Abstract
Phosphoinositide 3-kinase (PI3K) participates in extracellular signal-regulated kinase 1 and 2 (ERK1-2) activation according to signal strength, through unknown mechanisms. We report herein that Gab1/Shp2 constitutes a PI3K-dependent checkpoint of ERK1-2 activation regulated according to signal intensity. Indeed, by up- and down-regulation of signal strength in different cell lines and through different methods, we observed that Gab1/Shp2 and Ras/ERK1-2 in concert become independent of PI3K upon strong epidermal growth factor receptor (EGFR) stimulation and dependent on PI3K upon limited EGFR activation. Using Gab1 mutants, we observed that this conditional role of PI3K is dictated by the EGFR capability of recruiting Gab1 through Grb2 or through the PI3K lipid product PIP3, according to a high or weak level of receptor stimulation, respectively. In agreement, Grb2 siRNA generates, in cells with maximal EGFR stimulation, a strong dependence on PI3K for both Gab1/Shp2 and ERK1-2 activation. Therefore, Ras/ERK1-2 depends on PI3K only when PIP3 is required to recruit Gab1/Shp2, which occurs only under weak EGFR mobilization. Finally, we show that, in glioblastoma cells displaying residual EGFR activation, this compensatory mechanism becomes necessary to efficiently activate ERK1-2, which could probably contribute to tumor resistance to EGFR inhibitors.
Phosphoinositide 3-kinase (PI3K) and the Ras/extracellular signal-regulated protein kinases 1 and 2 (ERK1-2) are essential signaling pathways regulating biological and pathophysiological responses to growth factors, cytokines, or hormones. Each of these pathways is activated by specific mechanisms involving the recruitment of particular adaptor proteins: classically, Grb2 and Shc mediate Ras activation by mobilizing the Ras nucleotide exchange factor Sos, while larger docking proteins, such as insulin receptor substrate-1 or Grb2-associated binder-1 (Gab1), promote PI3K stimulation by providing binding sites for PI3K regulatory subunits (26).
Numerous data suggest the existence of intensive cross talks between these two pathways, yet their molecular interconnections remain incompletely defined. First, it is well accepted that oncogenic Ras mutants can stimulate PI3K through physical association, which activates PI3K-dependent antiapoptotic processes and thereby contributes to Ras mutant oncogenicity (5, 9). Conversely, PI3K was proposed to participate in ERK1-2 activation, since different research groups reported that PI3K inhibition prevents ERK1-2 stimulation, notably in response to growth factors (6, 24, 35, 36). Nevertheless, it is now well established that the canonical Shc/Grb2/Sos module links receptor tyrosine kinase (RTK) stimulation to Ras activation independently of PI3K (26, 31). Consequently, an open question is whether or not PI3K actively participates in ERK1-2 stimulation, a debate regularly fed by novel discordant reports showing that PI3K inhibition has or does not have consequences for ERK1-2 activation in response to growth factor or cytokine stimulation (16, 18, 21, 27, 34). Thus far, only a few studies have attempted to address this controversy at the molecular level (6, 24, 35). Their conclusions converged on the idea that PI3K plays a conditional, signal strength-dependent function in ERK1-2 activation. Actually, these studies showed that PI3K is necessary for ERK1-2 activation when cells are stimulated by a small number of RTK molecules (for example, in the case of cells stimulated with a low dose of growth factor or when cells express a few molecules of a given RTK). In contrast, in the case of cells abundantly expressing a certain RTK and stimulated with a high dose of its ligand, PI3K becomes unnecessary for ERK1-2 activation (6, 35). This suggested that a compensatory PI3K-dependent mechanism is capable of promoting efficient ERK1-2 activation under weak RTK stimulations, a mechanism becoming redundant with other ERK1-2-activating pathways under strong stimulation. To date, the nature of this PI3K-dependent process has remained largely hypothetical. One of these two studies suggested that the redundant pathway sequentially involved PI3K, protein kinase C (PKC), and Raf-1, the latter kinase being located downstream of Ras in the ERK1-2 cascade (6). This view found support in reports demonstrating that some PKC isoforms (e.g., PKCζ) constitute downstream targets of PI3K and are capable of stimulating Raf-1 independently of or in parallel to Ras (1, 28). Nevertheless, the concept that, under RTK stimulation, PKC could promote ERK1-2 activation more efficiently than Grb2/Sos is far from being well accepted. In addition, this concept was not supported by the second study, which proposed that a compensatory mechanism existed upstream of Ras, even though this mechanism has remained unknown (35).
To clarify this situation, the aim of this work was to identify the molecular processes that allow PI3K to participate in ERK1-2 activation according to signal intensity. Taking advantage of related cell lines with striking differences regarding PI3K requirement, we observed in this study that a major signal strength- and PI3K-dependent checkpoint of ERK1-2 activation is located upstream of Ras. This led us to investigate the role of the Grb2-bound adapter Gab1 and its downstream partner, the ubiquitous SH2 domain-containing protein tyrosine phosphatase Shp2. We and others have previously reported that Gab1 and Shp2 actively participate in Ras/ERK1-2 stimulation in response to epidermal growth factor (EGF), through different mechanisms (2, 14, 19, 30, 38) (see references 8 and 20 for reviews).
We report herein that, under various experimental conditions, the recruitment of Gab1 and Shp2 by the EGF receptor (EGFR) follows a signal strength-dependent regulation by PI3K strictly identical to that exerted by the lipid kinase on Ras/ERK1-2 activation. Moreover, we observed that this combined conditional regulation is in fact driven by the EGFR capability of differentially mobilizing Gab1, which is achieved through Grb2-mediated interactions during strong receptor stimulation and through an association between PI3K lipid products and the Gab1 PH domain under low EGFR stimulation. This identifies the Gab1/Shp2 signaling module as a signal strength-dependent effector of PI3K involved in Ras/ERK1-2 activation, which provides for the first time a molecular explanation of the conditional role of PI3K in this pathway. Most importantly, we observed that this compensatory mechanism, capable of promoting Ras activation only in response to a low level of EGFR stimulation, becomes indispensable to promote full ERK1-2 activation in tumor cells displaying a residual level of EGFR stimulation. Considering that this residual activation contributes to the resistance of certain tumors to EGFR pharmacological inhibition (12, 15, 17), this study provides a molecular illustration of this resistance and suggests that targeting PI3K-mediated recruitment of Gab1/Shp2 may therefore resensitize those cells to anticancer therapies directed against EGFR.
MATERIALS AND METHODS
Materials.
Human recombinant EGF was from Peprotech. The monoclonal anti-β-actin (catalog no. A5441), anti-Flag tag (catalog no. F3165), and polyclonal anti-phospho-ERK1-2 (catalog no. M8159) antibodies were from Sigma. The polyclonal anti-Gab1 (catalog no. sc-9049), anti-ERK2 (catalog no. sc-154), anti-Shp2 (catalog no. sc-280), anti-EGFR (catalog no. sc-03-G), anti-Grb2 (catalog no. sc-255), anti-ErbB3 (catalog no. sc-285), and monoclonal anti-Myc epitope tag (clone 9E10) antibodies were from Santa Cruz Biotechnology Inc. The monoclonal anti-pan-Ras antibody (catalog no. OP40) was from Oncogene Research Products, and the monoclonal anti-Shc (catalog no. 06-203) and anti-V5 (catalog no. 46-0705) antibodies were from Upstate Biotechnology and Invitrogen, respectively. The monoclonal 4G10 antiphosphotyrosine (anti-pTyr) antibody was produced in the laboratory. The monoclonal antihemagglutinin (anti-HA) epitope tag (clone 12CA5) was from Roche. The polyclonal anti-phospho-Akt (catalog no. 9271S) and anti-phospho-Gab1-Tyr627 (catalog no. 3231) and the monoclonal anti-phospho-EGFR (Tyr 1068; catalog no. 2234) antibodies were from Cell Signaling Technology. Horseradish peroxidase-conjugated antibodies anti-mouse (catalog no. 7076), -rabbit (catalog no. W4011), and -goat (catalog no. sc-2020) immunoglobulin G (IgG) were from Cell Signaling Technology, Promega, and Santa Cruz Biotechnology, respectively. LY294002 and wortmannin were from Sigma. AG1478 was from BioMol. Small interfering RNA (siRNA) targeting Gab1, Grb2, and Shp2 and negative-control siRNA were purchased from Qiagen (catalog no. 2654736, 300328, 2225902, and 1027310, respectively). Cell culture reagents were from Invitrogen.
Expression plasmids and adenoviruses.
The pcDNA3 plasmids encoding Myc-tagged wild-type (WT) Gab1, Gab1ΔPH (deleted of its PH domain), or Gab1 mutated on its Shp2 binding site (Gab1-Y627F); the pcDNA6/V5-HisA plasmids encoding V5-tagged WT Shp2 or catalytically inactive Shp2 (Shp2-C459G); and the plasmid encoding HA-tagged Ras were already described (10, 19, 36). The pBat-Flag plasmids encoding WT Gab1 and Gab1 lacking the sites for binding to Grb2 SH3 domains (Gab1ΔGrb2) were kindly provided by U. Schaeper (Berlin, Germany) (25). For adenovirus production, the sequences encoding HA-tagged EGFR or green fluorescent protein were subcloned into the plasmid pShuttle. Recombinant adenoviruses were then obtained according to the pAdEasy homologous recombination system (11).
Cell culture, stimulation, transfection, and adenoviral infection.
