Abstract
β-Catenin functions as a transcriptional regulator in Wnt signaling. Its function is regulated by a specific destruction system. Plakoglobin is a close homologue of β-catenin in mammalian cells and is regulated in a similar fashion. When β-catenin or plakoglobin is exogenously expressed in cells, endogenous β-catenin is stabilized, which complicates estimation of the transcriptional activities of exogenously expressed proteins. To facilitate the design of experiments aimed at investigating the transcriptional activities of β-catenin and plakoglobin, we utilized F9 cells in which we knocked out endogenous β-catenin and/or plakoglobin by gene deletion and exogenously expressed wild-type and mutant β-catenin and/or plakoglobin. We show that C-terminally deleted β-catenin, but not plakoglobin, has a strong dominant-negative effect on transcription without altering the nuclear accumulation of β-catenin. Moreover, we show that Wnt-3a activation of LEF/T-cell factor (TCF)-dependent transcription depends on β-catenin but not on plakoglobin. Using chimeras of β-catenin and plakoglobin, we demonstrate that plakoglobin has the potential to function in transcriptional regulation but is not responsible for Wnt-3a signaling in F9 cells. Our data show that preferential nuclear accumulation of β-catenin is not necessarily linked to its transcriptional activity. We also clearly demonstrate that plakoglobin is insufficient for LEF/TCF-dependent transcriptional activation by Wnt-3a in F9 cells.
Plakoglobin was originally discovered as a cytoplasmic component of two distinct intercellular junctions, the adherens junction and the desmosome (8). cDNA cloning revealed that plakoglobin is highly homologous to β-catenin, another cytoplasmic protein found in adherens junctions but not in desmosomes (8, 23, 31). Plakoglobin binds to the cytoplasmic domains of classical cadherins and desmosomal cadherins in adherens junctions and desmosomes, respectively (2, 17, 21). In adherens junctions, β-catenin and plakoglobin compete for binding to classical cadherins (2, 10).
β-Catenin and its Drosophila homologue, Armadillo, are also part of the Wnt signaling pathway, which is involved in cell proliferation and cell fate determination at various stages of development (30, 41). In the absence of a Wnt signal, nonjunctional β-catenin is actively ubiquitinated and destroyed by the proteasomal system (1). Targeting of nonjunctional β-catenin for destruction is dependent on phosphorylation of N-terminal serine/threonine residues by a complex including adenomatous polyposis coli, axin, and glycogen synthase kinase 3β (44). In the presence of a Wnt signal, however, the phosphorylation complex is inactivated and the intracellular level of β-catenin increases. Accumulated cytoplasmic β-catenin then enters the nucleus, where it activates the transcription of Wnt-responsive genes through a complex with LEF/T-cell factor (TCF) proteins (4, 12, 26).
It has been reported that plakoglobin binds to LEF-1 with an affinity similar to that of β-catenin (12, 35). This plakoglobin/LEF-1 complex, however, is inefficient in forming a ternary complex with the LEF-1 DNA-binding sequence (45). In accordance with these data, plakoglobin possesses LEF/TCF-dependent transcriptional activity (20), although the activity is lower than that of β-catenin. Furthermore, β-catenin-knockout mice show early embryonic lethality, likely due to Wnt signaling defects, suggesting that plakoglobin cannot substitute for this signaling function of β-catenin (13). It remains to be elucidated, however, whether plakoglobin functions in transcription in response to a Wnt signal.
A meticulous comparison between β-catenin and plakoglobin transcriptional activities has been difficult to accomplish because these proteins are regulated at the level of protein degradation, making it arduous to experimentally manipulate expression in a cell with endogenous protein. The armadillo repeat domain of β-catenin/plakoglobin interacts with adenomatous polyposis coli (14) and axin (3, 11, 15, 16, 27), which form a complex that targets β-catenin/plakoglobin for proteasome-dependent destruction. Overexpression of this armadillo repeat domain depletes the β-catenin/plakoglobin destruction complex, which prevents their degradation and activates LEF/TCF-dependent transcription. Thus, exogenous expression of a membrane-tethered form of β-catenin or plakoglobin increases LEF/TCF-dependent transcriptional activity through an increase in endogenous β-catenin/plakoglobin (24, 25). On the other hand, upregulation of transcriptional activity by membrane-tethered β-catenin or plakoglobin led to the suggestion that the major function of β-catenin/plakoglobin might be to interact with LEF/TCF in the cytoplasm to relieve the repressor activity of these transcriptional regulators. This idea, however, has been abandoned. Recently, a detailed analysis suggested that nuclear β-catenin (or Armadillo in Drosophila) is required for the transcriptional response to a Wnt signal (7, 38, 40).
Since the armadillo repeat domain directly interacts with LEF/TCF, it is possible that overexpression of this domain depletes LEF/TCF and prevents LEF/TCF-dependent transcription. In fact, it was reported that expression of C-terminally deleted β-catenin prevents LEF/TCF-dependent transcription (45). Thus, exogenous expression of β-catenin or plakoglobin might regulate LEF/TCF-dependent transcription both positively and negatively in the presence of endogenous β-catenin/plakoglobin. In other words, the presence of endogenous β-catenin/plakoglobin prevents the precise measurement of transcriptional activity mediated by exogenously expressed forms of these proteins. β-Catenin/plakoglobin-deficient cancer cell lines and RNA interference technology have been used to overcome this problem (7, 20). In most cases, however, a complete loss of β-catenin/plakoglobin expression was not accomplished. Even in Drosophila studies, functional analysis of Armadillo has not been successful in a complete loss-of-function background because of embryonic lethality (38, 40). Thus, for precise analysis of the signaling activity of exogenously expressed β-catenin and plakoglobin, we need β-catenin/plakoglobin-null cells.
F9 cells are derived from mouse teratocarcinoma (32) and are known to be responsive to a Wnt-3a signal (34). TCF-4 is expressed in these cells as a main partner of β-catenin (our unpublished observation). We recently succeeded in disrupting the genes encoding β-catenin and plakoglobin in F9 cells using gene-targeting technology (10). Since almost all of the exons encoding β-catenin and/or plakoglobin were deleted by our targeting vectors, we obtained cells that were completely null for β-catenin and/or plakoglobin. To date, we have reported on the adhesive activity of β-catenin-null (βT) and β-catenin/plakoglobin-null (BPD) F9 cells (10). For this study, we generated plakoglobin-null (PgD) F9 cells. Here we use this collection of β-catenin- and/or plakoglobin-null F9 cells to define the roles of β-catenin and plakoglobin in LEF/TCF-dependent transcription.
Expression of membrane-tethered β-catenin/plakoglobin in BPD cells confirmed that cytoplasmic/nuclear free β-catenin is required for the transcriptional activity mediated by these molecules. Comparison of F9, βT, PgD, and BPD cells showed that β-catenin but not plakoglobin is required for their response to a Wnt-3a signal. BPD cell derivatives expressing β-catenin, plakoglobin, and various mutant forms of these molecules showed that β-catenin possesses higher transcriptional activity than plakoglobin and that a C-terminal deletion mutant of β-catenin, but not that of plakoglobin, has a strong dominant-negative effect on LEF/TCF-dependent transcriptional activity. Close comparison of subcellular localization and transcriptional activity revealed that preferential nuclear accumulation of β-catenin is not necessarily coupled to the transcriptional activation. Functional analysis of β-catenin/plakoglobin chimeric molecules demonstrated that plakoglobin and its mutant molecules fail to activate LEF/TCF-dependent transcription in the presence of Wnt-3a.
MATERIALS AND METHODS
Expression vectors and isolation of stable transfectants.
