Abstract
Primary ciliary dyskinesia (PCD) results from ciliary dysfunction and is commonly characterized by sinusitis, male infertility, hydrocephalus, and situs inversus. Mice homozygous for the nm1054 mutation develop phenotypes associated with PCD. On certain genetic backgrounds, homozygous mutants die perinatally from severe hydrocephalus, while mice on other backgrounds have an accumulation of mucus in the sinus cavity and male infertility. Mutant sperm lack mature flagella, while respiratory epithelial cilia are present but beat at a slower frequency than wild-type cilia. Transgenic rescue demonstrates that the PCD in nm1054 mutants results from the loss of a single gene encoding the novel primary ciliary dyskinesia protein 1 (Pcdp1). The Pcdp1 gene is expressed in spermatogenic cells and motile ciliated epithelial cells. Immunohistochemistry shows that Pcdp1 protein localizes to sperm flagella and the cilia of respiratory epithelial cells and brain ependymal cells in both mice and humans. This study demonstrates that Pcdp1 plays an important role in ciliary and flagellar biogenesis and motility, making the nm1054 mutant a useful model for studying the molecular genetics and pathogenesis of PCD.
Primary ciliary dyskinesia (PCD), which was previously known as immotile cilia syndrome, affects approximately 1 in 16,000 newborn children worldwide and results from a defect in ciliary and flagellar motility (1, 2, 6, 13, 17, 66). Affected individuals often suffer from bronchiectasis, chronic sinusitis, and neonatal respiratory distress. In addition, males are infertile, and many individuals have situs inversus, a complete reversal of left-right asymmetry. The triad of sinusitis, bronchiectasis, and situs inversus is commonly known as Kartagener's syndrome. Some individuals with PCD also develop hydrocephalus (3, 15, 18, 28, 32, 70), otitis media (39, 40, 47), and retinitis pigmentosa (37, 65, 76).
Motile cilia are located on the surface of many types of eukaryotic cells and have a variety of functions (18, 22, 36, 46, 60, 63). For example, cilia on respiratory epithelial cells are responsible for movement of fluid and particles over the cell surface and are a critical component of host defense. Cilia on ependymal cells lining the ventricular surface of the brain facilitate cerebrospinal fluid flow, while those on the embryonic node play a critical role in left-right patterning during early development. The structurally related flagella are required for sperm motility.
Motile cilia elongate from the basal bodies of epithelial, ependymal, or nodal cells (1, 13, 17, 20, 22, 27, 36, 46, 60, 54). The core, or axoneme, of the cilia and flagella consists of a “9 + 2” microtubule structure with a ring of nine microtubule doublets surrounding a central pair of single microtubules. Several accessory proteins are associated with the microtubule pairs, including radial spokes and dynein arms, which generate the motor force required for ciliary motility. Although motile, nodal cilia have a 9 + 0 arrangement that lacks the central microtubule pair and resemble immotile cilia.
Human PCD linkage studies have demonstrated extensive genetic heterogeneity (7, 72). A number of genes have recently been implicated in human and mouse PCD. Predictably, human PCD mutations have been identified in three dynein chain genes: DNAI1 (19, 48, 71), DNAH11 (4), and DNAH5 (24, 44, 45). Similarly, loss of the mouse homolog of DNAH5 (Mdnah5) results in immotile cilia, chronic respiratory infections, hydrocephalus, and situs inversus (26), while loss of the dynein heavy chain gene Mdhc7 results in immotile cilia and male infertility (41). Additionally, mice lacking the gene encoding the axonemal filament protein Tektin-t also have immotile cilia due to impaired dynein arm function (64). PCD was also observed in mice lacking the genes encoding the transcription factor hepatocyte nuclear factor/forkhead homolog 4 (Hfh-4) (8, 10), DNA polymerase λ (31), and the novel sperm-associated antigen 6 (Spag6) (53). Mutations in the human retinitis pigmentosa GTPase regulator gene have also been found to result in PCD (37, 76).
Although a diverse array of genes has already been implicated in PCD pathogenesis, relatively little is known about the biochemical and cellular processes that underlie ciliary and flagellar formation and function. In this paper, we identify a novel protein important for ciliary and flagellar function in the mouse. We demonstrate primary ciliary dyskinesia in mice homozygous for the nm1054 mutation, a recessive, pleiotropic mutation caused by an approximately 400-kb deletion on chromosome 1 that contains six genes (42, 43). Transgenic rescue demonstrated that deletion of the genes six-transmembrane epithelial antigen of the prostate (Steap3) and acyl-CoA binding protein (Acbp) result in the previously characterized anemia (42, 43) and cutaneous (35) phenotypes, respectively. Here, we show that the PCD phenotypes of hydrocephalus, male infertility, and respiratory ciliary dysfunction result from the loss of a single, novel gene named primary ciliary dyskinesia protein 1 (Pcdp1). We also demonstrate expression of the gene in spermatogenic and motile ciliated cell types and show protein localization in flagella and motile cilia in both mice and humans.
MATERIALS AND METHODS
Mice.
The nm1054 mutation was maintained on both the C57BL/6J (B6) and the 129S6/SvEvTac (129) backgrounds as previously described (42). Hydrocephalus was analyzed at 3 weeks in B6 mice expressing RPCI-22 bacterial artificial chromosome (BAC) 11D19, which contains the Steap3 gene and rescues the anemia (43). All other phenotypic analyses were performed on (B6 × 129)F1 (B6129F1) mice at 8 weeks. Animal procedures were approved by the Animal Care and Use Committee at Children's Hospital Boston.
Transgenic rescue.
BAC transgenic animals were generated and identified as previously described (35, 43).
X-ray analysis.
Three-week-old mice were euthanized, and the heads were fixed in 4% paraformaldehyde. X-rays were taken at 55 KeV for 1 min using the Faxitron cabinet X-ray system 43855C (Faxitron).
Histology.
Testes from 8-week-old B6129F1 male mice were fixed in 10% buffered formalin and transferred to 70% ethanol after 24 h. Heads were fixed in Bouin's fixative until the bones were fully decalcified, and coronal sections were cut through the sinuses. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Electron microscopy.
Testes and heads from 8- and 24-week-old B6129F1 mice were fixed overnight in a modified Karnovsky's solution of 2.5% glutaraldehyde-2.0% paraformaldehyde, pH 7.4. After fixation, bony tissues in the heads were decalcified in 0.5 M EDTA. Testes and dissected respiratory mucosa were then rinsed in cold 0.1 M sodium cacodylate buffer, pH 7.4, and treated with cacodylate-buffered 2.0% osmium tetroxide for 1.5 h.
The tissue was rapidly dehydrated through increasing concentrations of ethanol and embedded in Polybed 812 (Polysciences Inc.). Thin sections were cut on a Leica EM UC-6 ultramicrotome and placed on uncoated copper grids. The grids were stained for 3 minutes with saturated uranyl acetate in a 1:1 mixture of 70% ethanol and 100% methanol (16) and for 3 min with Reynold's lead citrate (52). Stained sections were analyzed under a Phillips EM 208S electron microscope.
Ciliary beat frequency analysis.
Tracheae were isolated from 8-week-old B6129F1 mice in Dulbecco's modified Eagle medium supplemented with 1% penicillin-streptomycin. Ciliary beat frequency of tracheal rings was analyzed using the Sisson-Ammons video analysis system as previously described (57).
Northern analysis.