U87MG, U251MG (human malignant glioma), HEK293 (human embryonic kidney), and Vero (monkey kidney) cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. Before stimulation, cells were incubated overnight in serum-free medium (24 h in the case of U87MG cells). Stimulations were performed using 5 or 50 ng/ml of EGF for 5 min, as indicated. Before stimulation, cells were treated for 15 min with 100 nM wortmannin or 20 μM LY294002 or with 100 nM AG1478 for 30 min, as indicated.
For transient transfections of Vero cells, subconfluent 100-mm plates were incubated with 4 ml of DMEM containing 2 μg of total DNA, 16 μl of Lipofectamine, and 24 μl of Plus reagent (Invitrogen). After 3 h of incubation, the medium was changed to DMEM supplemented with 10% fetal bovine serum and antibiotics. For transient transfections of HEK 293 cells, subconfluent 100-mm plates were treated with a mixture containing 6 μl of FuGene6 reagent (Roche) with 2 μg of total DNA, according to the manufacturer's instructions. The mixture was then added to cells incubated in normal culture medium for 24 h, before serum deprivation and stimulation. For adenoviral infection, subconfluent plates were incubated for 24 h with 15 infectious particles per cell and then serum starved overnight and stimulated or not stimulated with EGF as indicated.
siRNA transfection.
Forty-percent-confluent 100-mm plates of HEK 293 cells were treated with a transfection mixture containing 3.4 ml DMEM and 600 μl Opti-MEM (Invitrogen) containing siRNA (30 nM in a 4-ml final volume) and 9 μl Oligofectamine (Invitrogen). Cells were incubated in the transfection mixture for 4 h, and then DMEM supplemented with 30% serum and antibiotics (2 ml) was added to each plate, and cells were again incubated for 24 h and then serum starved overnight and stimulated or not stimulated with EGF as indicated.
Cell lysis, immunoprecipitations, His-tag affinity precipitation, and immunoblotting.
Cells were scrapped off in lysis buffer containing 20 mM Tris, pH 7.4, 150 mM NaCl, 10 mM EDTA, 10% glycerol, 1% Nonidet P-40, 10 μg/ml each of aprotinin and leupeptin, and 1 mM orthovanadate. After shaking for 15 min at 4°C, followed by a 13,000 × g centrifugation for 15 min, soluble material was incubated with the appropriate antibody for 1 h at 4°C. The antigen-antibody complexes were incubated with protein A- or protein G-Sepharose (Amersham) for 1 h and then collected by centrifugation and washed three times with lysis buffer containing 0.1% Nonidet P-40, 0.1 mM orthovanadate, and 1 μg/ml each of aprotinin and leupeptin. Proteins were then resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblotting using a standard procedure. Blots were developed using enhanced chemiluminescence (Amersham Biosciences, Inc.). For direct immunoblotting analysis of crude cell lysates, cells were directly scrapped off in electrophoresis sample buffer and then boiled and processed for immunoblotting.
To measure ERK1-2 phosphorylation in transfected cells, cells were transfected with 0.2 μg of DNA encoding Myc-His-tagged ERK1 and 1.8 μg of the indicated effector protein. After stimulation, cells were harvested in lysis buffer supplemented with 300 mM NaCl. Soluble material was incubated with 30 μl of ProBond Resin (Invitrogen) and then washed with lysis buffer supplemented with 5 mM imidazole. Samples were then analyzed by immunoblotting.
Ras-GTP and GST-Grb2 affinity precipitation assay.
The glutathione S-transferase (GST) fusion protein containing the Ras-binding domain (RBD) of Raf-1 (GST-RBD) (4) and the GST fusion protein containing the Grb2 protein (GST-Grb2) were expressed in Escherichia coli and extracted using glutathione-Sepharose beads (Sigma) with a standard procedure (3, 37). For Ras-GTP pulldown experiments, cells were cotransfected with 0.5 μg and 1.5 μg of plasmids encoding HA-tagged Ras and the indicated construct (Gab1 and Shp2), respectively. Cells were then serum starved and scraped off in 1 ml lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 10 mM MgCl2, 0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride, and 1 μg/ml each of aprotinin and leupeptin. Cleared lysates were incubated at 4°C for 30 min with 3 μg of GST-RBD bound to glutathione-Sepharose beads. Beads were washed three times in lysis buffer and then boiled in standard electrophoresis sample buffer, and proteins were resolved by SDS-polyacrylamide gel electrophoresis and analyzed by immunoblotting. For Gab1 precipitation with GST-Grb2, the same protocol was applied, except that cells were transfected with 2 μg of Gab1 constructs, and the cleared lysates were incubated for 2 h with 3 μg of GST-Grb2.
Flow cytometry quantification of cell surface EGFR.
Cultured cells were rinsed and then harvested in phosphate-buffered saline (PBS) containing 2 mM EDTA, followed by fixation in 1% formaldehyde for 30 min at room temperature and then incubation for 30 min in PBS containing 1% bovine serum albumin. Cells were then incubated for 1 h with a monoclonal antibody directed against the extracellular portion of EGFR (clone LA1; Upstate Biotechnology) diluted 1/100 in PBS-1% bovine serum albumin. After being washed in PBS, cells were stained with rhodamine phycoerythrine-conjugated anti-mouse IgG (Caltag Laboratories). After being washed with PBS, cells were analyzed with a Coulter XL4 flow cytometer, with excitation and emission settings at 488 nm and 575 nm, respectively.
Membrane fractions.
Membrane fractions were prepared as previously described (36). Briefly, cells were scrapped off in hypotonic lysis buffer and then Dounce homogenized. The homogenate was centrifuged at 100,000 × g for 1 h. The pellet was dissolved in 1% Triton X-100 lysis buffer and the insoluble material spun out. This was taken as the solubilized membrane fraction.
RESULTS
Gab1/Shp2 and Ras/ERK1-2 follow the same cell line-specific regulation by PI3K.
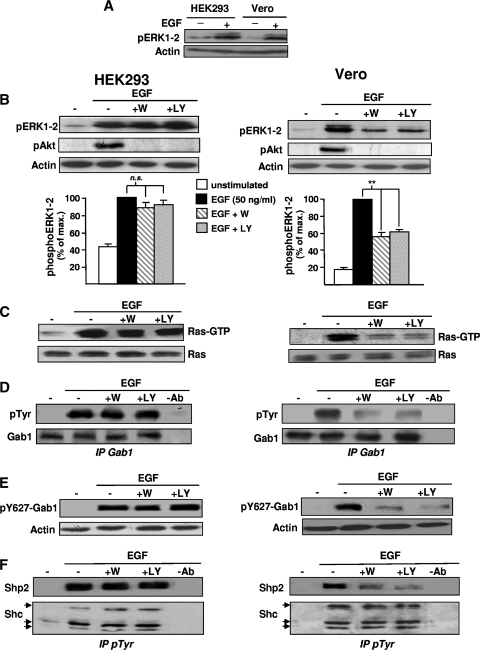
We addressed the question of PI3K intervention in the Ras/ERK1-2 pathway by taking advantage of two related cell lines from primate kidney, namely, HEK293 and Vero cells, which, despite displaying similar levels of EGF-induced ERK1-2 activation (Fig. 1A), are characterized by a profound difference regarding PI3K requirement for ERK1-2 stimulation. Indeed, Fig. 1B (left panels) shows that preincubation of HEK293 cells with PI3K inhibitors has no significant effect on ERK1-2 phosphorylation induced by 50 ng/ml EGF. In contrast, in Vero cells (right panels), both inhibitors reduced by >50% the ERK1-2 phosphorylation induced by the same EGF dose. As PI3K inhibitors abolished Akt phosphorylation in both cell lines (Fig. 1B), this disparity could be allocated to differential contributions of PI3K to ERK1-2 activation. Then, to localize which step of the ERK1-2 pathway was differentially blocked by PI3K inhibitors, we studied Ras activation, which represents a central position in this pathway. As shown in Fig. 1C, PI3K inhibitors strongly attenuate EGF-induced Ras activation in Vero cells (right panels), but they have no apparent effect in HEK293 cells (left panels).
FIG. 1.
Ras/ERK1-2 and Gab1/Shp2 conform to the same cell line-dependent regulation by PI3K. (A) HEK293 or Vero cells were stimulated with EGF (50 ng/ml) as indicated and then lysed. Lysate aliquots were then subjected to anti-phospho-ERK1-2 (pERK-1-2) and antiactin immunoblotting to control gel loading (bottom). (B to F) HEK293 (left panels and graph) or Vero (right panels and graph) cells. (B) Cells were treated with wortmannin (+W) or LY294002 (+LY) before stimulation with EGF (50 ng/ml) as indicated and then lysed. Lysate aliquots were then subjected to anti-pERK1-2, anti-phospho-Akt (pAkt), and antiactin immunoblotting to control efficiency of PI3K inhibitors and gel loading, respectively. For the graphs, measurements of ERK1-2 activation were obtained by a computer-assisted procedure. Data represent means ± standard errors from six experiments. **, P value of <0.01. ns, no significant difference (paired t test). (C) Cells were treated as described for panel B and then processed for a Ras-GTP precipitation assay using the GST fusion protein containing the RBD of Raf1 (GST-RBD). The amount of activated Ras (Ras-GTP) associated with GST-RBD was determined by anti-Ras immunoblotting (top). Bottom, anti-Ras immunoblotting of corresponding lysates was performed to control the amount of total Ras in each sample. (D) Cells were treated as described for panel B and then lysed and processed for Gab1 immunoprecipitation (IP Gab1) followed by anti-pTyr (top) and anti-Gab1 (bottom) immunoblotting. (E) Cell lysate aliquots processed as described for panel B were directly subjected to anti-phospho-Gab1-Tyr627 (top) and antiactin (bottom) immunoblotting. (F) Cells were treated as described for panel B and then processed for immunoprecipitation of pTyr-containing signaling complexes, using anti-pTyr antibody (IP pTyr). This was followed by anti-Shp2 (top) and anti-Shc immunoblotting as a control (bottom). Lanes −Ab: a mock immunoprecipitation was performed from a 5-min-stimulated cell lysate without adding the primary antibody. Unless otherwise indicated, the results shown are representative of at least three independent experiments.