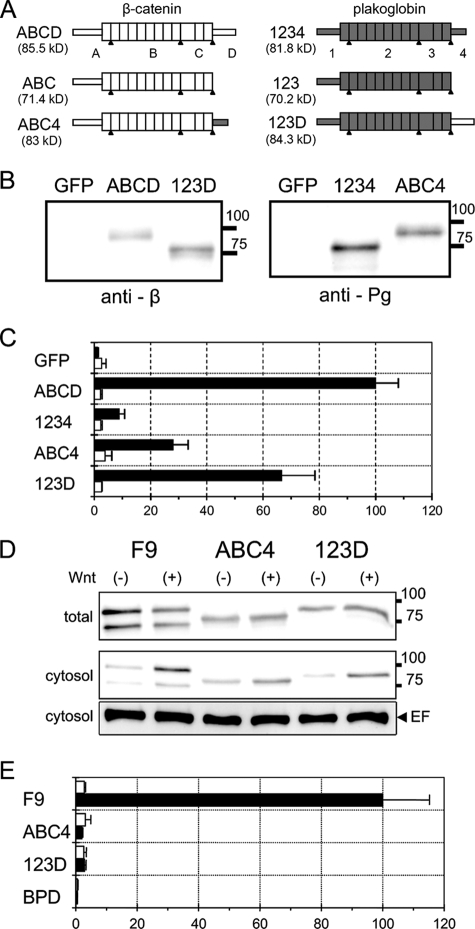
To construct the expression vectors for β-catenin, plakoglobin, and their mutant and chimeric molecules, we initially generated two cDNAs, β-ABCD and Pg-1234, using PCR. A, B, C, and D represent the amino-terminal α-catenin-binding domain including Arm1, the cadherin-binding domain including Arm2 to -9, the domain including Arm10 to -12, and the C-terminal domain, respectively. 1, 2, 3, and 4 represent similar domains in plakoglobin (see Fig. 7). In β-ABCD, KpnI, XbaI, NdeI, and SalI sites were generated at 10 base pairs before the initiation codon, at Ser184-Arg185, at Thr653-Ala655, and just after the stop codon, respectively. XbaI, NspV, and NdeI are boundaries of A-B, B-C, and C-D, respectively. In Pg-1234, KpnI, XbaI, NspV, NdeI, and SalI sites were generated at 12 base pairs before the initiation codon, at Ser175-Arg176, at Ile505-Asn507, at Thr643-Ala645, and just after the stop codon, respectively. XbaI, NspV, and NdeI are boundaries of domains 1-2, 2-3, and 3-4, respectively. Amino acid sequences of β-catenin and plakoglobin were not affected in β-ABCD and Pg-1234, since we used silent mutations for the generation of restriction enzyme sites.
FIG. 7.
LEF/TCF-dependent transcriptional activity in BPD cells transiently expressing β-catenin/plakoglobin chimeric molecules. (A) Schematic representation of the deletion and chimeric constructs of β-catenin and plakoglobin. The proteins are conceptually divided into four regions (see Materials and Methods for details). ABCD and 1234 are intact β-catenin and plakoglobin, respectively. ABC and 123 are C-terminal deleted β-catenin and plakoglobin, respectively. In ABC4 and 123D, the C-terminal domain of β-catenin and plakoglobin were interchanged. Portions of β-catenin and plakoglobin are represented as open and shaded boxes, respectively. The expected molecular mass of each protein is shown in parentheses. The apparent molecular masses of molecules including ABC and 123 are a little higher and lower, respectively, than the expected ones. (B) Expression of β-catenin/plakoglobin chimeras in BPD cells. Cells were transfected with expression vectors for β-catenin (ABCD), plakoglobin (1234), and two chimeric molecules, ABC4 and 123D. As a control, the expression vector for GFP was used. Western blots of total cell lysates were probed with anti-β-catenin (anti-β) or antiplakoglobin (anti-Pg) antibodies. The positions of molecular weight markers (in thousands) are indicated on the right. (C) Effect of β-catenin/plakoglobin chimeric molecules on TOPflash (closed bars) or FOPflash (open bars) luciferase reporter activity. Cells were transiently cotransfected with pTOPflash (or FOPflash) and pCMV-renilla luciferase reporters and, where indicated, with expression vectors for β-catenin (ABCD), plakoglobin (1234), and the chimeric molecules, ABC4 and 123D. The value for BPD cells transfected with β-catenin (ABCD) was set to 100. Error bars indicate standard deviations. (D) Expression of β-catenin and plakoglobin in wild-type F9 cells and of 123D and ABC4 in BPD transfectants. Cells were treated with conditioned medium from L cells expressing Wnt-3a (+) or with that from parental L cells (−) for 15 h. In the upper panel (total) and the middle panel (cytosol), Western blots of total cell lysate and cytosolic fraction, respectively, were probed with a mixture of anti-β-catenin and antiplakoglobin antibodies. The positions of molecular weight markers (in thousands) are indicated on the right. Eukaryotic elongation factor 2 (EF) levels were used as an internal control (lower panel). (E) Effect of Wnt-3a on TOPflash luciferase reporter activity in F9, BPD, and BPD cells stably expressing ABC4 and 123D. Cells were transiently transfected with pTOPflash and pCMV-renilla luciferase reporters and then treated with conditioned medium from L cells expressing Wnt-3a (black bars) or with that from parental L cells (white bars) for 15 h. The value for F9 cells treated with Wnt-3a was set at 100.
pCAG-neo-KS was used for construction of all expression vectors described below. In this vector, a multiple cloning site (KpnI-EcoRV-XbaI-SalI/HincII) was inserted into the EcoRI site of pCAGGSneodelEcoRI (28). For expression of wild-type β-catenin, pCAG-βABCD was constructed by inserting a β-ABCD fragment between the KpnI and SalI sites of pCAG-neo-KS. A similar method was used in constructing pCAG-Pg1234. To construct pCAG-mβ for expression of stabilized mutant β-catenin, the KpnI-PmaCI fragment of pCAG-βABCD was replaced with that of pEF-mβF (37). To construct pCAG-mPg for stabilized mutant plakoglobin, mutations that change Ser24, Ser28, Thr32, and Ser36 to alanine were introduced into pCAG-Pg1234 using the QuikChange XL site-directed mutagenesis kit (Stratagene) and a complementary primer set (5′-CGACGCGGGCATCCACGCCGGCGTCAATGCCTGTGTGCCCGCTGT-3′ [bold letters show mutated nucleotides]). To construct the expression vectors for chimeric molecules pCAG-ABC4 and pCAG-123D, the NdeI-SalI fragments of pCAG-βABCD and pCAG-Pg1234 were interchanged. To construct the expression vectors for C-terminally deleted β-catenin (pCAG-βABC) and plakoglobin (pCAG-Pg123), the NdeI-HincII fragments of pCAG-βABCD and pCAG-Pg1234 were removed. In this case, the ligation of blunted ends generated an artificial stop codon just after the connection. An NdeI-SalI/HincII fragment corresponds to domain D of β-catenin and domain 4 of plakoglobin. To construct the expression vectors for membrane-tethered β-catenin (pCAG-Emβ) and plakoglobin (pCAG-EmPg), we initially created an expression vector for E-cadherin, pCAG-EM2. In pCAG-EM2, a BglII-XbaI fragment of pBATEM2 (29) which contains the whole E-cadherin cDNA was inserted between the EcoRV and XbaI sites of pCAG-neo-KS. The BglII site was blunted before ligation. To construct pCAG-Emβ, a ClaI-SalI fragment of pCAG-EM2 corresponding to the E-cadherin C-terminal region was replaced with the SalI-XhoI fragment of pmBCII, which contains the whole cDNA for the stabilized mutant β-catenin. PmBCII was generated by inserting stabilizing mutations in pBCII, which contains β-catenin full-length cDNA in which the initiation codon was replaced with an SalI site (37). The ClaI site in pCAG-EM2 and the SalI site in the pmBCII fragment were blunt ended before ligation. To construct pCAG-EmPg, the ClaI-SalI fragment of pCAG-EM2 was replaced with the ClaI-SalI fragment of a pCAG-mPg1234 derivative in which the initiation codon of plakoglobin was changed to a ClaI site by PCR. pCAG-NGFP, a green fluorescent protein (GFP) expression vector, was kindly provided by M. Matsuda (22). The pTOPflash and pFOPflash reporter plasmids were kindly provided by M. van de Wetering and H. Clevers (19).
For the isolation of stable transfectants, expression vectors were transfected into BPD cells using the Lipofectamine 2000 system (GIBCO BRL). Cells were subjected to G418 selection at 400 μg/ml for 2 weeks. At least five clones were isolated for each transfectant, and the one which showed a common phenotype was finally chosen as the representative clone.
Cells and culture conditions.
Mouse L fibroblast cells, F9 teratocarcinoma, cells and their derivatives were cultured in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal calf serum (GIBCO BRL). Culture dishes or coverslips for F9 cells and their derivatives were precoated with 0.2% gelatin for 15 min.
Plakoglobin-null F9 cells (PgD cells) were generated by gene targeting. The targeting vector for the plakoglobin gene and the method of gene targeting have been described in our previous report (10). Following electroporation and selection with G418, three clones among 272 clones analyzed contained the correct recombination events. Hybridization with an internal ribosome entry site probe showed that these clones lacked random integration events. Clone E8 was selected for the subsequent analysis. In the course of subcloning E8, one subclone (N6) in which the expression of plakoglobin polypeptide was not detected was isolated. Southern blot analysis demonstrated that both alleles of the plakoglobin gene were disrupted in this clone. The drug resistance genes in disrupted alleles were removed using the Cre-pac method, and the G418-sensitive subclone, N6C, was isolated and used as plakoglobin-null (PgD) cells.