A 32P-labeled Pcdp1 probe was generated by PCR amplification of nucleotides 2184 to 2483 of the open reading frame from expressed sequence tag (EST) clone BF018525 (Invitrogen) with primers TGACCCCTCTATGGTGGAAG and GCTTTGCTTCGCAGATTCTT. A multitissue mouse Northern blot (OriGene Technologies, Inc.) was prehybridized, probed, washed, and viewed as previously described (35).
Reverse transcription-PCR (RT-PCR).
RNA was isolated from testes of 24-week-old B6129F1 mice using the RNAqueous kit (Ambion Inc.). First-strand cDNA was synthesized from 1 μg of wild-type or nm1054 testis RNA using the SuperScript II reverse transcriptase kit (Invitrogen). Pcdp1 was amplified by PCR using primers TGACCCCTCTATGGTGGAAG and GCTTTGCTTCGCAGATTCTT, which lie outside the deleted region.
In situ hybridization.
Nucleotides 1857 to 2484 of the Pcdp1 open reading frame were amplified from EST BF018525 and cloned into the pCRII-TOPO vector (Invitrogen). Ten micrograms of that plasmid was digested with BamHI and XhoI to generate antisense and sense probes, respectively. Riboprobes were labeled with 35S using an in vitro transcription kit (Roche Molecular Biochemicals). Wild-type and nm1054 testes were fixed in 4% paraformaldehyde. Heads were fixed in 0.5 M EDTA until fully decalcified, and coronal sections were cut through the sinuses. Radioactive in situ hybridization was performed on paraffin-embedded slides. After deparaffinization, the slides were fixed in 4% paraformaldehyde for 10 min and then treated with proteinase K for 10 min at 37°C. The slides were hybridized overnight at 60°C with labeled riboprobe diluted in hybridization buffer to 1 × 104 cpm/μl. The slides were washed at 65°C for 2 hours in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), immersed in Kodak NTB emulsion, and exposed for 2 to 4 weeks at 4°C. Following exposure, the slides were developed and counterstained with hematoxylin.
Immunohistochemistry.
Wild-type and nm1054 mouse tissues were isolated and fixed as described above for in situ hybridization. Human sections were from routine formalin-fixed, paraffin-embedded surgical and autopsy specimens. A rabbit polyclonal antiserum was raised to and affinity purified against the C-terminal 15 amino acids of Pcdp1 (KNLRSKALNTYLILD; Bethyl Laboratories, Inc.). Immunoperoxidase studies for Pcdp1 were performed manually on 5-μm paraffin sections. Following deparaffinization and quenching of endogenous peroxidase activity with aqueous 3% hydrogen peroxide for 5 min, Retrieve-All 2 (Signet Pathology Systems, Inc.) was used for heat-induced epitope retrieval in a Black & Decker HS80 steamer for 50 min. Slides were cooled at room temperature for 20 min, washed in water, and placed in 0.05 M Tris, pH 7.6, with 4% porcine serum. The slides were then incubated with anti-Pcdp1 antibody at a 1:2,000 dilution (0.5 μg/ml) for 2 hours, washed with a Tris-saline solution, and incubated with a horseradish peroxidase-labeled goat anti-rabbit secondary antibody (PowerVision, ImmunoVision Technologies Co.) for 30 min. Antibody localization was effected using the peroxidase reaction with 3,3′-diaminobenzidine tetrahydrochloride (DAB+; DakoCytomation) as the chromogen. Staining intensity was enhanced by brief immersion in DAB enhancer solution (Zymed Laboratories). Sections were counterstained with methyl green, dehydrated, and coverslipped.
Nucleotide sequence accession number.
The mouse Pcdp1 sequence was deposited and released under GenBank accession number EF632061.
RESULTS
nm1054 mice have primary ciliary dyskinesia.
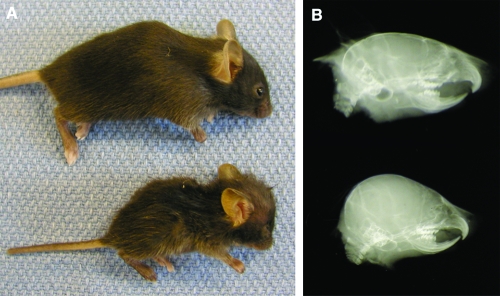
Mice homozygous for the nm1054 deletion have several phenotypes commonly associated with primary ciliary dyskinesia, including hydrocephalus, male infertility, and respiratory abnormalities. Homozygous mutants on the B6 background develop severe hydrocephalus, or dilatation of the ventricles of the brain, which results in a dramatic increase in the size of the cranial vault (Fig. 1). Mutants on this background usually die within the first week of life as a result of the hydrocephalus and severe anemia. Mutant mice transgenic for the Steap3 gene, which complements the anemia (43), occasionally live to 3 to 5 weeks of age before dying from the hydrocephalus. Mutants on the 129 background develop either mild or no hydrocephalus, indicating the presence of genetic modifiers segregating in diverse mouse strains.
FIG. 1.
Hydrocephalus in nm1054 mice. A lateral photograph (A) and radiograph (B) of 3-week-old C57BL/6J wild-type (top) and nm1054 (bottom) mice are shown. Note the expansion and doming of the cranial vault in the mutant animal. The greasy appearance of nm1054 hair is due to the loss of the Acbp gene, as previously described (35).
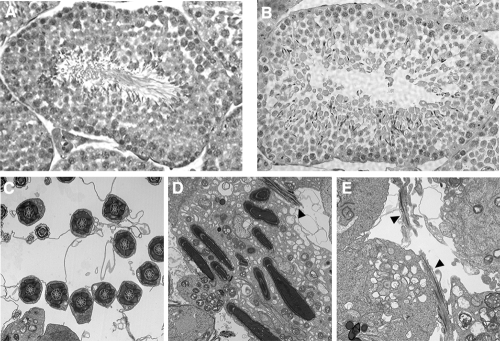
Mutant male mice on both the 129 and B6129F1 genetic backgrounds are infertile. Wild-type female mice paired with nm1054 mutant mice for 2 weeks form vaginal plugs but do not become pregnant. Histological analysis shows an absence of mature spermatozoa in the seminiferous tubules of nm1054 testes (Fig. 2A and B). While sperm heads are present and appear normal, there are no visible flagella. Transmission electron microscopy confirmed the absence of mature sperm, although abortive tail structures were occasionally visible (Fig. 2C to E). In addition, there are no sperm in the nm1054 epididymis (see Fig. S1A and B in the supplemental material). Reverse transcription-PCR showed that Protamine 1 (12, 30, 34), Protamine 2 (12, 11), TP1 (21, 69), and TP2 (55, 75), which are all markers of early to mid-spermiogenesis, the final stage of spermatogenesis, are expressed in the nm1054 testes (see Fig. S1C in the supplemental material). These data suggest that there is a spermatogenic defect late in spermiogenesis.
FIG. 2.
Spermiogenesis defect in B6129F1 nm1054 mice. (A and B) Sections of 8-week-old wild-type (A) and nm1054 (B) testes. Note the absence of mature sperm in the lumen of the nm1054 seminiferous tubule. Sections were stained with hematoxylin and eosin. Magnification, ×40. (C to E) Transmission electron microscopy of wild-type (C) and nm1054 (D and E) testes. Elongated dense bodies in panel D are nearly fully condensed sperm heads, but only occasional abortive tail structures can be identified in nm1054 testis sections (arrowheads in panels D and E).
