot analyses. The amounts of fibulin-1, -3, -4, and -5 mRNA were not significantly different in the Fbln2−/− and Fbln2+/+ animals (Fig. 7).
FIG. 7.
Northern blot analysis of total RNA isolated from skin and aorta of Fbln2+/+ and Fbln2−/− mice. Ten micrograms of RNA in each lane was hybridized with [32P]dCTP-labeled cDNA probes for fibulins and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each lane contains total RNA extracted from a separate animal. There is no significant difference between the two genotypes.
Matrix assembly by fibulin-2 null fibroblasts is not compromised.
Previous studies have shown that fibulin-2 can be incorporated into both elastic fibers and the fibronectin matrix (18, 23). To determine whether the absence of fibulin-2 alters the assembly of fibronectin or elastic fibers, embryonic fibroblasts from the Fbln2−/− and Fbln2+/+ animals grown for 7 days after confluence were immunostained with antibodies against elastin, fibrillin-1, and fibronectin. There were no significant differences in the intensity or pattern of immunostaining of the extracellular matrix produced by fibroblasts from the two genotypes (Fig. 8).
FIG. 8.
Immunofluorescence staining of extracellular matrix deposited by embryonic fibroblasts from Fbln2+/+ or Fbln2−/− mice. Primary antibodies used were against elastin (A and D), fibrillin-1 (B and E), or fibronectin (C and F). There is no noticeable difference between the two genotypes. Bars = 100 μm.
DISCUSSION
We have generated a mouse deficient in fibulin-2 in order to understand its biological role in vivo. Our previous studies demonstrate that fibulin-2 expression is prominent and highly specific at embryonic sites of epithelial-mesenchymal transformation (26, 28, 29). Expansion of the fibulin-2 extracellular network is associated with mesenchymal cells that have migrated into the endocardial cushion tissue, aortic arch vessels, and coronary vessels during cardiovascular development (26). High levels of fibulin-2 protein expression are also found in developing hair follicles and precartilage condensation sites (29). These observations suggest that fibulin-2 may promote proliferation, differentiation, and migration of mesenchymal cells during organogenesis. It is therefore surprising that a total absence of fibulin-2 does not have discernible effects on embryonic and postnatal development of the mice. In particular, the Fbln2−/− mice do not show developmental delay or abnormalities in the cardiovascular system.
Several lines of evidence suggest that fibulin-2 may have a role in male and female reproduction. A correlation has been found between postnatal development of testis and fibulin-2 expression in the basement membrane of seminiferous tubule in rats (11). Human ovary tissue has been shown to express high levels of fibulin-2 mRNA (30). Moreover, a recent study suggests that fibulin-2 and fibulin-1 may be involved in sequestering sex hormone-binding globulin within the uterine stroma and epididymis, thereby controlling sex-steroid access to target cells (15). However, the results presented here show that both male and female Fbln2−/− mice are fertile, indicating that fibulin-2 is not required for normal reproductive function.
Fibulin-2, like fibulin-4 and -5, binds to tropoelastin and fibrillin-1 in vitro and localizes to elastic fibers in vivo (18, 20). However, unlike the case with fibulin-4- or fibulin-5-deficient mice, histological and ultrastructural analyses of the fibulin-2-deficient mice demonstrate that a lack of fibulin-2 does not affect elastic fiber formation in vivo. Consistent with this finding, embryonic fibroblasts deficient in fibulin-2 are capable of depositing the fibrillar matrix of elastin, fibrillin-1, and fibronectin. Since members of the fibulin family display overlapping developmental expression, tissue distribution, and molecular interactions (4, 25), a loss of fibulin-2 likely can be compensated by other family members. In particular, the tissue distribution of fibulin-2 is substantially more restricted than that of the other four fibulins. For instance, in the lung, fibulin-2 is present only in the blood vessels, whereas the other four fibulins are also found, to various extents, in the airways and parenchyma (8). Moreover, the content of fibulin-2 in protein extracts from most organs, as determined by radioimmunoinhibition assays, is considerably less than that of fibulin-1 and -5 and is similar to that of fibulin-3 and -4 (8).
Northern blot analyses show that the mRNA levels of the other four fibulins are not changed for the fibulin-2 null mice from that for controls. On the other hand, immunostaining studies of the fibulin-2 null mice show increased fibulin-1 protein expression in the inner elastic lamina of the aorta, where fibulin-2 is normally localized. This indicates that a loss of fibulin-2 leads to an alteration in the localization of the fibulin-1 protein within the aorta rather than a change in its gene expression. It is possible that in the inner elastic lamina of the fibulin-2 null mice, the fibulin-1 protein is bound to molecules that normally interact with fibulin-2 and is thereby less prone to removal or degradation. Fibulin-2, like fibulin-1 but unlike fibulin-3, -4, and -5, binds fibronectin and several basement membrane and cartilage proteins, including laminin α2 and γ2 chains, nidogen, collagen XVIII, vesican, and aggrecan (8, 16, 21). It is thus conceivable that a loss of fibulin-2 could readily be compensated for by fibulin-1 but not by fibulin-3, -4, and -5. On the other hand, compensation by other fibulin family members, though not detected in this study, cannot be excluded.