The generation of β-catenin-null F9 cells (βT cells) and β-catenin/plakoglobin-null F9 cells (BPD cells) has been described in our previous report (10). Mouse L cells that secrete Wnt-3a were kindly provided by S. Takada (33). To prepare conditioned medium of L cells and L cells expressing Wnt-3a, 106 cells in a 10-cm dish were cultured for 3 days. After the medium was changed, cells were cultured for one more day and the supernatant diluted 1/4.
Transient transfection and luciferase assay.
F9 cells or their derivatives (24-well plates) were transfected with 1.0 to 1.1 μg plasmid with the Lipofectamine 2000 system (GIBCO BRL) for 5 h and then cultured in normal or conditioned medium for another 15 h. The cells were lysed for luciferase assay and Western blot assay or fixed for immuncytochemical staining. Luciferase activity was measured using a dual luciferase assay system (Promega) and a lumiphotometer. For Wnt-3a assays, cells were transfected with 1.0 μg of pTOPflash and 5 to 25 ng pCMV-renilla reporter vectors and then cultured in conditioned medium from L cells or from L cells secreting Wnt-3a. To examine the effects of β-catenin, plakoglobin, and their mutants, 0.1 or 0.5 μg of the indicated expression vectors was mixed with 0.5 μg pTOPflash (or pFOPflash) and 5 to 25 ng pCMV-renilla reporter vectors. Total DNA was adjusted to 1.1 μg with pCAG-NGFP if necessary.
Antibodies, Western blotting, and immunocytochemical analysis.
Mouse anti-β-catenin monoclonal antibody (MAb), mouse anti-γ-catenin (plakoglobin) MAb (Transduction Laboratories), and anti-eEF2 polyclonal antibody (Cell Signaling) were used for immunocytochemical and/or immunoblot analyses. As secondary antibodies for immunocytochemical analysis, Cy3-conjugated donkey anti-mouse immunoglobulin G (IgG) (Jackson Immunoresearch, West Grove, PA) was used. As secondary antibodies for use with the ECL Advance Western blotting detection kit, ECL anti-mouse IgG horseradish peroxidase-linked whole antibody (from sheep; GE Healthcare) and anti-rabbit IgG horseradish peroxidase-linked antibody (Cell Signaling) were used.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblotting were performed as previously described (18). Samples solubilized in SDS sample buffer were separated by SDS-polyacrylamide gel electrophoresis. For immunoblotting, proteins were electrophoretically transferred onto nitrocellulose sheets. Nitrocellulose membranes were then incubated with primary antibody. Antibody detection was performed using an ECL Advance Western blotting detection kit (GE Healthcare) according to the manufacturer's instructions or using an Amersham biotin-streptavidin kit.
For immunofluorescent staining, cells cultured on 15-mm gelatin-coated coverslips were washed, fixed, and then incubated with primary and secondary antibodies as previously described (18). Cells were fixed with 3.7% paraformaldehyde for 15 min. For DAPI (4′,6′-diamidino-2-phenylindole) staining, samples were incubated in 1 μg/ml DAPI in phosphate-buffered saline and washed three times in phosphate-buffered saline. Samples were embedded using a SlowFade Light antifade kit (Molecular Probes). Images were acquired using the AxioVision 4.4 equipped with a microscope (model Axioskop 2; Carl Zeiss, Inc.) and AxioCAM-cooled charge-coupled device camera with 20×/0.50NA and a 40×/0.75NA Plan-Neofluar objectives. Adjustment of brightness, contrast, color balance, and final image size were achieved using Adobe Photoshop CS.
Cell fractionation.
Cells were suspended in 150 μl of HEPES-buffered Mg2+-free saline. The cell suspension was frozen in liquid N2, thawed, and then centrifuged at 1,500 × g for 10 min. The supernatant was added to 1/3 volume of 4× SDS sample buffer and used as the cytosolic fraction.
RESULTS
Transcriptional activation by membrane-tethered β-catenin and plakoglobin.
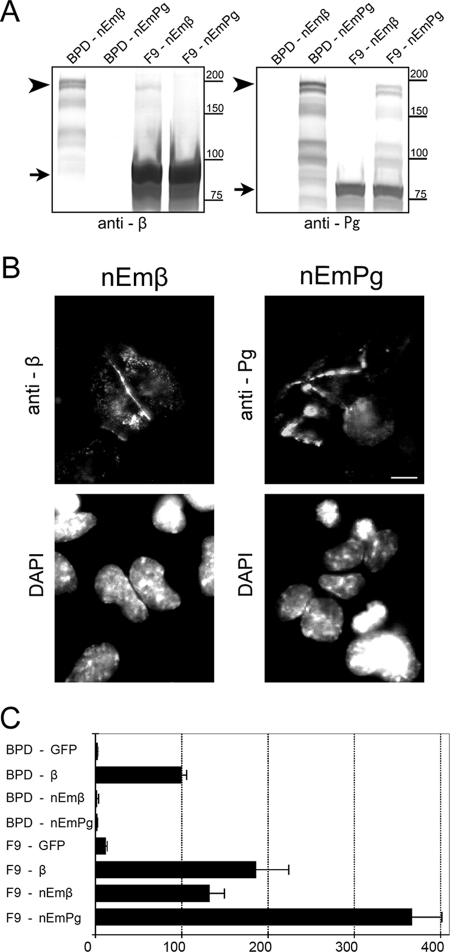
It has been shown by others that membrane-tethered plakoglobin (24) and membrane-tethered β-catenin (25) can induce a Wnt-like signal. It was recently shown that stabilization of endogenous β-catenin is required for the transcriptional activity of membrane-tethered mutants (39). It is still not known, however, whether membrane-tethered mutants are directly involved in the regulation of LEF/TCF-dependent transcriptional activity. Thus, our first goal was to address the transcriptional function of membrane-tethered β-catenin and plakoglobin using our β-catenin/plakoglobin-null BPD cells. For this purpose, we prepared constructs consisting of full-length β-catenin or plakoglobin fused to E-cadherin lacking the catenin-binding site. For these fusions, we used β-catenin and plakoglobin cDNAs mutated in the N-terminal phosphorylation sites to produce protein that is resistant to proteolytic degradation. We designated the E-cadherin-β-catenin fusion nEmβ and the E-cadherin-plakoglobin fusion nEmPg.
We transiently expressed these fusion proteins in β-catenin/plakoglobin-null BPD cells and in parental F9 cells and examined their expression and localization. Both nEmβ and nEmPg with the expected molecular weights were detected in immunoblots of extracts from BPD and F9 cells (Fig. 1A). In BPD cells, the fusion proteins formed complexes with endogenous E-cadherin and thus were stabilized. Transfection efficiency was higher in the sparse BPD cells than in closely packed F9 cells (our unpublished observation). Consequently, the expression level of each fusion protein was higher in BPD cells than in F9 cells. Immunocytochemical analysis showed that nEmβ and nEmPg were localized at cell-cell contact sites and cytoplasmic vesicles in BPD cells (Fig. 1B).
FIG. 1.
LEF/TCF-dependent transcriptional activity in F9 and BPD cells transiently expressing membrane-tethered β-catenin or plakoglobin. (A) Expression of membrane-tethered β-catenin and plakoglobin in parental F9 and BPD (β-catenin/plakoglobin-null F9) cells. F9 and BPD cells were transiently transfected with expression vectors for membrane-tethered β-catenin (nEmβ) or plakoglobin (nEmPg). Western blots of total cell lysate were probed with anti-β-catenin (anti-β) or antiplakoglobin (anti-Pg) antibodies. Arrows, endogenous β-catenin and plakoglobin in left and right panels, respectively. Arrowheads, membrane-tethered β-catenin and plakoglobin in left and right panels, respectively. The expression level of membrane-tethered proteins in F9 cells is relatively low (see text). The positions of molecular weight markers (in thousands) are indicated on the right. (B) Subcellular localization of membrane-tethered β-catenin and plakoglobin in BPD cells. At 20 h after transfection, cells were stained with anti-β-catenin or antiplakoglobin antibodies for detection of nEmβ and nEmPg, respectively. Nuclei were visualized with DAPI. Note that expressed molecules are localized predominantly at the plasma membrane. Bar, 20 μm. (C) Effect of membrane-tethered β-catenin and plakoglobin on TOPflash luciferase reporter activity. BPD and F9 cells were transiently cotransfected with pTOPflash and pCMV-renilla luciferase reporters and with the expression vector for each protein. As positive and negative controls, expression vectors for β-catenin and GFP, respectively, were used. The value for BPD cells transfected with β-catenin was set at 100. Error bars indicate standard deviations.
