Abstract
Interactions between monocytes/macrophages and endothelial cells play an important role in the pathogenesis of atherosclerosis, and the adherence of monocytes to the arterial endothelium is one of the early events in atherogenesis. In the present study, peritoneal macrophages harvested from green fluorescent protein (GFP) transgenic mice were used to analyze how Chlamydia pneumoniae infection affects the adherence of GFP-macrophages to mouse endothelial cells in vitro and to the aorta from normolipidemic and hyperlipidemic mice ex vivo. In vitro studies showed that C. pneumoniae-infected GFP-macrophages adhered better than uninfected macrophages to endothelial cells and GFP-macrophages adhered better to infected than uninfected endothelial cells. The ex vivo studies showed that C. pneumoniae-infected macrophages adhered better than uninfected macrophages to aortas from both normolipidemic and hyperlipidemic C57BL/6J mice and apolipoprotein E (ApoE)-deficient mice. In contrast, adherence of C. pneumoniae-infected macrophages to the aortas of intercellular adhesion molecule 1 (ICAM-1) knockout mice was not enhanced, suggesting that ICAM-1 is crucial for activation of the adherence of C. pneumoniae-infected macrophages to the endothelium. In conclusion, the present study defined a homing mechanism by which C. pneumoniae promotes the adherence of mononuclear phagocytes to the endothelium at the site of atherosclerotic lesion formation to promote the progression of atherosclerosis.
Chlamydia pneumoniae is an obligate intracellular gram-negative bacterium and is primarily a respiratory pathogen. Seroepidemiological studies have shown an association of C. pneumoniae antibody and atherosclerosis (28). The association of C. pneumoniae and atherosclerosis has been strengthened by detection (17) and isolation (26) of the organism from atherosclerotic lesions. Studies in animal models of atherosclerosis showed that intranasal inoculation of hyperlipidemic mice accelerates the progression of atherosclerosis (7, 21). In addition, animal experiments indicate that C. pneumoniae may be disseminated from the lungs to atherosclerotic lesions in the artery via circulating monocytes (5, 22).
Atherosclerosis is a disease of chronic inflammation and a major cause of coronary heart disease and stroke. Early events in lesion development include endothelial activation, which can be triggered by risk factors such as hypercholesterolemia. This results in leukocyte recruitment to the endothelium and migration into the subendothelium (18). The interaction of monocytes/macrophages with the endothelium is promoted by the expression of receptors for adhesion molecules on monocytes/macrophages, which mediate adherence to the corresponding adhesion molecules on endothelial cells, such as intercellular adhesion molecule 1 (ICAM-1), vascular adhesion molecule 1 (VCAM-1), E-selectin, and P-selectin (6, 8, 18). Leukocyte recruitment and activation of the expression of proinflammatory cytokines characterize the early process of atherosclerosis (18). Infection of human monocytes or macrophages with C. pneumoniae has been shown to enhance the adhesion of monocytes/macrophages to human endothelial cells (2, 10, 12, 20). Furthermore, infection of endothelial cells with C. pneumoniae has been shown to up-regulate the expression of E-selectin, ICAM-1, and VCAM-1 (11) and stimulate rolling, adhesion, and transmigration of human neutrophils or monocytes (14, 19, 23).
In this study, peritoneal macrophages from green fluorescent protein (GFP) transgenic mice on a C57BL/6J background were used as a tool to analyze how C. pneumoniae infection affects the adherence of GFP-macrophages to mouse endothelial cells in vitro and how hyperlipidemia affects the adherence of C. pneumoniae-infected macrophages to the mouse aorta ex vivo. In addition, the role of ICAM-1 in the adherence of C. pneumoniae-infected macrophages was studied with ICAM-1 knockout mice.
MATERIALS AND METHODS
Cells and animals.