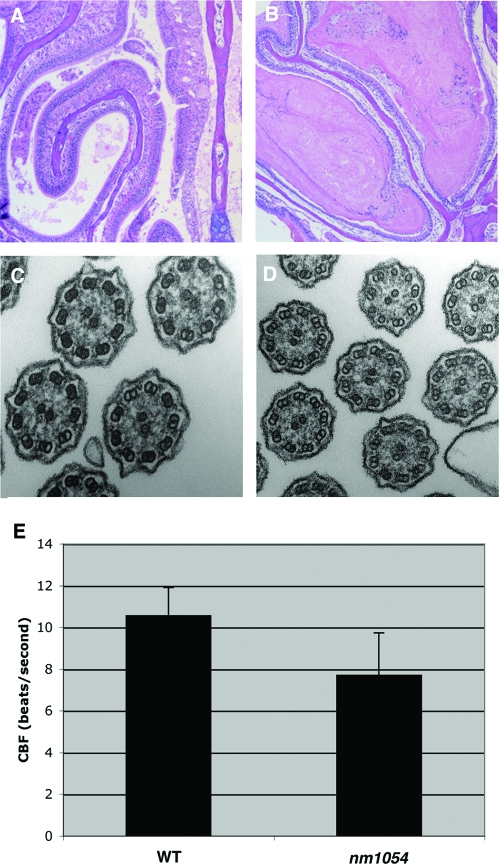
In addition to the hydrocephalus and male infertility, there is an accumulation of mucus in the sinuses of nm1054 mutant mice (Fig. 3A and B). Although there is no evidence of the inflammation seen in sinusitis, excessive mucus in the nasal passage could be indicative of the impaired epithelial clearance that predisposes PCD patients to chronic infection and respiratory distress. In dramatic contrast to the absence of sperm flagella, nm1054 sinus and tracheal epithelial cells possess cilia with a normal ultrastructure (Fig. 3C and D and data not shown). However, the beat frequency of nm1054 tracheal epithelial cilia is approximately 25% lower than that of wild-type cilia, with a difference of more than 2 beats per second (Fig. 3E). This impaired ciliary motility could account for the apparent defect in mucus clearance. Presumably, the hydrocephalus in nm1054 mice results from an accumulation of cerebrospinal fluid due to a similar decrease in motility of ependymal cilia. Situs inversus, otitis media, and retinitis pigmentosa are not present in nm1054 mutant mice (data not shown).
FIG. 3.
Respiratory abnormalities in B6129F1 nm1054 mice. (A and B) Coronal sections of 8-week-old wild-type (A) and nm1054 (B) maxillary sinus and turbinate samples. Note the accumulation of mucus in the nm1054 sinus (B). Sections were stained with hematoxylin and eosin. (C and D) Transmission electron microscopy of wild-type (C) and nm1054 (D) tracheal epithelial cilia, demonstrating a normal axonemal ultrastructure. (E) Ciliary beat frequency (CBF) of wild-type and nm1054 tracheal epithelial cilia, showing a decreased rate of beating in the mutant (n = 6 in each group; P < 0.01).
Transgenic rescue of nm1054 PCD.
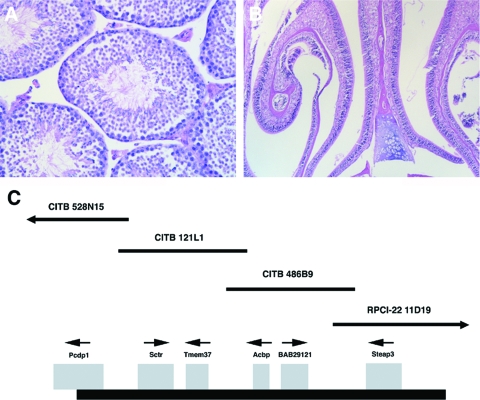
An overlapping BAC contig spanning the nm1054 deletion was previously developed (43). The PCD in nm1054 mice is rescued in a transgenic line derived from CITB BAC clone 528N15 (Fig. 4) (29), while the anemia (42, 43) and the cutaneous phenotype (35) are not affected. Transgenic mutant mice on the B6 background do not develop hydrocephalus (data not shown). Additionally, transgenic B6129F1 nm1054 males are fertile. Histology and electron microscopy of transgenic mutant testes demonstrate the presence of mature spermatozoa (Fig. 4A and data not shown), and there is no abnormal accumulation of mucus in the sinuses from transgenic mutant animals (Fig. 4B). None of the other transgenes spanning the nm1054 deletion rescues any of the PCD phenotypes.
FIG. 4.
Transgenic rescue of the nm1054 primary ciliary dyskinesia. (A and B) Sections of BAC 528N15 transgenic B6129F1 nm1054 testis (A) and maxillary sinus and turbinates (B), which are indistinguishable from the wild type. Sections were stained with hematoxylin and eosin. (C) Schematic map of overlapping BAC clones spanning the nm1054 deletion interval. The thick black line (bottom) represents the deleted interval on chromosome 1. Deleted genes are shown as gray boxes, with the direction of transcription indicated by a small arrow above each gene. Individual CITB and RPCI-22 BAC clones are represented as thin black lines and identified by clone identification numbers. BAC CITB 528N15 contains only Pcdp1.
Characterization of Pcdp1, a novel ciliary protein.
BAC 528N15, which rescues the PCD in nm1054 mutant mice, contains a single, novel gene we have named Pcdp1 (Fig. 4C). This strongly suggests that the PCD observed in nm1054 mutant mice results solely from the loss of Pcdp1. The Pcdp1 gene was originally defined by gene prediction tools in the Ensembl database (5), as well as partial testis ESTs with GenBank accession numbers BB616643 (9), BU937221, CF197896, BF018525, AV274480, and BF319649 and ensemble EST EMUST37840. We cloned the full-length Pcdp1 open reading frame from reverse-transcribed B6129F1 testis cDNA (data not shown) and determined that the 2,508-nucleotide gene is comprised of 23 exons and encodes an 836-amino-acid protein. This is consistent with a study identifying this gene by UniGene ID number 297290 as one of 28 novel genes expressed during spermatogenesis (23).
The Pcdp1 protein has orthologs in species ranging from ciliated unicellular eukaryotes to humans, although no Chlamydomonas reinhardtii ortholog has been identified. The protein sequence is highly conserved among higher eukaryotes (Fig. 5A). Although there are no identifiable domains, signals, or structural motifs in the Pcdp1 protein, the high sequence similarity suggests that the protein has an important function. The region spanning amino acids 53 to 231 is 22% identical to Hydin, a large protein localized to the central microtubule pair of cilia and flagella (Fig. 5B) (33). Loss of Hydin results in immotile flagella in Chlamydomonas reinhardtii (33) and hydrocephalus in the hy3 mouse (14).
FIG. 5.
Alignment of Pcdp1 proteins. (A) Alignment of the following Pcdp1 orthologs: mouse (GenBank accession number EF632061), rat (GenBank accession number XP_001054199), and human (assembled by alignment of GenBank accession numbers EAW95222 and NP_001025167 with the mouse Pcdp1 sequence) (62, 67). (B) Alignment of similar regions of mouse Pcdp1 and mouse Hydin (GenBank accession number AA044963) (14). Identical amino acids are shaded in gray.
Expression of Pcdp1 in mouse tissues was investigated by Northern analysis, RT-PCR, and radioactive in situ hybridization. A multitissue Northern blot assay showed weak expression of an approximately 2.5-kb transcript only in the testis (Fig. 6A). Pcdp1 expression in wild-type and nm1054 testis was investigated by RT-PCR using primers that lie outside the deleted region. Absence of Pcdp1 expression in nm1054 testes strongly suggests that the mutation is a null (Fig. 6B). In situ hybridization showed Pcdp1 expression specifically in developing spermatocytes and spermatids but not in immature spermatogonia (Fig. 6C), suggesting that it is only expressed in cells undergoing spermatogenesis. Given the multitissue phenotype, expression was also expected in other specialized cell types possessing motile cilia. In situ hybridization showed expression in the ciliated respiratory epithelial cells lining the sinuses, trachea, and bronchi of wild-type mice (Fig. 6E and data not shown). In contrast, no Pcdp1 expression was detected in nm1054 testis (Fig. 6D) or respiratory epithelial cells (Fig. 6F) when we used a probe that lies outside the deleted region. Additionally, no signal was observed from the sense controls (data not shown).