We next examined the effect of nEmβ and nEmPg fusion proteins on LEF/TCF-dependent transcriptional activity (Fig. 1C). Transcriptional activity of wild-type β-catenin expressed in BPD cells was used as a control. The transcriptional activities of nEmβ and nEmPg were negligible in BPD cells (Fig. 1C). Expression of nEmβ or nEmPg in parental F9 cells, on the other hand, highly activated LEF/TCF-dependent transcription. These data clearly demonstrate that endogenous β-catenin or endogenous plakoglobin is required for membrane-tethered β-catenin and plakoglobin to influence LEF/TCF-dependent transcription.
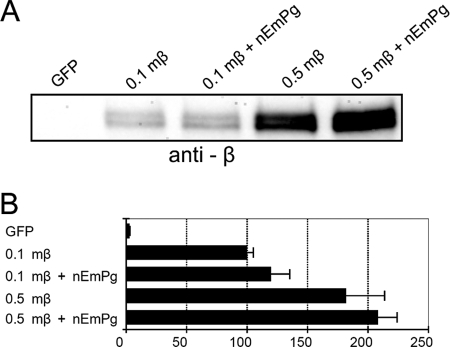
Membrane-tethered plakoglobin was most efficient in activating LEF/TCF-dependent transcription (Fig. 1C). In an effort to confirm that this hyperactivation depends on the efficient stabilization of β-catenin, stabilized mutant β-catenin was cotransfected with membrane-tethered plakoglobin in BPD cells (Fig. 2). Since the stabilized β-catenin mutant is protected from proteasomal destruction (44), we expected that the expression of nEmPg would not affect transcriptional activity of this mutant. To evaluate the effect of nEmPg on the transcriptional activity of stabilized β-catenin, we prepared two populations of cells expressing different amounts of stabilized β-catenin. Immunoblot analysis showed that the expression level of mutant β-catenin protein in cells transfected with 0.5 μg expression plasmid was severalfold higher than that in cells transfected with 0.1 μg plasmid (Fig. 2A). Luciferase analysis showed that the LEF/TCF-dependent transcriptional activity was also higher in cells transfected with 0.5 μg of expression plasmid, although the value did not exceed twofold (Fig. 2B). As expected, the expression level of stabilized mutant protein was not dramatically affected by expression of nEmPg. The transcriptional activity was also not severely affected by expression of nEmPg, with only a slight increase under both high- and low-expression conditions. Thus, the hyperactivation of LEF/TCF-dependent transcriptional activity observed in F9 cells was not detected in BPD cells expressing stabilized β-catenin. These data confirm that membrane-tethered plakoglobin regulates the transcriptional activity of β-catenin mainly by stabilizing endogenous β-catenin. It is possible that the membrane-tethered plakoglobin fusion protein more efficiently prevents degradation of endogenous β-catenin/plakoglobin than does the β-catenin fusion protein.
FIG. 2.
Effects of membrane-tethered plakoglobin on LEF/TCF-dependent transcription activated by stabilized mutant β-catenin. Cells were transiently cotransfected with expression vectors for pTOPflash and pCMV-renilla luciferase reporters and, where indicated, with expression vectors for stabilized mutant β-catenin (mβ) and membrane-tethered plakoglobin (nEmPg). A 0.1- or 0.5-μg amount of plasmid for stabilized mutant β-catenin (0.1mβ and 0.5mβ, respectively) or 0.1 μg of plasmid for membrane-tethered plakoglobin (nEmPg) was transfected as indicated. The total amount of plasmid DNA was adjusted to 1.1 μg by the addition of GFP expression vector, if necessary. (A) Effect of membrane-tethered plakoglobin on the expression level of stabilized mutant β-catenin protein. Note that only a small increase in stabilized mutant β-catenin expression was observed when membrane-tethered plakoglobin was expressed. (B) Effect of membrane-tethered plakoglobin on TOPflash luciferase reporter activity in the presence of stabilized mutant β-catenin. The value for BPD cells transfected with 0.1 μg plasmid for stabilized mutant β-catenin (0.1mβ) was set at 100. Error bars indicate standard deviations.
Effect of Wnt-3a on F9 cells and their derivatives.
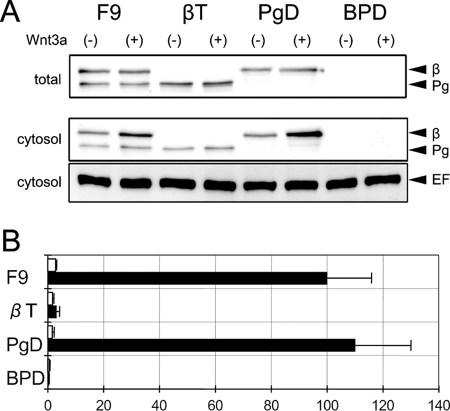
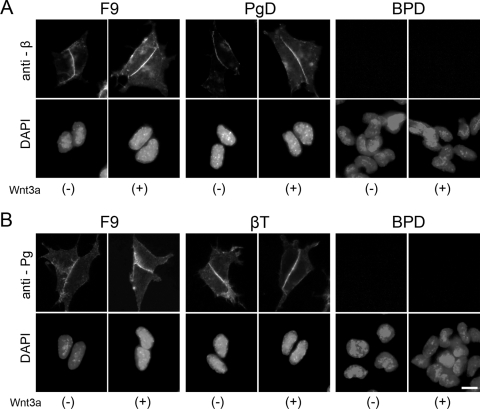
F9 mouse teratocarcinoma cells are known to be sensitive to Wnt-3a signal (34). To distinguish the roles of β-catenin and plakoglobin in response to Wnt-3a, we used β-catenin-null (βT), plakoglobin-null (PgD), and β-catenin/plakoglobin-null (BPD) F9 cells (10) (see Materials and Methods). Parental F9 cells express both β-catenin and plakoglobin. βT and PgD cells express plakoglobin and β-catenin, respectively. BPD cells express neither β-catenin nor plakoglobin (Fig. 3 A). The expression levels of β-catenin and plakoglobin protein were examined by Western blot analysis before and after Wnt-3a stimulation (Fig. 3A). In total cell lysate, the expression levels of the proteins seemed not to be severely affected, probably because a large amount of these molecules was associated with cadherin and thus was protected from degradation (Fig. 3A, total). In the cytosolic fraction, a slight increase in β-catenin protein was detected after Wnt-3a stimulation (Fig. 3A, cytosol). Using pTOPflash and pCMV-renilla as reporters, we examined the response of these cells to a Wnt-3a stimulus. F9 and PgD cells, which express β-catenin, showed strong LEF/TCF-dependent transcriptional activities (Fig. 3B). In contrast, an increase of the transcriptional activity was barely observed in βT and BPD cells, which lack β-catenin expression (Fig. 3B). Although a trace level of transcriptional activity was detected in βT cells, this seemed to be a nonspecific response since it was detected even after the addition of control conditioned medium from L cells. Thus, the expression of β-catenin but not that of plakoglobin is required for the response of F9 cells to a Wnt-3a signal.
FIG. 3.
LEF/TCF-dependent transcriptional activity in response to Wnt-3a. (A) Expression of β-catenin (β) and plakoglobin (Pg) in parental F9, β-catenin-null (βT), plakoglobin-null (PgD), and β-catenin/plakoglobin-null (BPD) cells. Cells were treated with conditioned medium from L cells expressing Wnt-3a (+) or with that from parental L cells (−) for 15 h. In the upper panel (total) and middle panel (cytosol), Western blots of total cell lysate and a cytosolic fraction, respectively, were probed with a mixture of anti-β-catenin and antiplakoglobin antibodies. Eukaryotic elongation factor 2 (EF) levels were used as an internal control (lower panel). (B) Effect of Wnt-3a on the TOPflash luciferase reporter in F9, βT, PgD, and BPD cells. Cells were transiently transfected with pTOPflash and pCMV-renilla luciferase reporters and then treated with conditioned medium from L cells expressing Wnt-3a (black bars) or with that from parental L cells (white bars) for 15 h. The value for F9 cells treated with Wnt-3a was set at 100. Error bars indicate standard deviations.
Next, we asked if there was a change in the subcellular localization of β-catenin and/or plakoglobin after Wnt-3a stimulation (Fig. 4). Since β-catenin functions in the nucleus to transduce signals, preferential nuclear accumulation of β-catenin has been considered a hallmark of Wnt signaling (5). In F9 and PgD cells, however, nuclear accumulation of β-catenin was not clearly observed even in the presence of Wnt-3a (Fig. 4), despite activation of LEF/TCF-dependent transcription (Fig. 3B). After Wnt-3a stimulation, β-catenin appeared to be slightly increased throughout F9 and PgD cells (Fig. 4A). We did not observe any change in plakoglobin expression or localization in F9 and βT cells (Fig. 4B).