Our previous finding that the fibulin-1 null mice display a perinatally lethal phenotype (10) suggests that fibulin-2 is unable to functionally compensate for the loss of fibulin-1. The lack of compensation for fibulin-1 could be explained by the following points. Fibulin-1 is an integral component of all basement membranes, and consequently its expression initiates very early during embryonic development (26). The lethal phenotype of the fibulin-1 null mice results largely from bleeding due to a defective endothelial basement membrane of small but not large blood vessels (10). On the other hand, fibulin-2 has a more restricted expression pattern and is not present in the small blood vessels (26). Its expression during embryogenesis initiates substantially later than that of fibulin-1 (26). Moreover, fibulin-2 is significantly less abundant than fibulin-1, present at a level of only 10 to 30% of that of fibulin-1 in various organs (8). Though fibulin-1 and fibulin-2 share similar modular structures and binding interactions, the temporal, spatial, and quantitative expression differences prevent fibulin-2 from serving the full functions of fibulin-1.
In conclusion, our studies demonstrate that fibulin-2 is not essential for development, fertility, and elastogenesis. This could be attributed to functional compensation by other members of the fibulin protein family. Testing this hypothesis will depend on the characterization of mice deficient in two or more fibulins.
Acknowledgments
We thank Takako Sasaki and Rupert Timpl for providing antibodies against fibulins and Lynn Sakai for the gift of the anti-fibrillin-1 antibody.
This work was supported in part by National Institutes of Health grant GM55625 (to M.-L.C.).
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Argraves, W. S., L. M. Greene, M. A. Cooley, and W. M. Gallagher. 2003. Fibulins: physiological and disease perspectives. EMBO Rep. 41127-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birk, D. E., and R. L. Trelstad. 1986. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 103231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown-Augsburger, P., C. Tisdale, T. Broekelmann, C. Sloan, and R. P. Mecham. 1995. Identification of an elastin cross-linking domain that joins three peptide chains. Possible role in nucleated assembly. J. Biol. Chem. 27017778-17783. [DOI] [PubMed] [Google Scholar]
- 4.Chu, M. L., and T. Tsuda. 2004. Fibulins in development and heritable disease. Birth Defects Res. C Embryo Today 7225-36. [DOI] [PubMed] [Google Scholar]
- 5.El-Hallous, E., T. Sasaki, D. Hubmacher, M. Getie, K. Tiedemann, J. Brinckmann, B. Batge, E. C. Davis, and D. P. Reinhardt. 2007. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adapter function to tropoelastin. J. Biol. Chem. 2828935-8946. [DOI] [PubMed] [Google Scholar]
- 6.Freeman, L. J., A. Lomas, N. Hodson, M. J. Sherratt, K. T. Mellody, A. S. Weiss, A. Shuttleworth, and C. M. Kielty. 2005. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem. J. 3881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassel, S., F. X. Sicot, S. Gotta, and M. L. Chu. 1999. Mouse fibulin-2 gene. Complete exon-intron organization and promoter characterization. Eur. J. Biochem. 263471-477. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi, N., G. Kostka, J. H. Garbe, D. R. Keene, H. P. Bachinger, F. G. Hanisch, D. Markova, T. Tsuda, R. Timpl, M. L. Chu, and T. Sasaki. 2007. A comparative analysis of the fibulin protein family: biochemical characterization, binding interactions and tissue localization. J. Biol. Chem. 28211805-11816. [DOI] [PubMed] [Google Scholar]
- 9.Kontgen, F., and C. L. Stewart. 1993. Simple screening procedure to detect gene targeting events in embryonic stem cells. Methods Enzymol. 225878-890. [DOI] [PubMed] [Google Scholar]
- 10.Kostka, G., R. Giltay, W. Bloch, K. Addicks, R. Timpl, R. Fassler, and M. L. Chu. 2001. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol. Cell. Biol. 217025-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loveland, K., S. Schlatt, T. Sasaki, M. L. Chu, R. Timpl, and M. Dziadek. 1998. Developmental changes in the basement membrane of the normal and hypothyroid postnatal rat testis: segmental localization of fibulin-2 and fibronectin. Biol. Reprod. 581123-1130. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin, P. J., B. Bakall, J. Choi, Z. Liu, T. Sasaki, E. C. Davis, A. D. Marmorstein, and L. Y. Marmorstein. 13 September 2007. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum. Mol. Genet. 163059-3070. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin, P. J., Q. Chen, M. Horiguchi, B. C. Starcher, J. B. Stanton, T. J. Broekelmann, A. D. Marmorstein, B. McKay, R. Mecham, T. Nakamura, and L. Y. Marmorstein. 2006. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol. Cell. Biol. 261700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura, T., P. R. Lozano, Y. Ikeda, Y. Iwanaga, A. Hinek, S. Minamisawa, C. F. Cheng, K. Kobuke, N. Dalton, Y. Takada, K. Tashiro, J. J. Ross, T. Honjo, and K. R. Chien. 2002. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415171-175. [DOI] [PubMed] [Google Scholar]