FIG. 6.
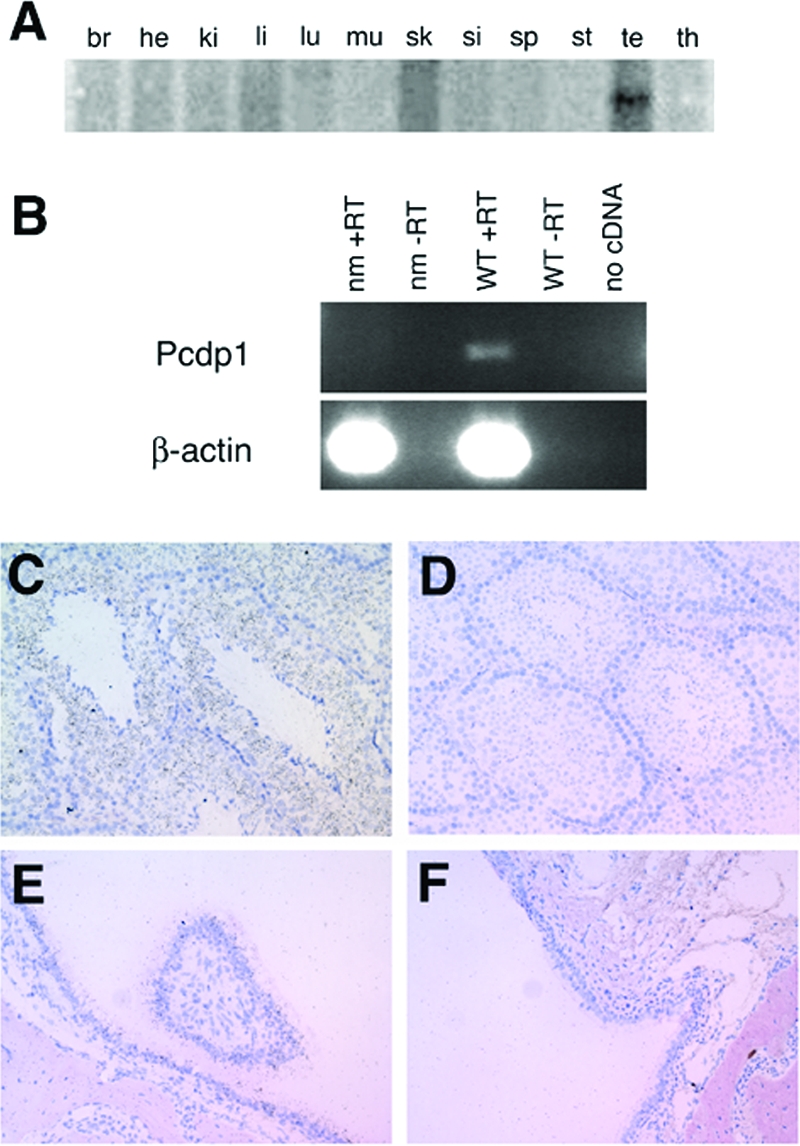
Pcdp1 mRNA expression. (A) Northern analysis of Pcdp1 mRNA in brain (br), heart (he), kidney (ki), liver (li) (38), lung (lu), muscle (mu), skin (sk), small intestine (si), spleen (sp), stomach (st), testis (te), and thymus (th). (B) RT-PCR of Pcdp1 in wild-type (WT) and nm1054 (nm) testes specimens. Reactions lacking either reverse transcriptase (-RT) or template (no cDNA) were used as negative controls. β-Actin was used as a loading control. (C to F) Radioactive in situ hybridization analysis of Pcdp1 expression in 8-week-old B6129F1 wild-type testis (C), nm1054 testis (D), wild-type sinus (E), and nm1054 sinus (F). Dusty, black, granular staining indicates Pcdp1 mRNA expression in wild-type spermatocytes and spermatids (C), as well as in wild-type sinus epithelium (E).
We also investigated Pcdp1 protein expression by immunohistochemistry using a rabbit polyclonal antibody raised to the C-terminal 15 amino acids. The antibody was validated by Western blotting of N- and C-terminal FLAG-tagged Pcdp1 constructs overexpressed in HEK293T cells (data not shown). Pcdp1 protein is expressed in spermatocytes and spermatids and localizes to the flagella of mature spermatozoa (Fig. 7A), the cilia of mouse (Fig. 7C) and human (Fig. 7E) respiratory epithelial cells, and the cilia of ependymal cells in human brain (Fig. 7F). Some cytoplasmic staining is observed near the apical surface of the respiratory epithelial and ependymal cells that may correspond to Pcdp1 protein in transit to the cilia. The Pcdp1 staining pattern in mouse testis and respiratory epithelia is identical to that of acetylated tubulin, a marker for motile cilia (data not shown). Pcdp1 was not detected in nm1054 sperm flagella (Fig. 7B) or respiratory epithelial cilia (Fig. 7D). Some staining was observed in the cytoplasm of nm1054 epithelial cells. Although the C-terminal antigenic region is not removed by the nm1054 deletion, absence of a transcript in nm1054 animals using RT-PCR primers (Fig. 6B) and an in situ hybridization probe (Fig. 6D and F) that lie outside the deleted region suggest that any staining observed in mutant tissues is nonspecific.
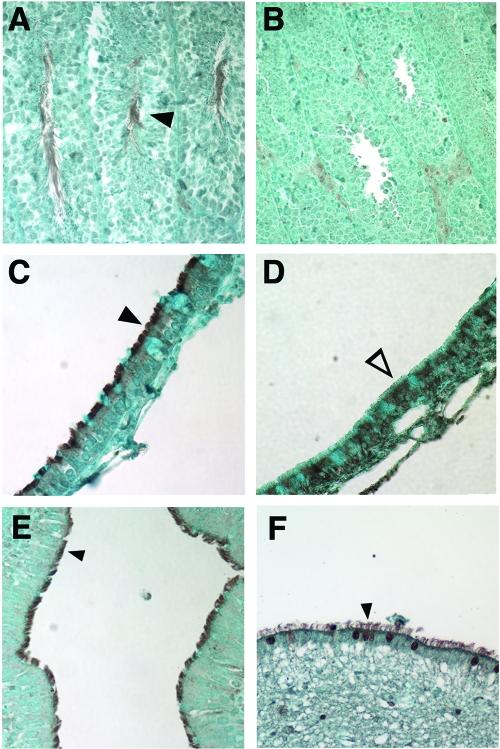
FIG. 7.
Immunohistochemical analysis of mouse and human Pcdp1 protein expression. (A and B) Expression of Pcdp1 in B6129F1 wild-type (A) and nm1054 (B) testes. (C and D) Expression of Pcdp1 in B6129F1 wild-type (C) and nm1054 (D) sinus epithelial cells. (E and F) Expression of Pcdp1 in human ciliated bronchial epithelial cells (E) and brain ependymal cells (F). Closed arrowheads indicate presence of mouse Pcdp1 in wild-type flagella (A) and cilia (C) and human PCDP1 in bronchial epithelial (E) and ependymal (F) cilia. The open arrowhead indicates an absence of Pcdp1 in nm1054 sinus epithelial cilia (D).