FIG. 4.
Subcellular localization of β-catenin and plakoglobin proteins in the presence (+) or absence (−) of Wnt-3a. F9, βT, PgD, and BPD cells were treated with the conditioned medium from L cells expressing Wnt-3a (+) or with that from parental L cells (−) for 15 h and then stained with anti-β-catenin (anti-β) or antiplakoglobin (anti-Pg) MAb. Nuclei were visualized with DAPI. Preferential nuclear accumulation of β-catenin and plakoglobin was not observed by immunofluorescent staining, even in the presence of Wnt-3a. Bar, 20 μm.
LEF/TCF-dependent transcriptional activity of β-catenin and plakoglobin in BPD cells.
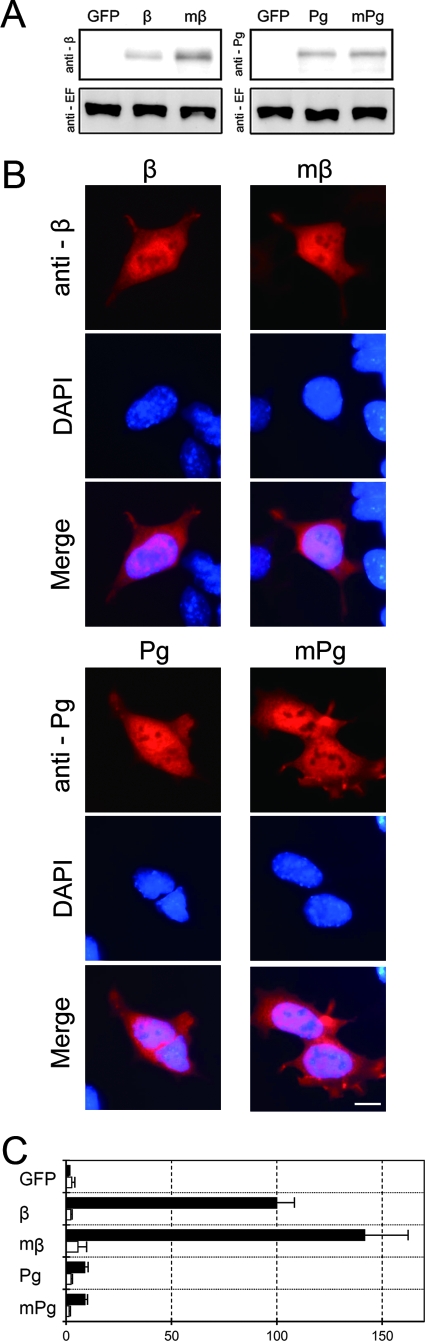
Using BPD cells, we examined the expression patterns and transcriptional activities of exogenously expressed β-catenin, plakoglobin, and their stabilized mutants in the absence of endogenous β-catenin/plakoglobin. When the expression vectors were transiently transfected into BPD cells, polypeptides with the expected molecular weight were expressed (Fig. 5A). Consistent with previous reports, the expression level of stabilized mutant β-catenin was significantly higher than that of wild-type β-catenin (Fig. 5A, anti-β). Immunocytochemical analysis demonstrated that overexpressed β-catenin, plakoglobin, and their mutants showed cytoplasmic and nuclear localization (Fig. 5B). In contrast to the case for Wnt signal activation, we observed preferential nuclear accumulation of both β-catenin and plakoglobin in a number of transfected BPD cells expressing high levels of these proteins. The expression level and localization of plakoglobin were similar to those of β-catenin. The transcriptional activities of β-catenin and plakoglobin, however, were distinctively dissimilar (Fig. 5C). When β-catenin or its stabilized mutant was transiently expressed in BPD cells, LEF/TCF-dependent transcriptional activity was highly induced. Expression of plakoglobin or its mutant, on the other hand, showed less than 10% induction of LEF/TCF-dependent transcriptional activity. The induction of transcriptional activity was negligible under any conditions when we used a control reporter, pFOPflash.
FIG. 5.
LEF/TCF-dependent transcriptional activity in BPD cells transiently expressing β-catenin, plakoglobin, and their stabilized mutants. BPD cells were transiently cotransfected with pTOPflash and pCMV-renilla luciferase reporter plasmids and, where indicated, with expression vectors for β-catenin (β), plakoglobin (Pg), stabilized mutant β-catenin (mβ), or stabilized mutant plakoglobin (mPg). As a control, an expression vector for GFP was used. (A) Expression of β-catenin, plakoglobin and their stabilized mutants in BPD cells. Western blots of total cell lysate were probed with anti-β-catenin (anti-β) or antiplakoglobin (anti-Pg) antibodies. Eukaryotic elongation factor 2 levels were used as an internal control (anti-EF; lower panel). (B) Subcellular localization of β-catenin, plakoglobin, and their stabilized mutants in BPD cells. Cells were stained with anti-β-catenin (anti-β) or antiplakoglobin (anti-Pg) MAb. Nuclei were visualized with DAPI. Occasionally, nuclear localization of β-catenin and plakoglobin was observed in cells expressing high levels of these proteins. Bar, 20 μm. (C) Effect of β-catenin, plakoglobin, and their stabilized mutants on TOPflash (closed bars) or FOPflash (open bars) luciferase reporter activity. The value for BPD cells transfected with β-catenin was set at 100. Error bars indicate standard deviations.
Dominant-negative effect of C-terminally deleted β-catenin.
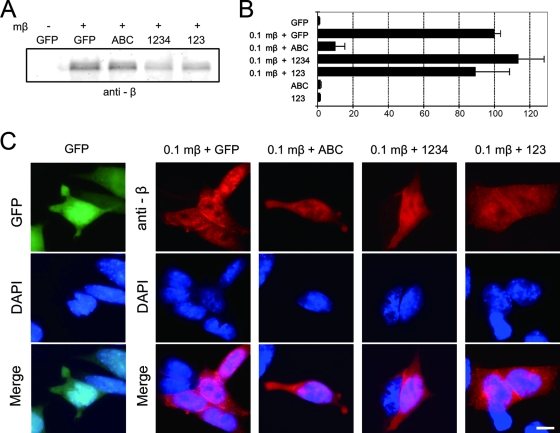
In an effort to estimate the dominant-negative effect of the armadillo repeat domains of β-catenin and plakoglobin, we examined the transcriptional activity of stabilized mutant β-catenin in the presence or absence of transiently expressed C-terminal deletion mutants. The dominant-negative effect of plakoglobin was also examined using the same assay system. (For the structures of wild-type plakoglobin [1234], C-terminally deleted β-catenin [ABC], and C-terminally deleted plakoglobin [123], see Fig. 7A.) Expression of the C-terminal deletion mutants could not be evaluated using anti-β-catenin or antiplakoglobin antibodies because these antibodies recognize the C-terminal regions which were deleted in mutant molecules. Thus, expression of these mutants was verified by their ability to stabilize wild-type β-catenin in BPD cells (data not shown). The expression of C-terminally deleted mutants by themselves showed no transcriptional activity (Fig. 6B). Since stabilized mutant β-catenin is protected from degradation, the expression level of this mutant was not significantly increased by the transient expression of C-terminally deleted mutants (Fig. 6A). However, the expression of C-terminally deleted β-catenin significantly decreased the LEF/TCF-dependent transcriptional activity of stabilized mutant β-catenin (Fig. 6B). The transcriptional activity was reduced to less than 10% of the activity seen in cells expressing stabilized β-catenin alone. In contrast, the expression of plakoglobin and C-terminally deleted mutant plakoglobin did not affect the transcriptional activity of stabilized mutant β-catenin (Fig. 6 B). Neither plakoglobin nor C-terminally deleted mutants affected the subcellular localization of stabilized mutant β-catenin. Stabilized mutant β-catenin localized in the nucleus even in the presence of these molecules (Fig. 6C). Thus, C-terminally deleted β-catenin functioned as a dominant-negative molecule for transcriptional activity even though it did not affect the expression and localization of stabilized mutant β-catenin.
FIG. 6.