- 15.Ng, K. M., M. G. Catalano, T. Pinos, D. M. Selva, G. V. Avvakumov, F. Munell, and G. L. Hammond. 2006. Evidence that fibulin family members contribute to the steroid-dependent extravascular sequestration of sex hormone-binding globulin. J. Biol. Chem. 28115853-15861. [DOI] [PubMed] [Google Scholar]
- 16.Olin, A. I., M. Morgelin, T. Sasaki, R. Timpl, D. Heinegard, and A. Aspberg. 2001. The proteoglycans aggrecan and Versican form networks with fibulin-2 through their lectin domain binding. J. Biol. Chem. 2761253-1261. [DOI] [PubMed] [Google Scholar]
- 17.Pan, T. C., T. Sasaki, R. Z. Zhang, R. Fassler, R. Timpl, and M. L. Chu. 1993. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J. Cell Biol. 1231269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinhardt, D. P., T. Sasaki, B. J. Dzamba, D. R. Keene, M. L. Chu, W. Gohring, R. Timpl, and L. Y. Sakai. 1996. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J. Biol. Chem. 27119489-19496. [DOI] [PubMed] [Google Scholar]
- 19.Roark, E. F., D. R. Keene, C. C. Haudenschild, S. Godyna, C. D. Little, and W. S. Argraves. 1995. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J. Histochem. Cytochem. 43401-411. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki, T., W. Gohring, N. Miosge, W. R. Abrams, J. Rosenbloom, and R. Timpl. 1999. Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS Lett. 460280-284. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki, T., W. Gohring, T. C. Pan, M. L. Chu, and R. Timpl. 1995. Binding of mouse and human fibulin-2 to extracellular matrix ligands. J. Mol. Biol. 254892-899. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki, T., K. Mann, H. Wiedemann, W. Gohring, A. Lustig, J. Engel, M. L. Chu, and R. Timpl. 1997. Dimer model for the microfibrillar protein fibulin-2 and identification of the connecting disulfide bridge. EMBO J. 163035-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki, T., H. Wiedemann, M. Matzner, M. L. Chu, and R. Timpl. 1996. Expression of fibulin-2 by fibroblasts and deposition with fibronectin into a fibrillar matrix. J. Cell Sci. 1092895-2904. [DOI] [PubMed] [Google Scholar]
- 24.Tillet, E., H. Wiedemann, R. Golbik, T. C. Pan, R. Z. Zhang, K. Mann, M. L. Chu, and R. Timpl. 1994. Recombinant expression and structural and binding properties of alpha 1(VI) and alpha 2(VI) chains of human collagen type VI. Eur. J. Biochem. 221177-185. [DOI] [PubMed] [Google Scholar]
- 25.Timpl, R., T. Sasaki, G. Kostka, and M. L. Chu. 2003. Fibulins: a versatile family of extracellular matrix proteins. Nat. Rev. Mol. Cell Biol. 4479-489. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda, T., H. Wang, R. Timpl, and M. L. Chu. 2001. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev. Dyn. 22289-100. [DOI] [PubMed] [Google Scholar]
- 27.Yanagisawa, H., E. C. Davis, B. C. Starcher, T. Ouchi, M. Yanagisawa, J. A. Richardson, and E. N. Olson. 2002. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415168-171. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, H. Y., M. L. Chu, T. C. Pan, T. Sasaki, R. Timpl, and P. Ekblom. 1995. Extracellular matrix protein fibulin-2 is expressed in the embryonic endocardial cushion tissue and is a prominent component of valves in adult heart. Dev. Biol. 16718-26. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, H. Y., R. Timpl, T. Sasaki, M. L. Chu, and P. Ekblom. 1996. Fibulin-1 and fibulin-2 expression during organogenesis in the developing mouse embryo. Dev. Dyn. 205348-364. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, R. Z., T. C. Pan, Z. Y. Zhang, M. G. Mattei, R. Timpl, and M. L. Chu. 1994. Fibulin-2 (FBLN2): human cDNA sequence, mRNA expression, and mapping of the gene on human and mouse chromosomes. Genomics 22425-430. [DOI] [PubMed] [Google Scholar]
- 31.Zheng, Q., E. C. Davis, J. A. Richardson, B. C. Starcher, T. Li, R. D. Gerard, and H. Yanagisawa. 2007. Molecular analysis of fibulin-5 function during de novo synthesis of elastic fibers. Mol. Cell. Biol. 271083-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]