DISCUSSION
In this study, we have demonstrated that loss of the novel gene Pcdp1 results in primary ciliary dyskinesia in the nm1054 mutant mouse. Homozygous mutants develop hydrocephalus on the C57BL/6J background, as well as male infertility and an accumulation of mucus in the sinus cavity, likely due to impaired clearance and slowed ciliary beat frequency. The Pcdp1 gene is expressed in spermatogenic cells and ciliated respiratory epithelial cells and encodes a conserved protein that localizes to sperm flagella and motile cilia in the respiratory system and the brain. Based on the phenotype, this novel protein likely plays an important role in ciliary biogenesis or function.
There is a striking difference between the spermatogenic and respiratory abnormalities in nm1054 mice. There is a complete absence of mature flagella in mutant testis and an absence of sperm in the epididymis. Presence of abortive tail structures and expression of spermiogenesis markers suggest that the block in spermatogenesis occurs late in spermiogenesis. In contrast, respiratory cilia are present and ultrastructurally normal, although the tracheal ciliary beat frequency is significantly lower than that of wild-type tracheal cilia. These findings suggest that Pcdp1 is essential for the biogenesis of flagella but is only required for the motility of respiratory epithelial cilia. Furthermore, this observation implies that although respiratory cilia and sperm flagella share a common axonemal structure, their formation and function are distinctly different. This hypothesis is supported by an identified mutation in the porcine KPL2 gene that results in truncated sperm tails but does not affect the axonemal structure of motile cilia (56).
Since the Pcdp1 protein is specifically localized along the length of cilia and flagella, it is not likely involved in initial elongation of the axoneme from the basal body. Such a function would likely require Pcdp1 in the cytoplasm rather than the full length of the cilia. This is supported by the apparent completion of ciliogenesis and the presence of flagellar structures that have likely passed the early stages of spermiogenesis. It is likely that Pcdp1 plays a role in ciliary and flagellar motility, as well as in flagellar axonemal assembly or stability late in spermiogenesis. However, biochemical data will be required to determine whether the role is structural or regulatory or if the protein is involved in another undefined pathway or process.
The absence of situs inversus in nm1054 deletion homozygotes suggests that Pcdp1 does not play an important role in nodal cilia and is not required for left-right patterning in the early embryo. Nodal cilia differ from other motile cilia in that they do not possess a central microtubule pair. Human mutations affecting the central microtubule pair have been shown to result in PCD without situs inversus (61). It is therefore possible that Pcdp1 function is associated with the central apparatus, which is composed of a microtubule pair and numerous accessory proteins and is thought to control and regulate the dynein motor force required for ciliary and flagellar motility (51, 58, 59). Disruption of central apparatus proteins in Chlamydomonas commonly results in immotile flagella (33, 51, 58, 59), while suppressor mutations in outer or inner dynein arm components restore motility by bypassing the inhibition (25, 49, 50). In the complete absence of the Chlamydomonas central apparatus, the flagella are paralyzed, but the microtubule doublets are still able to undergo the sliding motion that produces flagellar motility, albeit at a greatly reduced velocity (68). This suggests that the central apparatus plays a role in regulating the dynein motor force rather than generating it.
Even though the nm1054 ciliary ultrastructure is normal, the PCD in nm1054 mutants is similar to phenotypes observed in mice lacking dynein genes (26, 41). This suggests that Pcdp1 may directly or indirectly regulate dynein motor force. If Pcdp1 encodes a component of the central apparatus, loss of the gene in nm1054 mice could be disrupting ciliary motility by inhibiting microtubule sliding without destroying the axonemal ultrastructure. This effect has been observed when central apparatus components are mutated in the mouse. Male mice lacking the testis-specific isoform of the central apparatus protein sperm-associated antigen 16 (Spag16L) are infertile, and their sperm have flagella with a normal ultrastructure but decreased motility (73, 74). Amino acids 53 to 231 of Pcdp1 are 22% identical to the central apparatus protein Hydin. Loss of Hydin results in immotile flagella in Chlamydomonas and hydrocephalus in mice (33, 14), further supporting the possibility that Pcdp1 may encode a central pair protein. In addition, it is possible that Pcdp1 is also a key structural component in flagella, such that loss of the protein could cause flagellar instability during late spermiogenesis, resulting in the absence of mature sperm tails. Further experiments are required to decipher the differences between cilia and flagella to determine how the requirement for Pcdp1 may vary between these two organelles.
The nm1054 mutant is a useful model for studying PCD. To date, no mutations in the human ortholog of Pcdp1 have been identified that result in this disorder. However, given the PCD phenotype in nm1054 mice, understanding the function of the Pcdp1 protein may contribute greatly to the understanding of the molecular mechanisms perturbed in PCD, as well as to the diagnosis and treatment of the disorder.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (HL074247 for M.D.F. and AA008769 for J.H.S.) and a Department of Veteran's Affairs Merit Review Grant (T.A.W.). L.L. was supported in part by an NIH postdoctoral NRSA training grant (HL007574.23).
We gratefully thank James Edwards and Tonora Archibald in the Children's Hospital Boston Pathology Department histology core facility, as well as Yu Yang in the Dana Farber—Partners Cancer Center in situ core facility, for their technical assistance and expertise. We thank Hannah Kinney and Robin Haynes for human brain sections. We also thank Steven Margossian for reading the manuscript, as well as Nancy Andrews and members of the Andrews and Fleming laboratories for unending discussion and suggestions.