To identify the molecular explanation of this difference, we compared the mechanisms of Ras activation in these two cell lines. First, we verified that the EGF-induced association of the Shc/Grb2/Sos complex is insensitive to PI3K inhibition in both HEK293 and Vero cells (data not shown). We then studied the Gab1/Shp2 module, an alternate signaling pathway involved in Ras activation. In response to the same EGF dose, Gab1 phosphorylation (total in Fig. 1D or specific to the Shp2-binding site Tyr627 in Fig. 1E) was found to be resistant to PI3K inhibition in HEK293 (left panels) but strongly sensitive in Vero cells (right panels). To confirm this observation, we then examined the influence of PI3K inhibition on the EGF-induced recruitment of Shp2, the tyrosine phosphatase which mediates Ras activation downstream of Gab1. Shp2 recruitment to activated signaling complexes was assessed by measuring Shp2 amounts in anti-pTyr immunoprecipitates prepared from control or EGF-treated cells. Figure 1F shows that Shp2 recruitment induced by EGF is highly sensitive to PI3K inhibitors in Vero cells, whereas it is insensitive to the same compounds in HEK293 cells stimulated with an identical EGF concentration. The coimmunoprecipitation of Shc (Fig. 1F, bottom) (66, 52, and 46 kDa) in these anti-pTyr immunoprecipitates was used as a control for a signaling protein recruited by EGFR independently of PI3K (Fig. 1F, bottom). Taken together, the Fig. 1 results indicate that Gab1/Shp2 and Ras/ERK1-2 are both regulated by PI3K in Vero cells and that both are independent of the lipid kinase in HEK293 cells. This suggested that when Ras/ERK1-2 requires PI3K, it is because Gab1/Shp2 recruitment is dependent on PI3K.
Ras/ERK1-2 and Gab1/Shp2 become dependent on PI3K in HEK293 cells under submaximal EGFR activation.
Since PI3K participates in ERK1-2 activation upon weak, but not high, EGFR stimulation (6, 35), we hypothesized that this effect could rely on a PI3K-dependent mobilization of Gab1/Shp2 as a function of signal intensity. In favor of this view, as monitored by immunoblotting and by flow cytometry, EGFR expression and density at the cell surface were higher in HEK293 cells than in Vero cells (Fig. 2A and B). Moreover, none of those cells was found to express ErbB3 (Fig. 2C), supporting the idea of a quantitative difference, rather than a qualitative one, between those two cell lines in terms of EGF-induced signaling. Therefore, we hypothesized that under stimulation with a maximal (saturating) EGF dose (50 ng/ml), a larger number of EGFR molecules was engaged in HEK293 cells than in Vero cells. This was confirmed by monitoring EGFR autophosphorylation in these two cell lines, since this phosphorylation is undetectable in Vero cells when blots are processed to detect a nonsaturating signal in HEK293 cells (Fig. 2B, pEGFR).
FIG. 2.
Ras/ERK1-2 and Gab1/Shp2 in concert become sensitive to PI3K inhibition in HEK293 cells stimulated with a submaximal EGF dose. (A) EGFR expression at the surfaces of HEK293 and Vero cells was determined by flow cytometry as described in Materials and Methods. (B) Vero and HEK293 cells were stimulated or not stimulated with 5 or 50 ng/ml of EGF as indicated and then lysed and subjected to anti-phospho-ERK1-2 (pERK1-2), anti-phospho-EGFR (pEGFR), anti-EGFR, and antiactin immunoblotting to control gel loading. (C) Lysate aliquots of Vero, HEK293, and A431 cells were subjected to anti-ErbB3 and antitubulin immunoblotting, as indicated. (D) HEK293 cells were treated for 15 min with wortmannin (+W) or LY294002 (+LY) before stimulation with EGF (5 ng/ml) as indicated and then lysed. Lysate aliquots were then subjected to anti-pERK1-2 and antiactin immunoblotting. For the bottom graph, measurements of ERK1-2 phosphorylation were obtained by a computer-assisted procedure. (E) HEK293 cells were treated as described for panel D and then lysed and processed for Ras-GTP precipitation using GST-RBD. The amount of activated Ras (Ras-GTP) associated with the beads was determined by anti-Ras immunoblotting (top). Bottom, anti-Ras immunoblotting of corresponding lysates was performed to control the amount of total Ras in each sample. (F) Cells treated as described for panel D were processed for Gab1 immunoprecipitation (IP Gab1), followed by anti-pTyr (top) and -Gab1 (bottom) immunoblotting. (G) Cell lysates were subjected to anti-phospho-Gab1-Tyr627 (top) and antiactin (bottom) immunoblotting. (H) Cells treated as described for panel D were processed for immunoprecipitation of pTyr-containing signaling complexes by use of anti-pTyr antibody (IP pTyr), followed by anti-Shp2 (top) and anti-Shc (bottom) immunoblotting. Lanes −Ab: a mock immunoprecipitation was performed from a 5-min-stimulated cell lysate without adding the primary antibody. (I) HEK293 cells were incubated or not incubated with the indicated compounds, as shown, before stimulation with EGF and then lysis. Lysate aliquots were then subjected to immunoblotting with anti-pERK1-2, anti-phospho-Gab1-Tyr627 (pY627-Gab1), anti-phospho-EGFR (pEGFR), and antiactin antibodies.
To investigate whether this disparity in EGFR density could explain the differential requirements for PI3K in these two cell lines, we modulated the signal strength in each cell line. First, to dampen down the signal intensity in HEK293 cells, we stimulated them with a submaximal EGF dose (5 ng/ml). For a control, we verified that, in comparison with 50 ng/ml EGF, 5 ng/ml led to a decrease of EGFR autophosphorylation (Fig. 2B, pEGFR). Interestingly, this weaker EGF stimulation gave rise to a similar level of ERK1-2 activation (Fig. 2B, pERK1-2), suggesting that cells can effectively set up a mechanism to compensate for lower EGFR engagement and efficiently activate ERK1-2. Most interestingly, under this condition of lower stimulation, all markers of Gab1/Shp2 mobilization (Fig. 2F to H) became strongly sensitive to PI3K inhibition as well as all parameters of Ras/ERK1-2 activation (Fig. 2D and E). Consequently, these data showed that Gab1/Shp2 recruitment becomes dependent on PI3K under weak EGFR engagement. This regulation thus appears to be identical to that exerted by PI3K on the Ras/ERK1-2 pathway. In addition, we tried another approach to limit EGFR activation under maximal EGF dose, by partially inhibiting EGFR activity with AG1478. As expected, upon stimulation with 50 ng/ml EGF, a 50 nM AG1478 treatment results in a strong decrease of EGFR phosphorylation (Fig. 2I, pEGFR). More interestingly, ERK1-2 phosphorylation is poorly affected by such an EGFR inhibition, and Gab1 phosphorylation was reduced but not suppressed. Most importantly, under this inhibition, the phosphorylations of ERK1-2 and Gab1 become strongly dependent on PI3K activity (Fig. 2I, pERK1-2 and pGab1). This indicates that, under limited EGFR engagement, efficient ERK1-2 activation can occur through a PI3K-dependent mechanism. It also suggested that Gab1 was involved in this process since PI3K inhibitors suppressed the residual phosphorylation of both ERK1-2 and Gab1 that was not blocked by the EGFR inhibitor.
Ras/ERK1-2 and Gab1/Shp2 become independent on PI3K in Vero cells overexpressing the EGFR.
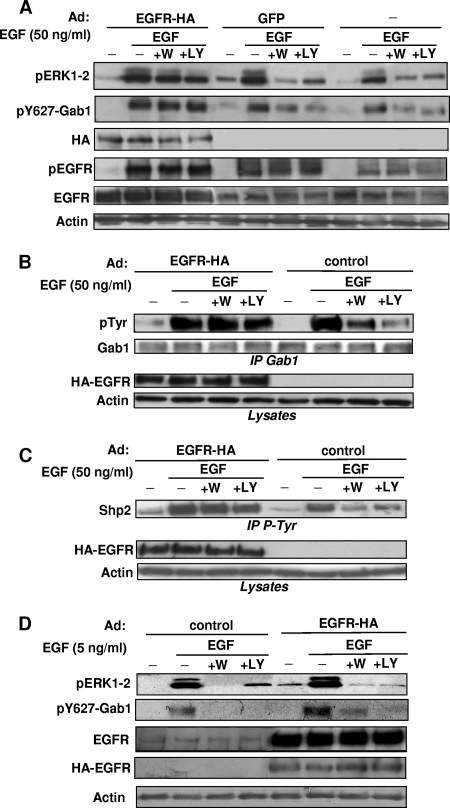
To further test the involvement of Gab1/Shp2 in the conditional role of PI3K in Ras/ERK activation, we modulated the signal strength in Vero cells by overexpressing EGFR with adenoviral infection. We set up conditions where the EGFR was only moderately overexpressed, to prevent its constitutive (EGF-independent) activation. Under these conditions, EGF-induced ERK1-2 activation becomes largely resistant to PI3K inhibition (Fig. 3A, top panel). Most interestingly, Gab1/Shp2 mobilization also becomes totally independent of PI3K activation in Vero cells overexpressing EGFR, as shown by monitoring total Gab1 tyrosine phosphorylation (Fig. 3B, top panel) or its specific phosphorylation on Tyr627 (Fig. 3A, second panel). The EGF-induced Shp2 recruitment is also turned into a PI3K-independent event under this condition of EGFR overexpression (Fig. 3C). Interestingly, upon similar EGFR overexpression and 5 ng/ml EGF stimulation, ERK1-2 activation as well as Gab1 phosphorylation remains sensitive to PI3K inhibitors, showing that submaximal EGF stimulation, despite nonlimiting EGFR expression, requires PI3K to promote Gab1 phosphorylation and ERK (Fig. 3D).