Effect of C-terminally deleted mutant β-catenin and C-terminally deleted mutant plakoglobin and full-length plakoglobin on LEF/TCF-dependent transcription activated with stabilized mutant β-catenin. BPD cells were transiently cotransfected with pTOPflash and pCMV-renilla luciferase reporter plasmids and, where indicated, with expression vectors for stabilized mutant β-catenin (mβ), plakoglobin (1234), C-terminally deleted mutant β-catenin (ABC), or plakoglobin (123). A 0.1-μg amount of plasmid for mβ (0.1mβ) and 0.5 μg of plasmid for 1234, ABC, and 123 were transfected as indicated. Total plasmid DNA was adjusted to 1.1 μg by the addition of GFP expression vector, if necessary. (A) Expression of stabilized mutant β-catenin in BPD cells. Cells were transfected as described above. Western blots of total cell lysate were probed with anti-β-catenin antibodies. Since the epitope recognized by anti-β-catenin is in the C-terminal domain of β-catenin, anti-β-catenin antibodies do not recognize ABC. Note that the expression level of stabilized mutant β-catenin is not significantly affected by the expression of ABC. (B) Effect of C-terminally deleted mutants on TOPflash luciferase reporter activity. Cells were transiently transfected as described above. The value for BPD cells transfected with stabilized mutant β-catenin and GFP (0.1mβ+GFP) was set at 100. Note that transcriptional activity of stabilized mutant β-catenin is suppressed by cotransfection of C-terminally deleted mutant β-catenin (0.1mβ+ABC) but not plakoglobin and its C-terminal deletion (0.1mβ+1234 and 0.1mβ+123). The expression of C-terminally deleted mutants by themselves showed no transcriptional activity (ABC and 123). Error bars indicate standard deviations. (C) Subcellular localization of stabilized mutant β-catenin in BPD cells transfected as described above. At 20 h after transfection, cells were stained with anti-β-catenin MAb. Nuclei were visualized with DAPI. Note that cotransfection of C-terminally deleted mutant β-catenin did not affect nuclear localization of stabilized mutant β-catenin (0.1mβ+ABC). Bar, 20 μm.
β-catenin/plakoglobin chimeric molecules.
As described above, plakoglobin did not facilitate LEF/TCF-mediated transcription in response to a Wnt-3a signal in F9 cells (Fig. 3B). Even when overexpressed, plakoglobin showed only weak transcriptional activity (Fig. 5C). Moreover, the C-terminal deletion mutant of plakoglobin did not show a dominant-negative effect on β-catenin-dependent transcription (Fig. 6B). These data suggest that the armadillo repeat domain and/or C-terminal transactivation domain of plakoglobin is nonfunctional in F9 cells. In an effort to examine the potential activity of each domain of plakoglobin in transcription, we made two β-catenin/plakoglobin chimeric molecules, in which their C-terminal transactivation domains were interchanged (Fig. 7A). ABC4 is a β-catenin mutant with its C-terminal domain replaced with that of plakoglobin. 123D is a plakoglobin mutant with its C-terminal domain replaced with that of β-catenin.
When expression vectors for these molecules were transfected into BPD cells, polypeptides with the expected molecular weights were expressed (Fig. 7B). Immunocytochemical analysis demonstrated that overexpressed ABCD (wild-type β-catenin) and 1234 (wild-type plakoglobin) showed cytoplasmic and nuclear localization (Fig. 5B and 8). ABC4 and 123D showed similar distributions in the cytoplasm and nucleus (Fig. 8). As with the expression of ABCD or 1234, we observed preferential nuclear accumulation of both ABC4 and 123D in a number of transfected cells expressing high levels of these proteins. As expected, examination of transcriptional activities of these molecules revealed strong activation of LEF/TCF-dependent transcription by ABCD but not by 1234 (Fig. 5C and 7C). ABC4 and 123D showed levels of activity between those of β-catenin and plakoglobin (Fig. 7C) which were consistent with a previous report (43). These results suggest that the armadillo repeat domain and the C-terminal domain of plakoglobin have the potential to function in LEF/TCF-dependent transcription in F9 cells, although they were less efficient than β-catenin.
FIG. 8.
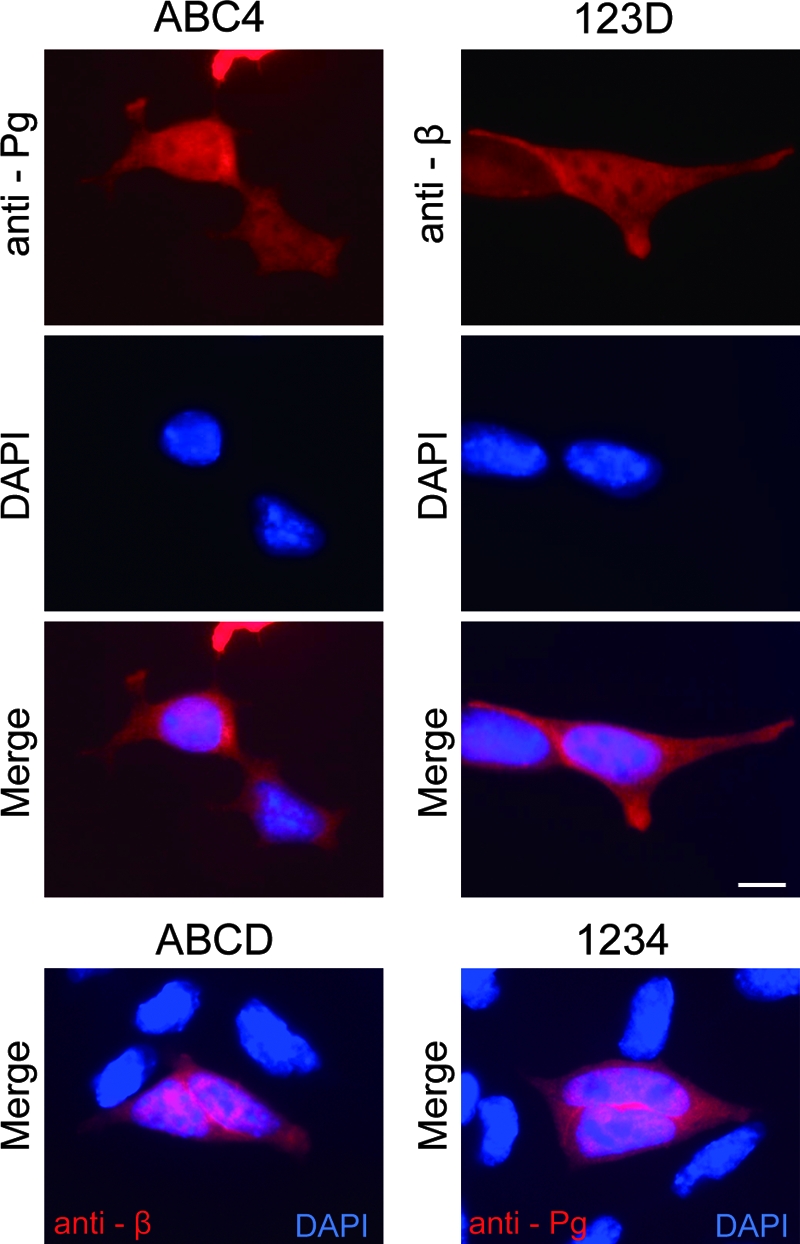
Subcellular localization of β-catenin/plakoglobin chimeric molecules in BPD cells. At 20 h after transfection, cells were stained with antiplakoglobin (anti-Pg) MAb for ABC4 or with anti-β-catenin (anti-β) MAb for 123D. Nuclei were visualized with DAPI. Occasionally, preferential nuclear accumulation of chimeric molecules was observed in cells expressing high levels of these proteins. For comparison, merged images of β-catenin or plakoglobin with DAPI for BPD cells expressing wild-type β-catenin (ABCD) or plakoglobin (1234) are also shown. Bar, 20 μm.
In an effort to examine the ability of the chimeric molecules to produce a response to Wnt-3a stimulation, we isolated BPD cells stably expressing ABC4 and 123D. Western blot analysis confirmed that each molecule was expressed in each transfectant (Fig. 7D). The expression levels of these molecules were similar to those of β-catenin and plakoglobin in F9 cells. Using these transfectants, we examined LEF/TCF-dependent transcriptional activity before and after Wnt-3a stimulation. As control, F9 cells showed strong LEF/TCF-dependent transcriptional activity after addition of Wnt-3a conditioned medium (Fig. 7E). In contrast, an increase of transcriptional activity was barely observed in cells expressing ABC4 and 123D (Fig. 7E). Although trace levels of transcriptional activity were detected in cells expressing ABC4 and 123D, this seemed to be a nonspecific response since it was detected even after the addition of control conditioned medium from L cells. It is possible that cells expressing ABC4 and 123D may have lost their Wnt-3a responsiveness during cloning of transfectants. To rule out this possibility, we examined the responsiveness of these cells to a Wnt-3a signal in the presence of β-catenin. When a small amount of wild-type β-catenin was transfected, the cells clearly responded to a Wnt-3a signal (data not shown). These results show that β-catenin/plakoglobin chimeric molecules do not respond to Wnt-3a signals despite their potential for the activation of LEF/TCF-dependent transcription.