Footnotes
Published ahead of print on 26 November 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Afzelius, B. A. 2004. Cilia-related diseases. J. Pathol. 204470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afzelius, B. A. 1976. A human syndrome caused by immotile cilia. Science 193317-319. [DOI] [PubMed] [Google Scholar]
- 3.al-Shroof, M., A. M. Karnik, A. A. Karnik, J. Longshore, N. A. Sliman, and F. A. Khan. 2001. Ciliary dyskinesia associated with hydrocephalus and mental retardation in a Jordanian family. Mayo Clin. Proc. 761219-1224. [DOI] [PubMed] [Google Scholar]
- 4.Bartoloni, L., J. L. Blouin, Y. Pan, C. Gehrig, A. K. Maiti, N. Scamuffa, C. Rossier, M. Jorissen, M. Armengot, M. Meeks, H. M. Mitchison, E. M. Chung, C. D. Delozier-Blanchet, W. J. Craigen, and S. E. Antonarakis. 2002. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA 9910282-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birney, E., D. Andrews, M. Caccamo, Y. Chen, L. Clarke, G. Coates, T. Cox, F. Cunningham, V. Curwen, T. Cutts, T. Down, R. Durbin, X. M. Fernandez-Suarez, P. Flicek, S. Graf, M. Hammond, J. Herrero, K. Howe, V. Iyer, K. Jekosch, A. Kahari, A. Kasprzyk, D. Keefe, F. Kokocinski, E. Kulesha, D. London, I. Longden, C. Melsopp, P. Meidl, B. Overduin, A. Parker, G. Proctor, A. Prlic, M. Rae, D. Rios, S. Redmond, M. Schuster, I. Sealy, S. Searle, J. Severin, G. Slater, D. Smedley, J. Smith, A. Stabenau, J. Stalker, S. Trevanion, A. Ureta-Vidal, J. Vogel, S. White, C. Woodwark, and T. J. Hubbard. 2006. Ensembl 2006. Nucleic Acids Res. 34D556-D561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisgrove, B. W., and H. J. Yost. 2006. The roles of cilia in developmental disorders and disease. Development 1334131-4143. [DOI] [PubMed] [Google Scholar]
- 7.Blouin, J. L., M. Meeks, U. Radhakrishna, A. Sainsbury, C. Gehring, G. D. Sail, L. Bartoloni, V. Dombi, A. O'Rawe, A. Walne, E. Chung, B. A. Afzelius, M. Armengot, M. Jorissen, D. V. Schidlow, L. van Maldergem, H. Walt, R. M. Gardiner, D. Probst, P. A. Guerne, C. D. Delozier-Blanchet, and S. E. Antonarakis. 2000. Primary ciliary dyskinesia: a genome-wide linkage analysis reveals extensive locus heterogeneity. Eur. J. Hum. Genet. 8109-118. [DOI] [PubMed] [Google Scholar]
- 8.Brody, S. L., X. H. Yan, M. K. Wuerffel, S. K. Song, and S. D. Shapiro. 2000. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am. J. Respir. Cell Mol. Biol. 2345-51. [DOI] [PubMed] [Google Scholar]
- 9.Carninci, P., T. Kasukawa, S. Katayama, J. Gough, M. C. Frith, N. Maeda, R. Oyama, T. Ravasi, B. Lenhard, C. Wells, R. Kodzius, K. Shimokawa, V. B. Bajic, S. E. Brenner, S. Batalov, A. R. Forrest, M. Zavolan, M. J. Davis, L. G. Wilming, V. Aidinis, J. E. Allen, A. Ambesi-Impiombato, R. Apweiler, R. N. Aturaliya, T. L. Bailey, M. Bansal, L. Baxter, K. W. Beisel, T. Bersano, H. Bono, A. M. Chalk, K. P. Chiu, V. Choudhary, A. Christoffels, D. R. Clutterbuck, M. L. Crowe, E. Dalla, B. P. Dalrymple, B. de Bono, G. Della Gatta, D. di Bernardo, T. Down, P. Engstrom, M. Fagiolini, G. Faulkner, C. F. Fletcher, T. Fukushima, M. Furuno, S. Futaki, M. Gariboldi, P. Georgii-Hemming, T. R. Gingeras, T. Gojobori, R. E. Green, S. Gustincich, M. Harbers, Y. Hayashi, T. K. Hensch, N. Hirokawa, D. Hill, L. Huminiecki, M. Iacono, K. Ikeo, A. Iwama, T. Ishikawa, M. Jakt, A. Kanapin, M. Katoh, Y. Kawasawa, J. Kelso, H. Kitamura, H. Kitano, G. Kollias, S. P. Krishnan, A. Kruger, S. K. Kummerfeld, I. V. Kurochkin, L. F. Lareau, D. Lazarevic, L. Lipovich, J. Liu, S. Liuni, S. McWilliam, M. Madan Babu, M. Madera, L. Marchionni, H. Matsuda, S. Matsuzawa, H. Miki, F. Mignone, S. Miyake, K. Morris, S. Mottagui-Tabar, N. Mulder, N. Nakano, H. Nakauchi, P. Ng, R. Nilsson, S. Nishiguchi, S. Nishikawa, et al. 2005. The transcriptional landscape of the mammalian genome. Science 3091559-1563. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., H. J. Knowles, J. L. Hebert, and B. P. Hackett. 1998. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J. Clin. Investig. 1021077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho, C., H. Jung-Ha, W. D. Willis, E. H. Goulding, P. Stein, Z. Xu, R. M. Schultz, N. B. Hecht, and E. M. Eddy. 2003. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol. Reprod. 69211-217. [DOI] [PubMed] [Google Scholar]
- 12.Cho, C., W. D. Willis, E. H. Goulding, H. Jung-Ha, Y. C. Choi, N. B. Hecht, and E. M. Eddy. 2001. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat. Genet. 2882-86. [DOI] [PubMed] [Google Scholar]
- 13.Chodhari, R., H. M. Mitchison, and M. Meeks. 2004. Cilia, primary ciliary dyskinesia and molecular genetics. Paediatr. Respir. Rev. 569-76. [DOI] [PubMed] [Google Scholar]
- 14.Davy, B. E., and M. L. Robinson. 2003. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum. Mol. Genet. 121163-1170. [DOI] [PubMed] [Google Scholar]
- 15.De Santi, M. M., A. Magni, E. A. Valletta, C. Gardi, and G. Lungarella. 1990. Hydrocephalus, bronchiectasis, and ciliary aplasia. Arch. Dis. Child. 65543-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst, S. A., and R. A. Ellis. 1969. The development of surface specialization in the secretory epithelium of the avian salt gland in response to osmotic stress. J. Cell Biol. 40305-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geremek, M., and M. Witt. 2004. Primary ciliary dyskinesia: genes, candidate genes and chromosomal regions. J. Appl. Genet. 45347-361. [PubMed] [Google Scholar]
- 18.Greenstone, M. A., R. W. Jones, A. Dewar, B. G. Neville, and P. J. Cole. 1984. Hydrocephalus and primary ciliary dyskinesia. Arch. Dis. Child. 59481-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guichard, C., M. C. Harricane, J. J. Lafitte, P. Godard, M. Zaegel, V. Tack, G. Lalau, and P. Bouvagnet. 2001. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome). Am. J. Hum. Genet. 681030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagiwara, H., N. Ohwada, T. Aoki, and K. Takata. 2000. Ciliogenesis and ciliary abnormalities. Med. Electron Microsc. 33109-114. [DOI] [PubMed] [Google Scholar]
- 21.Heidaran, M. A., R. M. Showman, and W. S. Kistler. 1988. A cytochemical study of the transcriptional and translational regulation of nuclear transition protein 1 (TP1), a major chromosomal protein of mammalian spermatids. J. Cell Biol. 1061427-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirokawa, N., Y. Tanaka, Y. Okada, and S. Takeda. 2006. Nodal flow and the generation of left-right asymmetry. Cell 12533-45. [DOI] [PubMed] [Google Scholar]
- 23.Hong, S., I. Choi, J. M. Woo, J. Oh, T. Kim, E. Choi, T. W. Kim, Y. K. Jung, D. H. Kim, C. H. Sun, G. S. Yi, E. M. Eddy, and C. Cho. 2005. Identification and integrative analysis of 28 novel genes specifically expressed and developmentally regulated in murine spermatogenic cells. J. Biol. Chem. 2807685-7693. [DOI] [PubMed] [Google Scholar]