FIG. 3.
Ras/ERK1-2 and Gab1/Shp2 in concert become resistant to PI3K inhibition in Vero cells overexpressing EGFR due to adenoviral infection. (A) Vero cells were infected or not infected with adenoviruses (Ad) encoding HA-tagged EGFR (EGFR-HA) or green fluorescent protein (GFP) at a concentration of 15 infectious particles per cell, as indicated. Following stimulation with EGF (50 ng/ml) and treatment with Wortmannin (+W) or LY294002 (+LY) as indicated, cells were lysed and subjected to immunoblotting with the indicated antibodies. (B) After infection with adenoviruses encoding HA-tagged EGFR or no infection (control), Vero cells were treated with wortmannin (+W) or LY294002 (+LY) and then stimulated with EGF (50 ng/ml), as indicated. Cells were then lysed and processed for Gab1 immunoprecipitation (IP Gab1), followed by anti-pTyr and anti-Gab1 immunoblotting, as indicated. The middle and bottom panels show corresponding lysates immunoblotted with anti-HA (HA-EGFR) and anti-actin antibodies. (C) Vero cells were infected, treated, and stimulated as described for panel B and then processed for immunoprecipitation of pTyr-containing signaling complexes (IP pTyr), followed by anti-Shp2 immunoblotting. The middle and bottom panels show corresponding lysates immunoblotted with the indicated antibodies. The results shown are representative of at least two independent experiments performed in duplicate. (D) Vero cells were infected or not infected with adenoviruses encoding HA-EGFR and then treated as described for panel A, except that they were stimulated with 5 ng/ml EGF. Lysate aliquots were then directly subjected to immunoblotting with the indicated antibodies.
Taken together, the results for Fig. 2 and 3 show that both Gab1/Shp2 and Ras/ERK1-2 become, in concert, dependent on PI3K in HEK293 cells stimulated with a submaximal EGF dose, and both are converted, again in concert, into PI3K-independent events in Vero cells overexpressing the EGFR. This attested that Gab1/Shp2 is recruited by PI3K as a function of signal intensity, a conditional regulation that is strictly identical to that exerted by PI3K on the Ras/ERK1-2 pathway. Consequently, this strongly suggested that Ras/ERK1-2 activation depends on PI3K only when Gab1/Shp2 recruitment requires PI3K, which occurs only under weak EGFR engagement.
Is Gab1/Shp2 redundant with other pathways for Ras activation under strong stimulation?
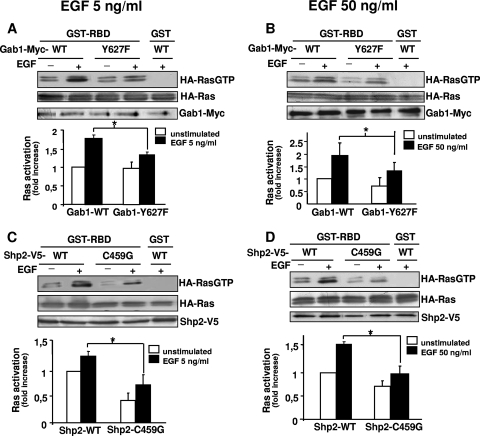
Although the involvement of Gab1/Shp2 in Ras/ERK1-2 activation is well accepted (8, 20), our above-stated observation that its PI3K-dependent recruitment correlates with ERK1-2 sensitivity to PI3K inhibitors raises the possibility that Gab1/Shp2 might be necessary to efficiently activate Ras under weak EGFR stimulation but is redundant with other Ras-activating pathways under strong stimulation. To test this possibility, we compared, under different levels of EGFR stimulation, the requirement for Gab1/Shp2 in Ras and ERK1-2 activation. This was performed with HEK293 cells stimulated with 5 or 50 ng/ml EGF. To block Gab1/Shp2, we used the dominant-negative mutants Gab1-Y627F (lacking the Shp2-binding site) and Shp2-C459G (catalytically inactive). As shown in Fig. 4, transfection of each mutant significantly repressed Ras activation in response to 5 (Fig. 4A and C) or to 50 (Fig. 4B and D) ng/ml EGF. Similar effects were observed on ERK1 phosphorylation (Fig. 5A and B). To further confirm these observations, we used an siRNA approach to inhibit Shp2 or Gab1 expression and to assess the consequences on ERK1-2 activation. As shown in Fig. 5C, loss of Shp2 or Gab1 expression resulted in a significant inhibition of ERK1-2 phosphorylation under low as well as under high EGF stimulation. Altogether, these data indicate that the Gab1/Shp2 module is necessary for Ras activation under low and high levels of EGFR activation, attesting the nonredundancy of Gab1/Shp2 with other pathways promoting Ras activation (i.e., Grb2/Sos), even under strong EGFR mobilization. Thus, this showed that Gab1/Shp2 is necessary for Ras activation independently of signal strength, which suggested that the mode of recruitment of Gab1/Shp2 could be regulated by PI3K as a function of signal intensity.
FIG. 4.
The Gab1/Shp2 module is required for complete Ras activation in HEK293 cells, even under high EGF concentrations. (A) HEK293 cells were cotransfected with plasmids encoding HA-tagged Ras and Myc-tagged Gab1 constructs (WT or mutated on the Shp2 binding site [Y627F]). After stimulation with EGF (5 ng/ml) as indicated, cells were lysed and incubated with beads bound to a GST-RBD fusion protein or GST alone, as indicated. The amount of activated HA-tagged Ras associated with the beads was determined by anti-HA immunoblotting (Ras-GTP, top). The middle and bottom panels show anti-HA-Ras and Gab1-Myc immunoblotting of corresponding lysates to control the expression levels of HA-Ras and Gab1-Myc constructs, respectively. The graphs show densitometric measurements of HA-Ras-GTP. Data represent the means ± standard errors from three experiments. *, P value of <0.05 (paired t test). (B) Same experiment as for panel A, except that cells were stimulated with 50 ng/ml EGF. (C) Same experiment as for panel A, except that cells were cotransfected with plasmids encoding, instead of Gab1 constructs, V5-tagged Shp2 constructs (WT or mutated on the catalytic site [C459G]), as indicated. (D) Same experiment as for panel C, except that cells were stimulated with 50 ng/ml EGF.
FIG. 5.
The Gab1/Shp2 module is required for complete ERK1-2 activation in HEK293 cells, even under high EGF concentrations. (A) HEK293 cells were cotransfected with plasmids encoding His-Myc-tagged ERK1 and Myc-tagged Gab1 constructs (WT or mutated on the Shp2 binding site [Y627F]). After stimulation with EGF (5 or 50 ng/ml) as indicated, cells were lysed and processed for His-tag affinity precipitation, followed by anti-phospho-ERK1-2 immunoblotting. Corresponding lysates were then subjected to anti-Myc immunoblotting to control the expression level of each construct. (B) Same experiment as for panel A, except that cells were cotransfected with plasmids encoding, instead of Gab1 constructs, V5-tagged Shp2 constructs (WT or mutated on the catalytic site [C459G]), as indicated. Associated lysates were then subjected to anti-V5 and anti-Myc immunoblotting. (C) Cells were treated or not treated (−) with siRNA against Gab1, Shp2, or a scrambled sequence (control). Following cell stimulation with EGF (5 or 50 ng/ml) as shown, lysates were subjected to immunoblotting with anti-pERK1-2, -Shp2, -Gab1, and -tubulin antibodies.
The EGFR recruits Gab1 through Grb2 under strong stimulation and through the PH domain under low stimulation.
We next tried to understand why Gab1/Shp2 is regulated by PI3K according to signal strength. We focused on the mechanisms of Gab1 mobilization by the EGFR. Indeed, Gab1, constitutively bound to Grb2, can be recruited through the association of Grb2 to phosphorylated EGFR (8) and through PI3K, whose lipid product phosphatidylinositol-3,4,5-trisphosphate (PIP3) has strong affinity for the Gab1 PH domain and can attract Gab1 in the EGFR vicinity (13, 22). We hypothesized that if the EGFR recruits Gab1 through the Grb2-dependent mechanism under strong receptor stimulation and through the PI3K-dependent process under weak EGFR engagement, this could explain the signal strength-dependent function of PI3K in Ras/ERK1-2 activation. To test this hypothesis, we first compared the results indicating whether or not Gab1 translocation to the plasma membrane was sensitive to PI3K inhibitors in HEK293 cells under strong or weak EGFR activation (induced by 50 or 5 ng/ml EGF, respectively). For 50 ng/ml EGF, we observed that Gab1 was translocated to the plasma membrane independently of PI3K, suggesting that Gab1 recruitment occurs essentially through Grb2 under strong stimulation (Fig. 6A). Quite interestingly, under 5 ng/ml EGF, the intensity of Gab1 redistribution to the membrane was similar to that observed under 50 ng/ml, and this translocation was strongly reduced by PI3K inhibitors. This suggests that a fraction of Gab1 is recruited through PIP3 under low, but not high, stimulation, leading to identical Gab1 mobilizations under both conditions.