The buffers used were Hanks’ balanced salt solution (HBSS; NaCl, 8 g; glucose, 1 g; KCl, 0.4 g; KH2PO4, 60 mg; Na2HPO4, 48 mg; MgSO4 · 7H2O, 0.2 g; CaCl2, 0.11 g [per liter, pH 7.2]), a glucose-potassium-sodium-phosphate (GKNP) solution (HBSS containing no Ca2+ or Mg2+), and chlamydia transport medium SPG (0.2 M sucrose, 0.8 mM KH2PO4, 6.7 mM Na2HPO4, 5 mM l-glutamic acid; pH 7.4). The culture media used were (i) RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum and 100 μg/ml each streptomycin and vancomycin and (ii) Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 5% fetal calf serum and 100 μg/ml each streptomycin and vancomycin. Cell lines used were a continuous human epithelial cell line (HL cells) obtained from Linda Cles (Department of Medicine, University of Washington, Seattle) and mouse endothelial cell line (MS1) from the American Type Culture Collection (Manassas, VA). MS1 is a pancreatic islet endothelial cell line transformed by retrovirus-mediated gene transfer in 1994 (1). The diets used were (i) regular chow and (ii) an atherogenic diet consisting of 15% fat, 1.25% cholesterol, and 0.5% sodium cholate (TD 88051; Harlan Teklad, Madison, WI). The mouse strains used were GFP transgenic mice (24) and ApoE (25) and ICAM-1 (13) knockout mice on a C57BL/6J background from Jackson Laboratories (Bar Harbor, ME).
Preparation of C. pneumoniae.
C. pneumoniae strain AR-39 was propagated in HL cells and purified by Hypaque-76 (Nycomed Inc., Princeton, NJ) linear gradient centrifugation (16). Organisms purified by this method contain less than 0.1% host cell materials (15). Purified organisms (>1 × 108 inclusion-forming units/ml) were resuspended in SPG buffer, aliquoted, and stored at −70°C until use. The infectivity titers were determined by titration in HL cells. Inoculum doses per macrophage or endothelial cell were expressed as multiplicities of infection (MOIs).
Preparation of mouse peritoneal macrophages.
GFP transgenic mice were injected intraperitoneally with 1 ml of 3% thioglycolate medium. After 4 to 5 days, peritoneal macrophages were harvested by injection of 10 ml of warm GKNP. The abdomen was massaged, and peritoneal fluids were withdrawn into a 50-ml tube and kept on ice. The macrophage suspension was centrifuged (700 × g, 5 min, 4°C) and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum. Peritoneal macrophages were plated into six-well plates. After adsorption at 37°C for 2 h in 5% CO2, the plates were washed once with RPMI 1640 medium to remove unattached cells. Adherent macrophages were cultured for 1 or 2 days at 37°C in 5% CO2 before use.
Infection of macrophages.
Murine peritoneal macrophages cultured for 1 day were used. Macrophages were inoculated with C. pneumoniae in 2 ml of RPMI 1640 medium by centrifugation at 700 to 800 × g at room temperature for 1 h. Inoculated cells were incubated for 1 h at 37°C in 5% CO2. Cells were washed once with 2 ml of RPMI 1640 medium and incubated with RPMI 1640 medium supplemented with 10% fetal calf serum for 1 day.
Infection of MS1 cells.
MS1 cells were infected with C. pneumoniae by the same method described above for macrophages, with minor modifications. MS1 monolayers on 24-well plates were inoculated with various doses of C. pneumoniae in 0.5 ml of DMEM, centrifuged at 700 to 800 × g at room temperature for 1 h, and incubated for 1 h at 37°C in 5% CO2. Cells were washed once with 1 ml of DMEM and incubated with DMEM supplemented with 5% fetal calf serum for 1 day.
In vitro adhesion assay.