DISCUSSION
We examined LEF/TCF-dependent transcriptional activity mediated by β-catenin, plakoglobin, and their mutants, using β-catenin-null (βT), plakoglobin-null (PgD), and β-catenin/plakoglobin-null (BPD) F9 cells. BPD cells allowed us to examine clearly this transcriptional activity in the absence of endogenous β-catenin and plakoglobin. Here we will discuss the defined roles of β-catenin and plakoglobin in LEF/TCF-dependent transcriptional activity in F9 cells. Furthermore, we will also discuss the difference in transcriptional induction mediated by Wnt-3a and by exogenous overexpression of β-catenin and plakoglobin.
Membrane-tethered-β-catenin/plakoglobin in LEF/TCF-dependent transcriptional activation.
In Drosophila, the effect of membrane-tethered mutants in the presence of hypomorphic Armadillo mutants has been examined. Previous reports and our present data showed that mutant β-catenin lacking the C-terminal region has a strong dominant-negative effect on Wnt signaling. Since this type of mutant Armadillo is expressed in common hypomorphic Armadillo mutants such as XM19 and XP33, it is possible that these mutants show a dominant-negative effect on the function of membrane-tethered Armadillo protein (9, 38). Expression of membrane-tethered β-catenin and plakoglobin in BPD cells, however, demonstrated that these molecules do not show transcriptional activity even in BPD cells which lack mutant β-catenin. Expression of these molecules in F9 cells confirmed that the presence of endogenous (wild-type) β-catenin is required for the activation of LEF/TCF-dependent transcription. Furthermore, we noted that membrane-tethered plakoglobin dramatically activated LEF/TCF-dependent transcription in F9 cells. This suggested a possible role for membrane-tethered plakoglobin in Wnt signaling besides the stabilization of β-catenin (5, 39). Expression of membrane-tethered plakoglobin, however, did not cause hyperactivation of LEF/TCF-dependent transcription mediated by stabilized mutant β-catenin. Thus, the stabilization of endogenous β-catenin is the main mechanism for transcriptional activation by membrane-tethered β-catenin and plakoglobin.
Uncoupling of nuclear localization and transcription activation.
In an attempt to elucidate the nuclear function of β-catenin, it was assumed that “preferential” nuclear accumulation of β-catenin served as a marker for activation of the Wnt signaling cascade (5). In the current study, we compared the transcriptional activity and subcellular localization of β-catenin both in Wnt-3a-treated F9 cells and in cells transiently overexpressing β-catenin and its mutants. Consistent with previous reports, when β-catenin was transiently overexpressed, preferential nuclear accumulation was occasionally observed in conjunction with high induction of LEF/TCF-dependent transcriptional activity. Despite significant induction of the transcription activity, however, preferential nuclear accumulation of β-catenin was barely detected in Wnt-3a-treated F9 cells. On the other hand, coexpression of stabilized mutant β-catenin and dominant-negative β-catenin lacking the C-terminal domain showed the opposite effect in BPD cells. Although clear nuclear accumulation of stabilized mutant β-catenin was observed, only a small amount of transcriptional activity was detected. Thus, the nuclear localization and accumulation of β-catenin detected by immunocytochemical analysis is not necessarily linked to LEF/TCF-dependent transcriptional activity.
Recently it has been reported that novel factors such as Legless and Pygopus are involved in the nuclear localization of β-catenin (38, 40). It is likely that β-catenin nuclear localization may be regulated independent of the LEF/TCF-dependent transcriptional activation machinery (6, 36, 42).
C-terminally deleted mutant β-catenin.
Consistent with previous reports, mutant β-catenin lacking the C-terminal domain functioned as a dominant-negative molecule for LEF/TCF-dependent transcription in F9 cells (45). A similar plakoglobin mutant lacking the C-terminal domain did not show this dominant-negative effect. These finding could be explained by the fact that the formation of a plakoglobin-LEF-DNA complex is less efficient than formation of a β-catenin-LEF-DNA complex (45). Aside from the dominant-negative effect, it has recently been reported that C-terminally deleted β-catenin retains some signaling activity, probably dependent on interactions with Legless/BCL-9 and Pygopus proteins (7, 38, 40). Taking advantage of β-catenin/plakoglobin-null F9 cells, we found out that removal of the C terminus completely abolishes the transcriptional activity of β-catenin and plakoglobin in F9 cells. These data suggest that, at least in F9 cell derivatives, a β-catenin/Legless/Pygopus complex is not formed or does not activate LEF/TCF-dependent transcription. The BPD cell line will be a useful tool to elucidate factors that are required for the function of a β-catenin/Legless/Pygopus complex in Wnt signaling in mammalian cells.
Insensitivity of plakoglobin to Wnt-3a signals.
As previously reported, overexpression of plakoglobin was associated with a significant increase in LEF/TCF-dependent transcriptional activity, although the level was less than that induced by β-catenin (20). When chimeric molecules with interchanged C-terminal domains of β-catenin and plakoglobin were overexpressed in BPD cells, they activated LEF/TCF-dependent transcription at a level intermediate between those seen for β-catenin and plakoglobin. These data are consistent with previous reports showing that plakoglobin is potentially functional in LEF/TCF-dependent transcription (20, 43, 45). These data also confirm that the armadillo repeat domain and C-terminal domain of plakoglobin are potentially functional, although their activities are weaker than that of full-length β-catenin. Thus, there is no doubt that plakoglobin has the potential to function in LEF/TCF-dependent transcription. However, it is unclear whether plakoglobin activates LEF/TCF-dependent transcription in response to a Wnt signal. In this report, we clearly showed that plakoglobin is insensitive to the addition of Wnt-3a. Removal of plakoglobin from F9 cells did not affect the Wnt-3a-induced LEF/TCF-dependent transcriptional activity. In contrast, removal of β-catenin caused a complete loss of reactivity to Wnt-3a even in the presence of endogenous plakoglobin. Interestingly, BPD cells stably expressing β-catenin/plakoglobin chimeric molecules did not respond to a Wnt-3a signal, although this chimera has the ability to activate LEF/TCF-dependent transcription. Thus, LEF/TCF-dependent transcriptional activity observed by the overexpression of plakoglobin and its chimeras does not necessarily reflect the Wnt-3a response of cells stably expressing these molecules. These results strongly suggest that higher-level regulation of β-catenin, which is often overlooked in overexpression experiments, is required for the response to Wnt signals (6, 36, 42). Furthermore, they also suggest that plakoglobin is not a physiological mediator of Wnt signaling in F9 cells.
The present study elegantly confirms the previous reports showing that free β-catenin is required for LEF/TCF-dependent transcription through Wnt signaling. In addition, we demonstrate here that preferential nuclear accumulation of β-catenin is not necessarily linked to activation of LEF/TCF-dependent transcription and highlight the point that we cannot use nuclear localization of β-catenin as an indicator of Wnt signal activation. On the other hand, our data strongly suggest that plakoglobin is not a physiological mediator in the Wnt signaling cascade in F9 cells, although it has the potential to mediate LEF/TCF-dependent transcription, as reported. The cryptic behavior of plakoglobin in response to a Wnt signal is puzzling. Using the benefits of an in vitro culture system, we successfully established BPD cells that are completely null for β-catenin/plakoglobin, which provide an ideal tool to analyze regulatory mechanisms of β-catenin and plakoglobin in Wnt signaling.
Acknowledgments
We are grateful to all laboratory members (Division of Cellular Interactions of the Institute of Molecular Embryology and Genetics at Kumamoto University) for their participation in helpful discussions. We also thank M. Matsuda for pCAG-CGFP; M. van de Wetering and H. Clevers for pTOPflash and pFOPflash; H. Niwa for pCAGGSneodelEcoRI; S. Takada for Wnt-3a-secreting L cells; C. Fujiwara, E. Morikawa, and T. Magarikaji for excellent technical assistance; and M. J. Wheelock for critical reading of the manuscript. A.N. thanks the late Shoichiro Tsukita for his encouragement at the initiation of this work.
Part of this work at the Department of Cellular Interactions of the Institute of Molecular Embryology and Genetics of Kumamoto University was supported by a grant to A.N. from the Core Research for Evolutional Science and Technology (CREST), Grants-in-Aid for Scientific Research and Cancer Research, and a Grant-in-Aid for 21st Century COE Research “Cell Fate Regulation Research and Education Unit” from the Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Published ahead of print on 5 November 2007.