FIG. 6.
EGFR recruits Gab1 through Grb2 under strong stimulation and through its PH domain under low stimulation. (A) HEK293 cells were stimulated or not stimulated with EGF (5 or 50 ng/ml) after treatment with the PI3K inhibitors. Membrane fractions were then prepared as described in Materials and Methods, and samples were analyzed with anti-Gab1 and anti-EGFR immunoblotting to verify that equal amounts of membrane proteins were present in all samples. (B) HEK293 cells were transfected with constructs encoding Myc-tagged Gab1, either WT or deleted of its PH domain (ΔPH), as indicated. Then, following serum starvation, cells were treated or not treated with wortmannin (+W) or LY294002 (+LY), followed by stimulation with EGF (50 ng/ml) or no stimulation, as shown. Cells were then lysed and processed for Gab1-Myc immunoprecipitation, followed by anti-pTyr (top) and anti-Myc (bottom) immunoblotting. (C) Same experiment as for panel B, except that cells were stimulated with 5 ng/ml EGF. (D) HEK293 cells were transfected with constructs encoding Myc-tagged Gab1, either WT or deleted of its PH domain (ΔPH), or Flag-tagged Gab1, either WT or mutated on its Grb2-binding sites (ΔGrb2). After stimulation with EGF (50 ng/ml), cells were lysed and incubated with beads bound to GST alone (−) or GST-Grb2 protein, as indicated. The amount of Gab1 associated with the beads was determined by anti-Myc or anti-Flag immunoblotting. Bottom panels, anti-Myc and anti-Flag immunoblotting of corresponding lysates. (E) HEK293 cells were transfected with Flag-tagged WT Gab1 or Gab1-ΔGrb2, as indicated. Then, cells were treated or not treated with wortmannin (+W) or LY294002 (+LY), followed by stimulation with EGF (50 ng/ml), as shown. Cells were then lysed and processed for Gab1-Flag immunoprecipitation, followed by anti-pTyr (top) and anti-Flag (bottom) immunoblotting. (F) Same experiment as for panel E, except that cells were stimulated with 5 ng/ml EGF. The data are representative of at least two independent experiments.
To further dissect the mechanism of Gab1 recruitment, we took advantage of two Gab1 mutants, one being deleted of its PH domain (ΔPH) and the other being deficient for binding to Grb2 (ΔGrb2), and we analyzed their levels of tyrosine phosphorylation, as markers of their recruitment, under strong and weak stimulations. Quite interestingly, Fig. 6B shows that, under 50 ng/ml EGF, the level of Gab1-ΔPH phosphorylation is identical to that for WT Gab1. This indicated that, under strong EGFR stimulation, the PH domain is dispensable for efficient recruitment of Gab1. For a control, Fig. 5A also shows that Gab1-ΔPH phosphorylation is insensitive to PI3K inhibition, as expected. Quite remarkably, when cells were stimulated with 5 ng/ml EGF (Fig. 6C), Gab1-ΔPH phosphorylation was much lower than that of WT Gab1, in sharp contrast with the results obtained under 50 ng/ml EGF (Fig. 6B). This attested that, under low EGFR activation, the PH domain becomes critical to achieve an efficient Gab1 mobilization. Figure 6C also shows, as controls, that the residual phosphorylation of Gab1-ΔPH induced by 5 ng/ml EGF is insensitive to PI3K inhibition, and its level is similar to that of WT Gab1 in cells treated with PI3K inhibitors, which confirmed that the PI3K-dependent phosphorylation of WT Gab1 is due to its PH domain functionality (22, 36).
From the results shown in Fig. 6B and C, we conclude first that the Gab1 PH domain is not necessary for Gab1 recruitment under strong EGFR engagement, which is in full accordance with the fact that Gab1 is recruited independently of PI3K under strong receptor stimulation (Fig. 1B to F, left panels, 3A to C, and 6A). We also conclude that, under weak EGFR stimulation, the PH domain becomes crucial for Gab1 recruitment, which is also in total agreement with the observations that PI3K is required for Gab1 recruitment under weak mobilization of the receptor (Fig. 1B to F, right panels, 2D to H, and 6A).
Because these conclusions implied that Grb2 was necessary and sufficient for recruiting Gab1 under strong EGFR activation, we verified the ability of Gab1-ΔPH to bind Grb2. Figure 6D shows that Gab1-ΔPH was pulled down by GST-Grb2 as readily as WT Gab1, whereas, as a negative control, Gab1-ΔGrb2 was not precipitated by GST-Grb2. Therefore, this was compatible with the idea that Gab1 is essentially recruited through Grb2 under strong stimulation, which provides additional support to the above-stated conclusions.
To further strengthen these conclusions, we finally compared the recruitments of Gab1-ΔGrb2 under weak and strong EGFR activation. Quite interestingly, Fig. 6E shows that Gab1-ΔGrb2 is not phosphorylated under 50 ng/ml EGF, indicating that Gab1 recruitment is entirely dependent on Grb2 during strong EGFR activation, which fully supports the above data. In contrast, under 5 ng/ml EGF, phosphorylation of Gab1-ΔGrb2 was detected (Fig. 6F), demonstrating that Gab1 can be recruited independently of Grb2 upon weak stimulation. Moreover, Fig. 6F also shows that, under this low stimulation condition, the phosphorylation of Gab1-ΔGrb2 was abolished by PI3K inhibitors, indicating that, under weak EGFR engagement, Gab1 recruitment occurs through a PI3K-dependent process. Therefore, the interpretation of Fig. 6E and F fully supports the conclusion that interaction with Grb2 is necessary and sufficient to promote Gab1 recruitment during strong stimulation of EGFR, whereas the binding of Gab1 PH domain to PI3K lipid products is critical to efficiently mobilize the adapter under low engagement of EGFR.
Inhibition of Grb2-mediated Gab1 recruitment creates a dependence on PI3K for both ERK1-2 activation and Gab1/Shp2 recruitment.
To further strengthen the above-stated conclusions, we have tested the effects of Gab1-ΔGrb2 and Gab1-ΔPH on Ras/ERK1-2 activation. Interestingly, these constructs failed to exert a dominant-negative effect, in contrast with the Gab1-Y627F mutant (see Fig. S1 in the supplemental material). This suggests that those two mutants do not display the characteristics of dominant-negative mutants, one of the required features being the capacity to compete with the recruitment of endogenous Gab1. Indeed, as previously reported in the case of Gab1-ΔPH, it is likely that, even when Gab1-ΔGrb2 or Gab1-ΔPH is overexpressed, it cannot prevent the recruitment of endogenous Gab1 by Grb2 or by PIP3, respectively, whereas Gab1-Y627F can do it (36).
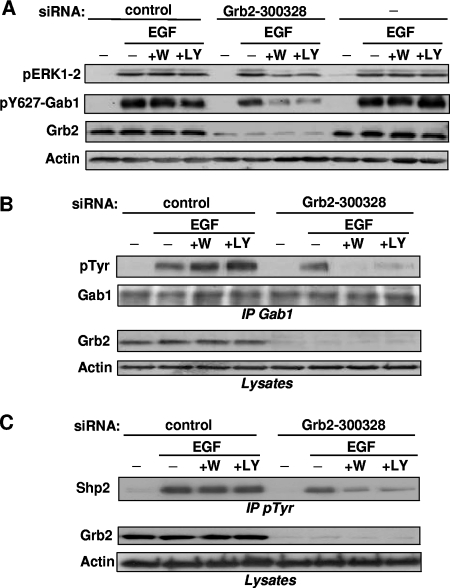
Consequently, we sought to provide additional evidence that the importance of PI3K in ERK1-2 activation is inversely related to the capacity of activated EGFR to mobilize a sufficient amount of Gab1 through Grb2. We thus performed a different strategy to block Grb2-mediated Gab1 recruitment, using siRNA to downregulate Grb2. Figure 7A (third panel) shows that a treatment of HEK293 cells with siRNA 300328 strongly reduced, but did not abolish, Grb2 expression, whereas a control siRNA had no apparent effect. For these treatments, we monitored ERK1-2 activation and Gab1/Shp2 mobilization induced by 50 ng/ml EGF in the presence of PI3K inhibitors. As a prerequisite for continuing this strategy, we set up conditions of treatment with siRNA 300328 (see Materials and Methods) which allowed a level of ERK1-2 activation comparable to that for untreated or for control siRNA-treated cells (Fig. 7A, top panel). This indicated that the residual amount of Grb2 was sufficient to promote ERK1-2 activation.
FIG. 7.