- 24.Hornef, N., H. Olbrich, J. Horvath, M. A. Zariwala, M. Fliegauf, N. T. Loges, J. Wildhaber, P. G. Noone, M. Kennedy, S. E. Antonarakis, J. L. Blouin, L. Bartoloni, T. Nublein, P. Ahrens, M. Griese, H. Kuhl, R. Sudbrak, M. R. Knowles, R. Reinhardt, and H. Omran. 2006. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am. J. Respir. Crit. Care Med. 174120-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, B., Z. Ramanis, and D. J. Luck. 1982. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell 28115-124. [DOI] [PubMed] [Google Scholar]
- 26.Ibanez-Tallon, I., S. Gorokhova, and N. Heintz. 2002. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum. Mol. Genet. 11715-721. [DOI] [PubMed] [Google Scholar]
- 27.Ibanez-Tallon, I., N. Heintz, and H. Omran. 2003. To beat or not to beat: roles of cilia in development and disease. Hum. Mol. Genet. 12(Spec. issue 1)R27-R35. [DOI] [PubMed] [Google Scholar]
- 28.Jabourian, Z., F. D. Lublin, A. Adler, C. Gonzales, B. Northrup, and D. Zwillenberg. 1986. Hydrocephalus in Kartagener's syndrome. Ear Nose Throat J. 65468-472. [PubMed] [Google Scholar]
- 29.Kim, U. J., B. W. Birren, T. Slepak, V. Mancino, C. Boysen, H. L. Kang, M. I. Simon, and H. Shizuya. 1996. Construction and characterization of a human bacterial artificial chromosome library. Genomics 34213-218. [DOI] [PubMed] [Google Scholar]
- 30.Kleene, K. C., R. J. Distel, and N. B. Hecht. 1984. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev. Biol. 10571-79. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi, Y., M. Watanabe, Y. Okada, H. Sawa, H. Takai, M. Nakanishi, Y. Kawase, H. Suzuki, K. Nagashima, K. Ikeda, and N. Motoyama. 2002. Hydrocephalus, situs inversus, chronic sinusitis, and male infertility in DNA polymerase lambda-deficient mice: possible implication for the pathogenesis of immotile cilia syndrome. Mol. Cell. Biol. 222769-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosaki, K., K. Ikeda, K. Miyakoshi, M. Ueno, R. Kosaki, D. Takahashi, M. Tanaka, C. Torikata, Y. Yoshimura, and T. Takahashi. 2004. Absent inner dynein arms in a fetus with familial hydrocephalus-situs abnormality. Am. J. Med. Genet. A 129308-311. [DOI] [PubMed] [Google Scholar]
- 33.Lechtreck, K. F., and G. B. Witman. 2007. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J. Cell Biol. 176473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, K., H. S. Haugen, C. H. Clegg, and R. E. Braun. 1995. Premature translation of protamine 1 mRNA causes precocious nuclear condensation and arrests spermatid differentiation in mice. Proc. Natl. Acad. Sci. USA 9212451-12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, L., C. A. DeBono, D. R. Campagna, D. C. Young, D. B. Moody, and M. D. Fleming. 2007. Loss of the acyl-CoA binding protein (Acbp) results in fatty acid metabolism abnormalities in mouse hair and skin. J. Investig. Dermatol. 12716-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath, J., and M. Brueckner. 2003. Cilia are at the heart of vertebrate left-right asymmetry. Curr. Opin. Genet. Dev. 13385-392. [DOI] [PubMed] [Google Scholar]
- 37.Moore, A., E. Escudier, G. Roger, A. Tamalet, B. Pelosse, S. Marlin, A. Clement, M. Geremek, B. Delaisi, A. M. Bridoux, A. Coste, M. Witt, B. Duriez, and S. Amselem. 2006. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J. Med. Genet. 43326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moran, J. L., A. D. Bolton, P. V. Tran, A. Brown, N. D. Dwyer, D. K. Manning, B. C. Bjork, C. Li, K. Montgomery, S. M. Siepka, M. H. Vitaterna, J. S. Takahashi, T. Wiltshire, D. J. Kwiatkowski, R. Kucherlapati, and D. R. Beier. 2006. Utilization of a whole genome SNP panel for efficient genetic mapping in the mouse. Genome Res. 16436-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mygind, N., and M. Pedersen. 1983. Nose-, sinus- and ear-symptoms in 27 patients with primary ciliary dyskinesia. Eur. J. Respir. Dis. Suppl. 12796-101. [PubMed] [Google Scholar]
- 40.Nastasi, K. J., and M. S. Blaiss. 1994. A seven-year-old boy with sinusitis, otitis media, and asthma. Ann. Allergy 7315-20. [PubMed] [Google Scholar]
- 41.Neesen, J., R. Kirschner, M. Ochs, A. Schmiedl, B. Habermann, C. Mueller, A. F. Holstein, T. Nuesslein, I. Adham, and W. Engel. 2001. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum. Mol. Genet. 101117-1128. [DOI] [PubMed] [Google Scholar]
- 42.Ohgami, R. S., D. R. Campagna, B. Antiochos, E. B. Wood, J. J. Sharp, J. E. Barker, and M. D. Fleming. 2005. nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. Blood 1063625-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohgami, R. S., D. R. Campagna, E. L. Greer, B. Antiochos, A. McDonald, J. Chen, J. J. Sharp, Y. Fujiwara, J. E. Barker, and M. D. Fleming. 2005. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 371264-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olbrich, H., K. Haffner, A. Kispert, A. Volkel, A. Volz, G. Sasmaz, R. Reinhardt, S. Hennig, H. Lehrach, N. Konietzko, M. Zariwala, P. G. Noone, M. Knowles, H. M. Mitchison, M. Meeks, E. M. Chung, F. Hildebrandt, R. Sudbrak, and H. Omran. 2002. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat. Genet. 30143-144. [DOI] [PubMed] [Google Scholar]
- 45.Omran, H., K. Haffner, A. Volkel, J. Kuehr, U. P. Ketelsen, U. H. Ross, N. Konietzko, T. Wienker, M. Brandis, and F. Hildebrandt. 2000. Homozygosity mapping of a gene locus for primary ciliary dyskinesia on chromosome 5p and identification of the heavy dynein chain DNAH5 as a candidate gene. Am. J. Respir. Cell Mol. Biol. 23696-702. [DOI] [PubMed] [Google Scholar]
- 46.Palmblad, J., B. Mossberg, and B. A. Afzelius. 1984. Ultrastructural, cellular, and clinical features of the immotile-cilia syndrome. Annu. Rev. Med. 35481-492. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen, M., and N. Mygind. 1982. Rhinitis, sinusitis and otitis media in Kartagener's syndrome (primary ciliary dyskinesia). Clin. Otolaryngol. Allied Sci. 7373-380. [DOI] [PubMed] [Google Scholar]
- 48.Pennarun, G., E. Escudier, C. Chapelin, A. M. Bridoux, V. Cacheux, G. Roger, A. Clement, M. Goossens, S. Amselem, and B. Duriez. 1999. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am. J. Hum. Genet. 651508-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porter, M. E., J. A. Knott, L. C. Gardner, D. R. Mitchell, and S. K. Dutcher. 1994. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the beta-dynein heavy chain. J. Cell Biol. 1261495-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter, M. E., J. Power, and S. K. Dutcher. 1992. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J. Cell Biol. 1181163-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porter, M. E., and W. S. Sale. 2000. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 151F37-F42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sapiro, R., I. Kostetskii, P. Olds-Clarke, G. L. Gerton, G. L. Radice, and I. J. Strauss. 2002. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol. Cell. Biol. 226298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satir, P., and S. T. Christensen. 2007. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69377-400. [DOI] [PubMed] [Google Scholar]