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 163797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle, H., S. Butz, J. Stappert, H. Weissig, R. Kemler, and H. Hoschuetzky. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 1073655-3663. [DOI] [PubMed] [Google Scholar]
- 3.Behrens, J., B. A. Jerchow, M. Wurtele, J. Grimm, C. Asbrand, R. Wirtz, M. Kuhl, D. Wedlich, and W. Birchmeier. 1998. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280596-599. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382638-642. [DOI] [PubMed] [Google Scholar]
- 5.Bienz, M., and H. Clevers. 2003. Armadillo/β-catenin signals in the nucleus—proof beyond a reasonable doubt? Nat. Cell Biol. 5179-182. [DOI] [PubMed] [Google Scholar]
- 6.Clevers, H. 2006. Wnt/β-catenin signaling in development and disease. Cell 127469-480. [DOI] [PubMed] [Google Scholar]
- 7.Cong, F., L. Schweizer, M. Chamorro, and H. Varmus. 2003. Requirement for a nuclear function of β-catenin in Wnt signaling. Mol. Cell. Biol. 238462-8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowin, P., H. P. Kapprell, W. W. Franke, J. Tamkun, and R. O. Hynes. 1986. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 461063-1073. [DOI] [PubMed] [Google Scholar]
- 9.Cox, R. T., L. M. Pai, J. R. Miller, S. Orsulic, J. Stein, C. A. McCormick, Y. Audeh, W. Wang, R. T. Moon, and M. Peifer. 1999. Membrane-tethered Drosophila Armadillo cannot transduce Wingless signal on its own. Development 1261327-1335. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga, Y., H. Liu, M. Shimizu, S. Komiya, M. Kawasuji, and A. Nagafuchi. 2005. Defining the roles of β-catenin and plakoglobin in cell-cell adhesion: isolation of catenin/plakoglobin-deficient F9 cells. Cell Struct. Funct. 3025-34. [DOI] [PubMed] [Google Scholar]
- 11.Hart, M. J., R. de los Santos, I. N. Albert, B. Rubinfeld, and P. Polakis. 1998. Downregulation of β-catenin by human axin and its association with the APC tumor suppressor, β-catenin and GSK3 β. Curr. Biol. 8573-581. [DOI] [PubMed] [Google Scholar]
- 12.Huber, O., R. Korn, J. McLaughlin, M. Ohsugi, B. G. Herrmann, and R. Kemler. 1996. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech. Dev. 593-10. [DOI] [PubMed] [Google Scholar]
- 13.Huelsken, J., R. Vogel, V. Brinkmann, B. Erdmann, C. Birchmeier, and W. Birchmeier. 2000. Requirement for β-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 148567-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulsken, J., W. Birchmeier, and J. Behrens. 1994. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J. Cell Biol. 1272061-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 171371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh, K., V. E. Krupnik, and S. Y. Sokol. 1998. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and β-catenin. Curr. Biol. 8591-594. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen, K. A., and M. J. Wheelock. 1992. Plakoglobin, or an 83-kD homologue distinct from β-catenin, interacts with E-cadherin and N-cadherin. J. Cell Biol. 118671-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komiya, S., M. Shimizu, J. Ikenouchi, S. Yonemura, T. Matsui, Y. Fukunaga, H. Liu, F. Endo, S. Tsukita, and A. Nagafuchi. 2005. Apical membrane and junctional complex formation during simple epithelial cell differentiation of F9 cells. Genes Cells 101065-1080. [DOI] [PubMed] [Google Scholar]
- 19.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 2751784-1787. [DOI] [PubMed] [Google Scholar]
- 20.Maeda, O., N. Usami, M. Kondo, M. Takahashi, H. Goto, K. Shimokata, K. Kusugami, and Y. Sekido. 2004. Plakoglobin (γ-catenin) has TCF/LEF family-dependent transcriptional activity in β-catenin-deficient cell line. Oncogene 23964-972. [DOI] [PubMed] [Google Scholar]
- 21.Mathur, M., L. Goodwin, and P. Cowin. 1994. Interactions of the cytoplasmic domain of the desmosomal cadherin Dsg1 with plakoglobin. J. Biol. Chem. 26914075-14080. [PubMed] [Google Scholar]
- 22.Matsuda, M., A. Kubo, M. Furuse, and S. Tsukita. 2004. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 1171247-1257. [DOI] [PubMed] [Google Scholar]
- 23.McCrea, P. D., C. W. Turck, and B. Gumbiner. 1991. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 2541359-1361. [DOI] [PubMed] [Google Scholar]
- 24.Merriam, J. M., A. B. Rubenstein, and M. W. Klymkowsky. 1997. Cytoplasmically anchored plakoglobin induces a WNT-like phenotype in Xenopus. Dev. Biol. 18567-81. [DOI] [PubMed] [Google Scholar]
- 25.Miller, J. R., and R. T. Moon. 1997. Analysis of the signaling activities of localization mutants of β-catenin during axis specification in Xenopus. J. Cell Biol. 139229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86391-399. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura, T., F. Hamada, T. Ishidate, K. Anai, K. Kawahara, K. Toyoshima, and T. Akiyama. 1998. Axin, an inhibitor of the Wnt signalling pathway, interacts with β-catenin, GSK-3β and APC and reduces the β-catenin level. Genes Cells 3395-403. [DOI] [PubMed] [Google Scholar]
- 28.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108193-199. [DOI] [PubMed] [Google Scholar]
- 29.Nose, A., A. Nagafuchi, and M. Takeichi. 1988. Expressed recombinant cadherins mediate cell sorting in model systems. Cell 54993-1001. [DOI] [PubMed] [Google Scholar]
- 30.Nusslein-Volhard, C., and E. Wieschaus. 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287795-801. [DOI] [PubMed] [Google Scholar]
- 31.Peifer, M., and E. Wieschaus. 1990. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell 631167-1176. [DOI] [PubMed] [Google Scholar]
- 32.Sherman, M. I., and R. A. Miller. 1978. F9 embryonal carcinoma cells can differentiate into endoderm-like cells. Dev. Biol. 6327-34. [DOI] [PubMed] [Google Scholar]
- 33.Shibamoto, S., K. Higano, R. Takada, F. Ito, M. Takeichi, and S. Takada. 1998. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3659-670. [DOI] [PubMed] [Google Scholar]
- 34.Shibamoto, S., J. Winer, M. Williams, and P. Polakis. 2004. A blockade in Wnt signaling is activated following the differentiation of F9 teratocarcinoma cells. Exp. Cell Res. 29211-20. [DOI] [PubMed] [Google Scholar]
- 35.Simcha, I., M. Shtutman, D. Salomon, J. Zhurinsky, E. Sadot, B. Geiger, and A. Ben-Ze'ev. 1998. Differential nuclear translocation and transactivation potential of β-catenin and plakoglobin. J. Cell Biol. 1411433-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stadeli, R., R. Hoffmans, and K. Basler. 2006. Transcription under the control of nuclear Arm/β-catenin. Curr. Biol. 16R378-R385. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi, N., S. Ishihara, S. Takada, S. Tsukita, and A. Nagafuchi. 2000. Posttranscriptional regulation of α-catenin expression is required for wnt signaling in L cells. Biochem. Biophys. Res. Commun. 277691-698. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, B. J. 2004. A complex of Armadillo, Legless, and Pygopus coactivates dTCF to activate wingless target genes. Curr. Biol. 14458-466. [DOI] [PubMed] [Google Scholar]
- 39.Tolwinski, N. S., and E. Wieschaus. 2004. A nuclear escort for β-catenin. Nat. Cell Biol. 6579-580. [DOI] [PubMed] [Google Scholar]
- 40.Tolwinski, N. S., and E. Wieschaus. 2004. A nuclear function for armadillo/β-catenin. PLoS Biol. 2E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieschaus, E., and R. Riggleman. 1987. Autonomous requirements for the segment polarity gene armadillo during Drosophila embryogenesis. Cell 49177-184. [DOI] [PubMed] [Google Scholar]
- 42.Willert, K., and K. A. Jones. 2006. Wnt signaling: is the party in the nucleus? Genes Dev. 201394-1404. [DOI] [PubMed] [Google Scholar]
- 43.Williams, B. O., G. D. Barish, M. W. Klymkowsky, and H. E. Varmus. 2000. A comparative evaluation of β-catenin and plakoglobin signaling activity. Oncogene 195720-5728. [DOI] [PubMed] [Google Scholar]
- 44.Yost, C., M. Torres, J. R. Miller, E. Huang, D. Kimelman, and R. T. Moon. 1996. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 101443-1454. [DOI] [PubMed] [Google Scholar]
- 45.Zhurinsky, J., M. Shtutman, and A. Ben-Ze'ev. 2000. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by β-catenin and plakoglobin. Mol. Cell. Biol. 204238-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]