Downregulation with siRNA of Grb2-mediated Gab1 recruitment creates a dependence on PI3K for both ERK1-2 activation and Gab1/Shp2 recruitment in HEK293 cells maximally stimulated. (A) Cells were treated or not treated (−) with siRNA against Grb2 (Grb2-300328) or against a scrambled sequence (control). Following cell treatment with wortmannin (+W) or LY294002 (+LY) or no treatment (−) and then stimulation with EGF (50 ng/ml) as shown, lysates were subjected to immunoblotting with the indicated antibodies. (B) Cells were transfected with Grb-2300328 or control siRNA and then treated with PI3K inhibitors and EGF (50 ng/ml), as described above. Cell lysates were then processed for Gab1 immunoprecipitation (IP Gab1), followed by anti-pTyr and anti-Gab1 immunoblotting, as indicated. For the middle and bottom panels, corresponding lysates were immunoblotted with anti-Grb2 and antiactin immunoblotting. (C) HEK293 cells were transfected, treated, and stimulated as described for panel B and then processed for immunoprecipitation of pTyr-containing signaling complexes (IP pTyr), followed by anti-Shp2 immunoblotting (top). The middle and bottom panels show anti-Grb2 and antiactin immunoblotting of corresponding lysates. Data in this figure are representative of three independent experiments.
Therefore, for these experimental conditions of incomplete Grb2 silencing, we tested the requirement for PI3K in ERK1-2 activation and Gab1/Shp2 recruitment. Quite interestingly, ERK1-2 phosphorylation induced by 50 ng/ml EGF was severely repressed by PI3K inhibitors in cells partially deprived of Grb2 (Fig. 7A, top panel). Moreover, Gab1 tyrosine phosphorylation, either as a whole (Fig. 7B) or specific to its Shp2-binding site (Fig. 7A, second panel), as well as Shp2 recruitment monitored by Shp2 mobilization in anti-pTyr immunoprecipitates (Fig. 7C), became strongly sensitive to PI3K inhibitors in these cells, despite maximal stimulation of EGFR. Taken together, the Fig. 7 results indicate that, in HEK293 cells stimulated with the highest EGF dose, Grb2 silencing creates a dependence on PI3K for both ERK1-2 activation and Gab1/Shp2 mobilization. This further confirmed our finding that the role of PI3K in ERK1-2 activation is necessary only when the EGFR is unable to recruit, through Grb2, a sufficient amount of Gab1/Shp2.
PI3K, upstream of Gab1/Shp2, promotes efficient ERK1/2 activation in glioblastoma cells resistant to EGFR inhibition.
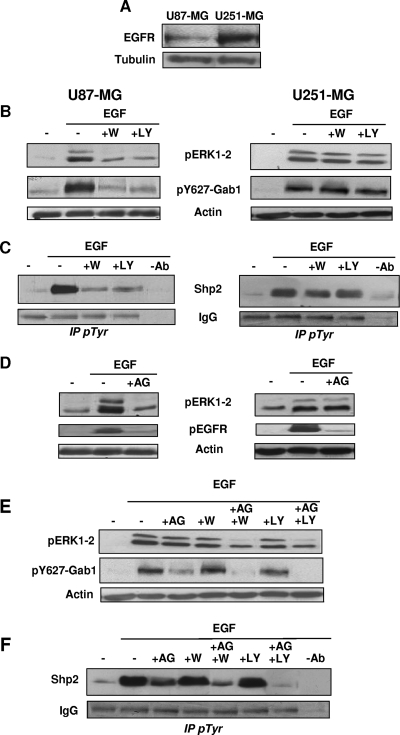
Tumors overexpressing the EGFR, notably glioblastomas, are frequently insensitive to drugs blocking EGFR kinase activity. This resistance seems to be due to an incomplete inhibitory action of these drugs, the residual EGFR activity being sufficient to promote ERK1-2 activation (15, 17). This suggests that, upon EGFR inhibition, resistant tumor cells can set up compensatory mechanisms to maintain efficient ERK1-2 activation. To define whether PI3K upstream of Gab1/Shp2 could participate in such mechanisms, we compared the human malignant glioma cell line U87MG with its close relative U251MG, which overexpresses the EGFR (32) (Fig. 8A).
FIG. 8.
PI3K, upstream of Gab1/Shp2, promotes efficient ERK1/2 activation in glioblastoma cells resistant to EGFR inhibition. (A) Aliquots of U87MG and U251MG cell lysates were analyzed by anti-EGFR and antitubulin immunoblotting. (B to D) U87MG (left panels) or U251MG (right panels) cells. (B) Cells were treated with wortmannin (+W) or LY294002 (+LY) before stimulation with EGF (50 ng/ml) and then lysed. Lysate aliquots were then subjected to immunoblotting with the indicated antibodies. (C) Cells treated as described for panel B were processed for anti-pTyr immunoprecipitation (IP pTyr), followed by anti-Shp2 immunoblotting (top) and IgG staining to control the immunoprecipitation and gel loading (bottom). (D) Serum-starved cells were treated with AG1478 before stimulation with EGF, as indicated. Lysate aliquots were then subjected to immunoblotting with the indicated antibodies. (E) U251MG cells were incubated or not incubated with the indicated compounds, as shown, before stimulation with EGF and then lysis and immunoblotting with the indicated antibodies. (F) Cells treated as described for panel E were processed for anti-pTyr immunoprecipitation, followed by anti-Shp2 immunoblotting (top) and IgG staining to control the immunoprecipitation and gel loading (bottom).
First, using PI3K inhibitors, we observed that, in response to 50 ng/ml EGF, ERK1-2 activation, as well as Gab1 phosphorylation and Shp2 recruitment, were altogether strongly dependent on PI3K activity in U87MG cells (Fig. 8B and C, left panels). In contrast, in U251MG cells, these three parameters were not significantly sensitive to PI3K inhibition (right panels). These results provide, for other cell types, a supplementary indication that Ras/ERK1-2 requires PI3K when Gab1/Shp2 recruitment is dependent of PI3K, which occurs only under weak EGFR engagement.
We then compared the consequences of EGFR inhibition on ERK1-2 activation in U87MG and U251MG cells, using AG1478, a highly specific EGFR inhibitor. Figure 8D (second row of panels) shows, as a control, that treatment of both cell lines with AG1478 led to a massive reduction of EGFR autophosphorylation. This EGFR inhibition resulted in an important reduction of ERK1-2 phosphorylation in U87MG cells (Fig. 8D, top left panel), but quite interestingly, it produced an insignificant effect on ERK1-2 activation in U251MG cells (Fig. 8D, top right panel). Thus, the latter cells seem to display the features of resistance to EGFR inhibition.
To determine whether PI3K upstream of Gab1/Shp2 could participate in this resistance, we assessed the effect of combined AG1478 and PI3K inhibitors on ERK1-2 activation, as well as on Gab1 and Shp2 recruitment, in U251MG cells. First, for AG1478 alone, Fig. 8E and F show that this compound reduced the mobilizations of both Gab1 and Shp2, but only partially, suggesting that this residual recruitment of Gab1/Shp2 could somehow participate in promoting ERK1-2 activation in the presence of AG1478. In agreement with this, and most interestingly, the EGF-induced Gab1 phosphorylation and Shp2 recruitment were nearly abrogated under the double inhibition by AG1478 and PI3K inhibitors (Fig. 8E and F). This also resulted in a dramatic reduction of ERK1-2 activation (Fig. 8E, lanes 5 and 7), a decrease which appeared much more pronounced than a simple addition of the marginal inhibitory effect of each inhibitor alone (Fig. 8E, lanes 3, 4, and 6).
Taken together, these data indicate that in glioblastoma cells overexpressing EGFR and treated with an EGFR inhibitor, PI3K becomes critical for both ERK1-2 activation and Gab1/Shp2 recruitment. This implies that in these cells, the high level of ERK1-2 activation achieved despite EGFR inhibition results from the implementation of the PI3K-dependent recruitment of Gab1/Shp2 to efficiently promote ERK1-2 activation.
DISCUSSION
ERK1-2 and PI3K are critical elements of the biological and pathophysiological functions of growth factors. In this study, we attempted to solve an open controversy regarding the cross talk between these pathways. Earlier studies showed that PI3K plays a signal strength-dependent role in ERK1-2 activation, but the mechanisms of this conditional regulation remained unclear. We addressed this question by exploring with different strategies how related cell lines can display profound differences regarding their dependence on PI3K for ERK1-2 activation. We thus obtained several instances of evidence that this difference is dictated by the ability of EGFR to recruit the Gab1/Shp2 module through two distinct mechanisms, according to signal intensity. Gab1, a major EGFR substrate, was known to be recruited by the receptor through Grb2- or PI3K-dependent processes (8, 22, 36). Our current findings indicate that each of these mechanisms is differentially utilized in the function of the EGFR stimulation level in order to promote a significant mobilization of the Gab1/Shp2 pathway under a high or low level of EGFR engagement, respectively.
Our conclusions are based on the observations that, under a variety of experimental conditions, PI3K exerts strictly identical regulations both on Ras/ERK1-2 activation and on Gab1/Shp2 mobilization. We observed first that Ras/ERK1-2 and Gab1/Shp2 are both sensitive to PI3K inhibition in Vero cells and are both resistant in HEK293 cells that contain an EGFR amount larger than that in Vero cells. Identical observations were also obtained for glioblastoma cell lines, where both ERK1-2 activation and Gab1/Shp2 recruitment appeared strongly sensitive to PI3K inhibition in U87MG cells and resistant to PI3K inhibition in U251MG cells that overexpress EGFR. In addition, by modulating the level of EGFR stimulation, we have found that both Ras/ERK1-2 and Gab1/Shp2 become, in concert, dependent on PI3K in HEK293 cells stimulated with a submaximal EGF dose and independent of PI3K in Vero cells overexpressing the EGFR due to adenoviral infection. Finally, in HEK293 cells, silencing Grb2 with siRNA created a strong dependence on PI3K, again in concert, for both ERK1/2 activation and Gab1/Shp2 mobilization. Consequently, we propose that Gab1/Shp2 might be considered the major signal strength-dependent effector of PI3K, which allows this lipid kinase to regulate the ERK1-2 pathway as a function of signal intensity.