- 55.Shih, D. M., and K. C. Kleene. 1992. A study by in situ hybridization of the stage of appearance and disappearance of the transition protein 2 and the mitochondrial capsule seleno-protein mRNAs during spermatogenesis in the mouse. Mol. Reprod. Dev. 33222-227. [DOI] [PubMed] [Google Scholar]
- 56.Sironen, A., B. Thomsen, M. Andersson, V. Ahola, and J. Vilkki. 2006. An intronic insertion in KPL2 results in aberrant splicing and causes the immotile short-tail sperm defect in the pig. Proc. Natl. Acad. Sci. USA 1035006-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sisson, J. H., J. A. Stoner, B. A. Ammons, and T. A. Wyatt. 2003. All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 211103-111. [DOI] [PubMed] [Google Scholar]
- 58.Smith, E. F., and P. A. Lefebvre. 1997. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil. Cytoskel. 381-8. [DOI] [PubMed] [Google Scholar]
- 59.Smith, E. F., and P. Yang. 2004. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil. Cytoskel. 578-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stannard, W., and C. O'Callaghan. 2006. Ciliary function and the role of cilia in clearance. J. Aerosol Med. 19110-115. [DOI] [PubMed] [Google Scholar]
- 61.Stannard, W., A. Rutman, C. Wallis, and C. O'Callaghan. 2004. Central microtubular agenesis causing primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 169634-637. [DOI] [PubMed] [Google Scholar]
- 62.Strausberg, R. L., E. A. Feingold, L. H. Grouse, J. G. Derge, R. D. Klausner, F. S. Collins, L. Wagner, C. M. Shenmen, G. D. Schuler, S. F. Altschul, B. Zeeberg, K. H. Buetow, C. F. Schaefer, N. K. Bhat, R. F. Hopkins, H. Jordan, T. Moore, S. I. Max, J. Wang, F. Hsieh, L. Diatchenko, K. Marusina, A. A. Farmer, G. M. Rubin, L. Hong, M. Stapleton, M. B. Soares, M. F. Bonaldo, T. L. Casavant, T. E. Scheetz, M. J. Brownstein, T. B. Usdin, S. Toshiyuki, P. Carninci, C. Prange, S. S. Raha, N. A. Loquellano, G. J. Peters, R. D. Abramson, S. J. Mullahy, S. A. Bosak, P. J. McEwan, K. J. McKernan, J. A. Malek, P. H. Gunaratne, S. Richards, K. C. Worley, S. Hale, A. M. Garcia, L. J. Gay, S. W. Hulyk, D. K. Villalon, D. M. Muzny, E. J. Sodergren, X. Lu, R. A. Gibbs, J. Fahey, E. Helton, M. Ketteman, A. Madan, S. Rodrigues, A. Sanchez, M. Whiting, A. C. Young, Y. Shevchenko, G. G. Bouffard, R. W. Blakesley, J. W. Touchman, E. D. Green, M. C. Dickson, A. C. Rodriguez, J. Grimwood, J. Schmutz, R. M. Myers, Y. S. Butterfield, M. I. Krzywinski, U. Skalska, D. E. Smailus, A. Schnerch, J. E. Schein, S. J. Jones, and M. A. Marra. 2002. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl. Acad. Sci. USA 9916899-16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabin, C. 2005. Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J. Mol. Histol. 36317-323. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka, H., N. Iguchi, Y. Toyama, K. Kitamura, T. Takahashi, K. Kaseda, M. Maekawa, and Y. Nishimune. 2004. Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol. Cell. Biol. 247958-7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Dorp, D. B., A. F. Wright, A. D. Carothers, and E. M. Bleeker-Wagemakers. 1992. A family with RP3 type of X-linked retinitis pigmentosa: an association with ciliary abnormalities. Hum. Genet. 88331-334. [DOI] [PubMed] [Google Scholar]
- 66.Van's Gravesande, K. S., and H. Omran. 2005. Primary ciliary dyskinesia: clinical presentation, diagnosis and genetics. Ann. Med. 37439-449. [DOI] [PubMed] [Google Scholar]
- 67.Venter, J. C., M. D. Adams, E. W. Myers, P. W. Li, R. J. Mural, G. G. Sutton, H. O. Smith, M. Yandell, C. A. Evans, R. A. Holt, J. D. Gocayne, P. Amanatides, R. M. Ballew, D. H. Huson, J. R. Wortman, Q. Zhang, C. D. Kodira, X. H. Zheng, L. Chen, M. Skupski, G. Subramanian, P. D. Thomas, J. Zhang, G. L. Gabor Miklos, C. Nelson, S. Broder, A. G. Clark, J. Nadeau, V. A. McKusick, N. Zinder, A. J. Levine, R. J. Roberts, M. Simon, C. Slayman, M. Hunkapiller, R. Bolanos, A. Delcher, I. Dew, D. Fasulo, M. Flanigan, L. Florea, A. Halpern, S. Hannenhalli, S. Kravitz, S. Levy, C. Mobarry, K. Reinert, K. Remington, J. Abu-Threideh, E. Beasley, K. Biddick, V. Bonazzi, R. Brandon, M. Cargill, I. Chandramouliswaran, R. Charlab, K. Chaturvedi, Z. Deng, V. Di Francesco, P. Dunn, K. Eilbeck, C. Evangelista, A. E. Gabrielian, W. Gan, W. Ge, F. Gong, Z. Gu, P. Guan, T. J. Heiman, M. E. Higgins, R. R. Ji, Z. Ke, K. A. Ketchum, Z. Lai, Y. Lei, Z. Li, J. Li, Y. Liang, X. Lin, F. Lu, G. V. Merkulov, N. Milshina, H. M. Moore, A. K. Naik, V. A. Narayan, B. Neelam, D. Nusskern, D. B. Rusch, S. Salzberg, W. Shao, B. Shue, J. Sun, Z. Wang, A. Wang, X. Wang, J. Wang, M. Wei, R. Wides, C. Xiao, C. Yan, et al. 2001. The sequence of the human genome. Science 2911304-1351. [DOI] [PubMed] [Google Scholar]
- 68.Witman, G. B., J. Plummer, and G. Sander. 1978. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J. Cell Biol. 76729-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu, Y. E., Y. Zhang, E. Unni, C. R. Shirley, J. M. Deng, L. D. Russell, M. M. Weil, R. R. Behringer, and M. L. Meistrich. 2000. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc. Natl. Acad. Sci. USA 974683-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zammarchi, E., C. Calzolari, M. S. Pignotti, P. Pezzati, E. Lignana, and A. Cama. 1993. Unusual presentation of the immotile cilia syndrome in two children. Acta Paediatr. 82312-313. [DOI] [PubMed] [Google Scholar]
- 71.Zariwala, M., P. G. Noone, A. Sannuti, S. Minnix, Z. Zhou, M. W. Leigh, M. Hazucha, J. L. Carson, and M. R. Knowles. 2001. Germline mutations in an intermediate chain dynein cause primary ciliary dyskinesia. Am. J. Respir. Cell Mol. Biol. 25577-583. [DOI] [PubMed] [Google Scholar]
- 72.Zariwala, M. A., M. R. Knowles, and H. Omran. 2007. Genetic defects in ciliary structure and function. Annu. Rev. Physiol. 69423-450. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, Z., I. Kostetskii, W. Tang, L. Haig-Ladewig, R. Sapiro, Z. Wei, A. M. Patel, J. Bennett, G. L. Gerton, S. B. Moss, G. L. Radice, and J. F. Strauss III. 2006. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol. Reprod. 74751-759. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, Z., R. Sapiro, D. Kapfhamer, M. Bucan, J. Bray, V. Chennathukuzhi, P. McNamara, A. Curtis, M. Zhang, E. J. Blanchette-Mackie, and J. F. Strauss III. 2002. A sperm-associated WD repeat protein orthologous to Chlamydomonas PF20 associates with Spag6, the mammalian orthologue of Chlamydomonas PF16. Mol. Cell. Biol. 227993-8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao, M., C. R. Shirley, Y. E. Yu, B. Mohapatra, Y. Zhang, E. Unni, J. M. Deng, N. A. Arango, N. H. Terry, M. M. Weil, L. D. Russell, R. R. Behringer, and M. L. Meistrich. 2001. Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice. Mol. Cell. Biol. 217243-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zito, I., S. M. Downes, R. J. Patel, M. E. Cheetham, N. D. Ebenezer, S. A. Jenkins, S. S. Bhattacharya, A. R. Webster, G. E. Holder, A. C. Bird, D. E. Bamiou, and A. J. Hardcastle. 2003. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J. Med. Genet. 40609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.