MS1 cells were seeded onto a 24-well plate 1 to 2 days before inoculation. Murine macrophages cultured in six-well plates were washed twice with GKNP and harvested by scraping with a rubber policeman. Harvested macrophages were centrifuged at 700 × g for 5 min at 4°C. Macrophage pellets were resuspended with HBSS. Each MS1 monolayer was inoculated with 0.5 ml of a suspension of 3 × 104 macrophages at 37°C with rocking. After 30 min of adsorption, the plate was washed twice with HBSS to remove nonadherent macrophages and fixed with 10% buffered formalin for 30 min at 4°C. The adherent macrophages were quantified by a fluorescence microscope at a magnification of ×200 with a fluorescein isothiocyanate-based filter. Twenty fields were counted per well. The results were expressed as the mean ± standard deviation of three or four wells.
Ex vivo adhesion assay.
Ex vivo adhesion assays were performed as described by Ishida et al. (9). Briefly, the thoracic aortas of 13-week-old female mice were isolated and pinned on a 3% agarose-coated six-well plate with fine needles. Macrophages (2 × 105 to 5 × 105) left uninfected or infected with C. pneumoniae in HBSS at an MOI of 0.5 were inoculated onto each aortic strip. The plate was rocked gently for 30 min at 37°C. Unbound macrophages were removed by washing twice with HBSS. Bound macrophages were fixed with 10% buffered formalin for 30 min at 4°C. The bound macrophages were counted.
Assay of plasma cholesterol.
Blood was collected in heparinized tubes. Plasma was separated from blood cells by centrifugation and frozen at −70°C until tested. Cholesterol levels were measured by a commercial enzymatic test kit (Sigma, St. Louis, MO). Cholesterol levels were determined in triplicate and averaged.
Statistical analysis.
Data are expressed as the mean ± the standard deviation. An unpaired two-tailed Student t test was used for determination of statistical significance. The P value was determined with Microsoft Excel (Microsoft, Redmond, WA).
RESULTS
Adherence of C. pneumoniae-infected macrophages to endothelial cells in vitro.
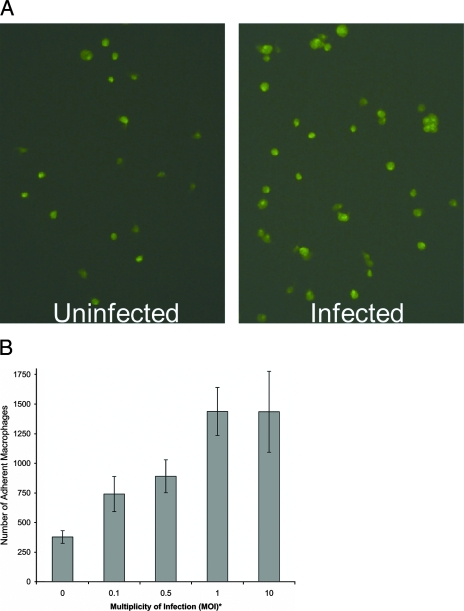
C. pneumoniae infection of macrophages was shown to stimulate adherence of macrophages to uninfected endothelial cells. Figure 1A shows adherent green macrophages under a fluorescence microscope. The increase in the number of adherent green macrophages in the infected group (infected at an MOI of 0.1) over the uninfected group can be readily discerned (Fig. 1A). In addition, in the infected group, clusters of two to four macrophages were observed. Quantification of adherent macrophages showed significant increases of 2.0- to 3.8-fold in the number of macrophages that adhered to endothelial cells when macrophages were infected at MOIs of 0.1, 0.5, 1.0, and 10 (Fig. 1B; done in triplicate).
FIG. 1.
Adherence of C. pneumoniae-infected mouse peritoneal GFP-macrophages to murine endothelial cells (MS1). (A) Fluoroscopic image showing that infected GFP-macrophages adhere better than uninfected macrophages to MS1. (B) Determination of the infectious dose (MOI) required for promotion of adherence. Macrophages were infected with C. pneumoniae at an MOI of 0.1 (A) or at various MOIs (B) for 1 day and assayed for adherence. Macrophages (3 × 104 per well) were added to MS1 monolayers, incubated for 30 min, and washed, and adherent macrophages were counted with a fluorescence microscope at a magnification of ×200 with a fluorescein isothiocyanate-based filter. *, P < 0.01 (MOIs of 0.5, 1.0, and 10) and P < 0.02 (MOI of 0.1).