We explored in detail why the Gab1/Shp2 module displays this feature. First, we could exclude the possibility that, under strong stimulation, Gab1/Shp2 becomes redundant with other Ras-activating pathways. Indeed, two different dominant-negative mutants of the Gab1/Shp2 module were found to impair Ras activation under both low and high EGFR stimulation. siRNA targeting of Shp2 or Gab1 expression also resulted in a significant inhibition of ERK activation under both conditions. Thus, it appears that, in parallel with other Ras-activating pathways (e.g., Grb2/Sos), Gab1/Shp2 is necessary for Ras/ERK activation independently of signal strength. Therefore, this suggested that it is the recruitment of Gab1/Shp2 that is conditionally regulated by PI3K.
Considering the possible roles of Grb2 and PI3K in Gab1 recruitment (8, 13, 22), we thus hypothesized that the EGFR could recruit Gab1 through Grb2 under strong receptor stimulation and through PI3K under weak stimulation, which could explain the conditional role of PI3K in ERK1-2 activation. Supporting this view are our observations that the same amount of Gab1 was relocalized to the plasma membrane under low and high EGFR engagement and that this translocation was highly sensitive to PI3K inhibitors only under weak EGF stimulation. This suggested that a fraction of Gab1 is recruited through PIP3 under low, but not high, stimulation, leading to identical Gab1 mobilizations under both conditions.
Moreover, our study of the behaviors of Gab1 mutants deficient for binding to Grb2 or deleted of the PH domain fully supports this mechanism. Actually, we observed that Gab1 interaction with Grb2 was necessary and sufficient to promote Gab1 recruitment during high EGFR stimulation and that, under low EGFR engagement, the Gab1 PH domain, which displays specific affinity for PIP3, became essential for efficient Gab1 mobilization.
Interestingly, we also observed that Gab1-ΔGrb2 was not phosphorylated under high EGFR stimulation, whereas it appeared moderately phosphorylated under low EGFR mobilization. This is in accordance with our hypothesis, since we propose that, under low EGFR stimulation, most Gab1 recruitment occurs through its PH domain. Therefore, Gab1-ΔGrb2 should be recruited, which is what we actually observed in Fig. 6F. In contrast, under high EGFR mobilization, Gab1 is recruited essentially through Grb2; therefore, Gab1-ΔGrb2 cannot be recruited, as shown in Fig. 6E. So this behavior of Gab1-ΔGrb2 appears to be consistent with the mechanism described in this paper.
Nevertheless, one may still wonder why, under high stimulation, Gab1-ΔGrb2 is not recruited through its PH domain. This same question can be asked in the case of endogenous Gab1, in which redistribution is not driven by PI3K lipid products under strong stimulation (Fig. 6A). This is certainly not due to a lack of PI3K activation under high stimulation, since Akt is readily phosphorylated under this condition (Fig. 1B). A possible explanation could be that strong stimulations create a massive recruitment of signaling proteins (including Gab1) in the EGFR vicinity, which could prevent access to the EGFR by additional Gab1 molecules potentially recruitable through PIP3. Another possibility could be that, at a high EGF dose, Gab1 molecules associated with EGFR through Grb2 also bind PIP3. The latter association probably does not influence the recruitment of these Gab1 molecules already associated with Grb2/EGFR (as suggested by Fig. 6A, high-dose results), but it could block the access of additional Gab1 molecules to PIP3, which therefore cannot be recruited. However, further studies are required to address in detail these questions relevant to the respective roles of Grb2 and PIP3 in the dynamic of Gab1 recruitment.
Additional evidence in favor of our hypothesis is brought by the fact that, in HEK293 cells, impairing Grb2-mediated Gab1 recruitment with Grb2 siRNA created a strong dependence on PI3K for Gab1/Shp2 mobilization, even under full stimulation by EGF. These experiments thus attested that, when Grb2-mediated recruitment is not sufficient to promote efficient Gab1 mobilization (i.e., when EGFR is weakly autophosphorylated or when Grb2 function is impaired), the EGFR must utilize PI3K to attract Gab1 in its vicinity. Consequently, this leads to the conclusion that the conditional role of PI3K in Gab1 recruitment, and hence in Ras/ERK1-2 activation, is in fact dictated by the ability of EFGR to differentially recruit Gab1 in function of its level of stimulation.
Most likely, this regulation occurs according to the following mechanism (schematized in Fig. 9). Under strong stimulation, the EGFR can mobilize Gab1/Shp2 independently of PI3K since a large number of EGFR molecules are autophosphorylated under this condition. This certainly generates numerous Grb2-binding sites on EGFR molecules, docking sites which are capable of massively recruiting Grb2-bound Gab1. In contrast, under low stimulation, fewer EGFR molecules are autophosphorylated. Consequently, the number of Grb2-binding sites is limited. The EGFR must therefore utilize an alternate process to effectively mobilize Gab1/Shp2. The possibility of attracting Gab1 through PI3K lipid products presumably represents a process more efficient, stoichiometrically, than recruiting Gab1 through Grb2. Indeed, while each Grb2-docking site of EGFR can associate with one molecule of Grb2-bound Gab1 (at best), PI3K activation in the vicinity of EGFR certainly results in a large number of PIP3 molecules, preferential ligands for the Gab1 PH domain. This induces a very efficient recruitment of Gab1, as shown by previous studies reporting that PI3K plays a pivotal role in EGFR-mediated Gab1 recruitment (22, 36).
FIG. 9.
Model of conditional activation of Ras/ERK by PI3K. See Discussion for comments.
Finally, an important progression brought by this study resides in the observation that this PI3K-mediated compensatory mechanism can also be used by tumor cells that are resistant to EGFR inhibition to efficiently activate ERK1-2. Indeed, recent reports showed that a large proportion of tumors overexpressing EGFR, notably glioblastomas, are not clinically responsive to EGFR-targeting drugs (e.g., gefitinib) (23). This resistance may be due to the fact that these compounds fail to fully inhibit EGFR, which allows a residual EGFR activity in these cells. Apparently, this residual activity is sufficient to promote efficient ERK1-2 stimulation and tumor development (15, 17). To improve the clinical efficiency of these drugs, it thus seems essential to identify the molecular details of this tumor resistance.
This study could significantly contribute to this objective since we describe herein a compensatory mechanism for Ras/ERK1-2 activation, i.e., a mechanism from which cells can escape when signal intensity increases. Indeed, However, our results show that, when signal strength decreases, the cells are forced to utilize this PI3K-dependent process to efficiently activate Ras/ERK1-2. Most importantly, we found that this mechanism is operational in glioblastoma cells apparently resistant to EGFR inhibition, i.e., cells displaying full ERK1-2 activation in response to EGF even in the presence of an EGFR inhibitor. Indeed, we observed that PI3K inhibitors dramatically potentialized the inhibitory effect of the EGFR inhibitor on ERK1-2 activation, while they abolished the recruitment of Gab1/Shp2 (Fig. 8E and F). Moreover, this strong dependence on PI3K attests that, in cells resistant to EGFR inhibition, the PI3K-dependent Gab1/Shp2 recruitment cannot be compensated for by other ERK1-2-activating processes, strongly suggesting that these cells acquired this resistance because they had the capacity to implement this PI3K-dependent mechanism. Consequently, we assume that this mechanism could constitute a novel and very attractive pharmacological target for improving the treatment of tumors resistant to EGFR inhibition. In favor of this view, a selective inhibitor of PI3Kα was recently found to overcome the resistance of lung cancer cells to gefitinib (12), even though it is not excluded at this stage that this effect could be due to blockage of other PI3K-dependent pathways involved in tumor development (29, 33).
In addition to incomplete inhibition of the receptor, resistance of tumor cells to EGFR inhibition can be due to other mechanisms, such as secondary mutations or mobilization of other receptors. An interesting illustration of this is the findings that lung tumor cells can amplify the gene encoding the RTK Met, which then causes gefitinib resistance by driving ErbB3-dependent activation of PI3K (7), a pathway which can “bypass” the EGFR/Gab1 pathway to stimulate PI3K. However, in our study using cultured cells on which we applied short-term treatment of EGF and/or inhibitors, it is unlikely that secondary mutations of EGFR could be responsible for the insensitivity of ERK activation to EGFR inhibition since we could resensitize the cells using PI3K inhibitors. It is also improbable that other RTKs are mobilized as we did not observe any ERK activation in the absence of EGF. Conversely, whether the mechanism proposed herein could be involved in cases of EGFR inhibitor resistance due to EGFR-activating mutations or to mobilization of other receptors appears as an extremely interesting question.
In conclusion, this study should attenuate the debate on the involvement of PI3K in ERK1-2 activation by providing for the first time a molecular explanation of the signal strength-dependent function of this lipid kinase. The mechanism involved is certainly a rare example of illustration at the molecular level of how a membrane receptor can activate specific processes according to its degree of mobilization, a feature that is certainly not unique to the EGFR and that probably plays a fundamental role in the multiplicity of functions of a given RTK. Moreover, this work suggests that pharmacological targeting of PI3K-mediated Gab1/Shp2 recruitment may resensitize EGFR-overexpressing tumors displaying low sensitivity to EGFR inhibition to anticancer therapies directed against EGFR.
Supplementary Material
Acknowledgments
We thank U. Schaeper for providing the Gab1-ΔGrb2 construct, S. Roche for the plasmid encoding GST-Grb2, and C. Delmas, C. Toulas, and M. O. Jauberteau for providing the glioblastoma cell lines. We are grateful to the vector core of the University Hospital of Nantes, supported by the Association Française contre les Myopathies, for providing the adenovirus vectors.
This work was supported by grants from Association pour la Recherche sur le Cancer and Ligue Nationale Contre le Cancer (comités de Aude, Haute-Garonne, Tarn et Tarn-et-Garonne).
Footnotes
Published ahead of print on 19 November 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Chou, M. M., W. Hou, J. Johnson, L. K. Graham, M. H. Lee, C. S. Chen, A. C. Newton, B. S. Schaffhausen, and A. Toker. 1998. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr. Biol. 81069-1077. [DOI] [PubMed] [Google Scholar]
- 2.Cunnick, J. M., J. F. Dorsey, T. Munoz-Antonia, L. Mei, and J. Wu. 2000. Requirement of SHP2 binding to Gab1 for MAPK activation in response to LPA and EGF. J. Biol. Chem. 27513842-13848. [DOI] [PubMed] [Google Scholar]
- 3.Dance, M., A. Montagner, A. Yart, B. Masri, Y. Audigier, B. Perret, J. P. Salles, and P. Raynal. 2006. The adaptor protein Gab1 couples the stimulation of vascular endothelial growth factor-2 to the activation of phosphoinositide 3-kinase. J. Biol. Chem. 28123285-23295. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij, J., and J. L. Bos. 1997. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene 14623-625. [DOI] [PubMed] [Google Scholar]
- 5.Downward, J. 2003. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 311-22. [DOI] [PubMed] [Google Scholar]
- 6.Duckworth, B. C., and L. C. Cantley. 1997. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. Dependence of signal strength. J. Biol. Chem. 27227665-27670. [DOI] [PubMed] [Google Scholar]
- 7.Engelman, J. A., K. Zejnullahu, T. Mitsudomi, Y. Song, C. Hyland, J. O. Park, N. Lindeman, C. M. Gale, X. Zhao, J. Christensen, T. Kosaka, A. J. Holmes, A. M. Rogers, F. Cappuzzo, T. Mok, C. Lee, B. E. Johnson, L. C. Cantley, and P. A. Janne. 2007. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 3161039-1043. [DOI] [PubMed] [Google Scholar]
- 8.Gu, H., and B. G. Neel. 2003. The “Gab” in signal transduction. Trends Cell Biol. 13122-130. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, S., A. R. Ramjaun, P. Haiko, Y. Wang, P. H. Warne, B. Nicke, E. Nye, G. Stamp, K. Alitalo, and J. Downward. 2007. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell 129957-968. [DOI] [PubMed] [Google Scholar]
- 10.Hanna, N., A. Montagner, W. H. Lee, M. Miteva, M. Vidal, M. Vidaud, B. Parfait, and P. Raynal. 2006. Reduced phosphatase activity of SHP-2 in LEOPARD syndrome: consequences for PI3K binding on Gab1. FEBS Lett. 5802477-2482. [DOI] [PubMed] [Google Scholar]
- 11.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 952509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihle, N. T., G. Paine-Murrieta, M. I. Berggren, A. Baker, W. R. Tate, P. Wipf, R. T. Abraham, D. L. Kirkpatrick, and G. Powis. 2005. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol. Cancer Ther. 41349-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isakoff, S. J., T. Cardozo, J. Andreev, Z. Li, K. M. Ferguson, R. Abagyan, M. A. Lemmon, A. Aronheim, and E. Y. Skolnik. 1998. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 175374-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh, M., Y. Yoshida, K. Nishida, M. Narimatsu, M. Hibi, and T. Hirano. 2000. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 203695-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassouf, W., C. P. Dinney, G. Brown, D. J. McConkey, A. J. Diehl, M. Bar-Eli, and L. Adam. 2005. Uncoupling between epidermal growth factor receptor and downstream signals defines resistance to the antiproliferative effect of Gefitinib in bladder cancer cells. Cancer Res. 6510524-10535. [DOI] [PubMed] [Google Scholar]
- 16.Kiyatkin, A., E. Aksamitiene, N. I. Markevich, N. M. Borisov, J. B. Hoek, and B. N. Kholodenko. 2006. Scaffolding protein GAB1 sustains epidermal growth factor-induced mitogenic and survival signaling by multiple positive feedback loops. J. Biol. Chem. 28119925-19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, B., C. M. Chang, M. Yuan, W. G. McKenna, and H. K. Shu. 2003. Resistance to small molecule inhibitors of epidermal growth factor receptor in malignant gliomas. Cancer Res. 637443-7450. [PubMed] [Google Scholar]
- 18.Mattoon, D. R., B. Lamothe, I. Lax, and J. Schlessinger. 2004. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montagner, A., A. Yart, M. Dance, B. Perret, J. P. Salles, and P. Raynal. 2005. A novel role for Gab1 and SHP2 in EGF-induced Ras activation. J. Biol. Chem. 2805350-5360. [DOI] [PubMed] [Google Scholar]
- 20.Neel, B. G., H. Gu, and L. Pao. 2003. The ′Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28284-293. [DOI] [PubMed] [Google Scholar]
- 21.Qiao, M., P. Shapiro, R. Kumar, and A. Passaniti. 2004. Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3-kinase/ERK-dependent and Akt-independent signaling pathway. J. Biol. Chem. 27942709-42718. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues, G. A., M. Falasca, Z. Zhang, S. H. Ong, and J. Schlessinger. 2000. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 201448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin, B. P., and A. Duensing. 2006. Mechanisms of resistance to small molecule kinase inhibition in the treatment of solid tumors. Lab. Investig. 86981-986. [DOI] [PubMed] [Google Scholar]
- 24.Rubio, I., and R. Wetzker. 2000. A permissive function of PI3K in Ras activation mediated by inhibition of GTPase-activating proteins. Curr. Biol. 101225-1228. [DOI] [PubMed] [Google Scholar]
- 25.Schaeper, U., N. H. Gehring, K. P. Fuchs, M. Sachs, B. Kempkes, and W. Birchmeier. 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 1491419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103211-225. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt, E. K., S. Fichelson, and S. M. Feller. 2004. PI3 kinase is important for Ras, MEK and Erk activation of Epo-stimulated human erythroid progenitors. BMC Biol. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schönwasser, D. C., R. M. Marais, C. J. Marshall, and P. J. Parker. 1998. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol. 18790-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sergina, N. V., M. Rausch, D. Wang, J. Blair, B. Hann, K. M. Shokat, and M. M. Moasser. 2007. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi, Z. Q., D. H. Yu, M. Park, M. Marshall, and G. S. Feng. 2000. Molecular mechanisms for the Shp-2 tyrosine function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol. 201526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sondermann, H., S. M. Soisson, S. Boykevisch, S. S. Yang, D. Bar-Sagi, and J. Kuriyan. 2004. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell 119393-405. [DOI] [PubMed] [Google Scholar]
- 32.Stea, B., R. Falsey, K. Kislin, J. Patel, H. Glanzberg, S. Carey, A. A. Ambrad, E. J. Meuillet, and J. D. Martinez. 2003. Time and dose-dependent radiosensitization of the glioblastoma multiforme U251 cells by the EGF receptor tyrosine kinase inhibitor ZD1839 (′Iressa'). Cancer Lett. 20243-51. [DOI] [PubMed] [Google Scholar]
- 33.Toker, A., and M. Yoeli-Lerner. 2006. Akt signaling and cancer: surviving but not moving on. Cancer Res. 663963-3966. [DOI] [PubMed] [Google Scholar]
- 34.Wandzioch, E., C. E. Edling, R. H. Palmer, L. Carlsson, and B. Hallberg. 2004. Activation of the MAP kinase pathway by c-Kit is PI-3 kinase dependent in hematopoietic progenitor/stem cell lines. Blood 10451-57. [DOI] [PubMed] [Google Scholar]
- 35.Wennström, S., and J. Downward. 1999. Role of phosphoinositide 3-kinase in activation of Ras and mitogen-activated protein kinase by epidermal growth factor. Mol. Cell. Biol. 194279-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yart, A., M. Laffargue, P. Mayeux, S. Chretien, C. Peres, N. K. Tonks, S. Roche, B. Payrastre, H. Chap, and P. Raynal. 2001. A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of Ras and mitogen-activated protein kinases by epidermal growth factor. J. Biol. Chem. 2768856-8864. [DOI] [PubMed] [Google Scholar]
- 37.Yart, A., S. Roche, R. Wetzker, M. Laffargue, N. K. Tonks, P. Mayeux, H. Chap, and P. Raynal. 2002. A function for phosphoinositide 3-kinase beta lipid products in coupling beta gamma to Ras activation in response to lysophosphatidic acid. J. Biol. Chem. 27721167-21178. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, S. Q., W. Yang, M. I. Kontaridis, T. G. Bivona, G. Wen, T. Araki, J. Luo, J. A. Thompson, B. L. Schraven, M. R. Philips, and B. G. Neel. 2004. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13341-355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.