Adherence of macrophages to C. pneumoniae-infected endothelial cells in vitro.
C. pneumoniae infection of endothelial cells was shown to increase the adherence of uninfected macrophages from female mice to endothelial cells. Endothelial cells were infected at MOIs of 0.1, 0.5, 1, and 10 (Table 1). Significant adherence increases of 2.0- and 1.8-fold were observed at MOIs of 1 and 10, respectively (P < 0.01). A similar magnitude of enhancement was observed when GFP-macrophages from male mice were used. The increases were 1.7- and 2.3-fold at MOIs of 1 and 10, respectively (P < 0.01 and P < 0.05).
TABLE 1.
C. pneumoniae infection of endothelial cells increases adherence of uninfected GFP-macrophages
| MOI | No. of adherent macrophagesa | Fold increase |
|---|---|---|
| 0 | 375 ± 58 | 1.0 |
| 0.1 | 440 ± 33 | 1.2 |
| 0.5 | 522 ± 173 | 1.4 |
| 1.0 | 763 ± 72b | 2.0 |
| 10 | 682 ± 87b | 1.8 |
Average cell count per 20 fields at a magnification of ×200; n = 3.
P < 0.01.
Adherence of C. pneumoniae-infected macrophages to the aortas of normo- and hyperlipidemic mice ex vivo.
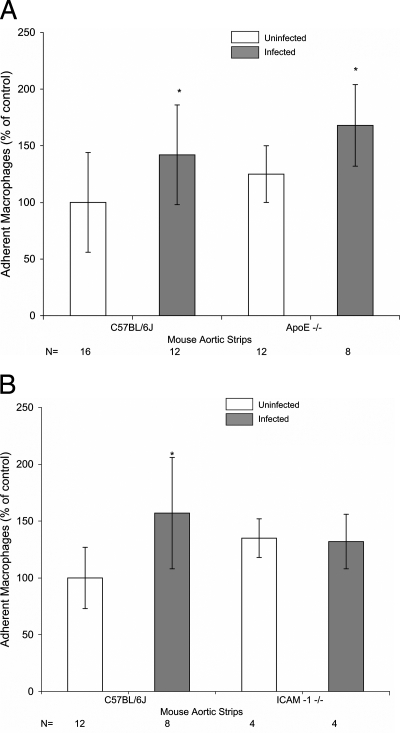
C. pneumoniae infection of macrophages resulted in significant increases in the adherence of macrophages to the aortas of normolipidemic C57BL/6J mice fed a chow diet (1.4-fold, Fig. 2A), hyperlipidemic ApoE-deficient mice fed a chow diet (1.3-fold, Fig. 2A), and C57BL/6J mice fed an atherogenic diet (1.6-fold, Fig. 2B). The adherence of uninfected macrophages to the aortas of hyperlipidemic, ApoE-deficient mice in comparison to those of normolipidemic C57BL/6J mice was also shown to increase slightly, but the increase was not statistically significant (Fig. 2A). The plasma cholesterol levels were 54 ± 7 mg/dl in C57BL/6J mice fed a chow diet, 310 ± 41 mg/dl in ApoE-deficient mice fed a chow diet, and 112 ± 22 mg/dl in C57BL/6J mice fed an atherogenic diet.
FIG. 2.
Adherence of C. pneumoniae-infected mouse peritoneal GFP-macrophages to the murine aorta. Mouse aortic strips were isolated from 13-week-old female C57BL/6J and ApoE−/− mice fed a chow diet (A) and C57BL/6J and ICAM-1 knockout mice fed an atherogenic diet for 5 weeks (B). The aortas were incubated with murine peritoneal GFP-macrophages. The adherent macrophages were counted under a fluorescence microscope. Data are presented as the percentage of adherent macrophages compared to that of the control (uninfected C57BL/6J mice). *, P < 0.05.
Adherence of C. pneumoniae-infected macrophages to the aortas of ICAM-1 knockout mice ex vivo.
To study the role of adhesion molecules in C. pneumoniae-associated atherosclerosis, the adherence of C. pneumoniae-infected macrophages to the aortas of ICAM-1 knockout mice fed an atherogenic diet was evaluated. The plasma cholesterol levels of ICAM-1 knockout mice were 159 ± 12 mg/dl. Infected macrophages adhered no better than uninfected macrophages to the aortas of ICAM-1 knockout mice (Fig. 2B). This was in contrast to the 1.6-fold increase in adherence (P < 0.05) observed with C. pneumoniae-infected macrophages in comparison to uninfected macrophages in hyperlipidemic C57BL/6J mice (Fig. 2B). These findings indicate that ICAM-1 may play a role in C. pneumoniae-accelerated atherosclerosis. In contrast, no decrease in the adherence of uninfected macrophages was observed in ICAM-1-deficient mice in comparison to C57BL/6J mice. This finding may suggest a compensatory effect from other adhesion molecules which were activated in ICAM-1 knockout mice by hyperlipidemia induced by the atherogenic diet (4). Hyperlipidemia has been shown to activate the expression of endothelial adhesion molecules VCAM-1 and ICAM-1 (8).
DISCUSSION
This study demonstrates that C. pneumoniae infection of macrophages promotes the adherence of macrophages to endothelial cells in vitro and ex vivo in the mouse system. The experimental findings from this study support our hypothesis that C. pneumoniae disseminates from the lungs to atherosclerotic lesions and that the homing process is facilitated by an increased adherence of C. pneumoniae-infected macrophages to endothelial cells and aortic tissue. Although the experiments were conducted with macrophages, the in vitro findings were similar to those of experiments performed with monocytes by other investigators (2, 10, 12, 20). Therefore, we would expect similar ex vivo findings if experiments were conducted with mouse monocytes. Nevertheless, the uniqueness of this study was in the use of GFP-macrophages as a tool to facilitate the visualization and enumeration of adherent macrophages.
In this study, adherence of macrophages to endothelial cells was also increased in C. pneumoniae-infected endothelial cells in comparison to uninfected endothelial cells. Similar findings were observed by other investigators (14, 19, 23). These reports showed that infection of human endothelial cells with C. pneumoniae stimulates rolling, adhesion, and transmigration of human neutrophils or monocytes (14, 19, 23).
How C. pneumoniae infection promotes the adherence of GFP-macrophages was not analyzed in this study. Previously, Kalayoglu et al. showed that C. pneumoniae infection of human monocytes activates the expression of the integrin β2 adhesion molecules (10). Subsequently, May et al. (20) reported that C. pneumoniae infection of human monocytes up-regulates very late antigen 4, lymphocyte function-associated antigen 1 (LFA-1), and macrophage antigen 1 (Mac-1) or urokinase receptor and increases the adhesion of human monocytes to human umbilical vein endothelial cells. It has been shown that C. pneumoniae infection induces gamma interferon (27) and that exposure of monocytes to gamma interferon enhances their adhesiveness to endothelial cells and activates LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18), CD14, and l-selectin on monocytes (30). Therefore, it is logical to conclude that C. pneumoniae may use this pathway to promote macrophage adherence.
The ex vivo studies were consistent with the in vitro studies described in this report and reports by other investigators (2, 10, 12). The significant findings from the present study were twofold. (i) C. pneumoniae infection of macrophages increased adherence to the aortas of normolipidemic and hyperlipidemic mice (Fig. 2A and B), and (ii) C. pneumoniae infection failed to enhance the adherence of GFP-macrophages to the aortas of ICAM-1 knockout hyperlipidemic mice (Fig. 2B). The first finding is consistent with our previous in vivo studies showing that C. pneumoniae accelerates hyperlipidemia-induced atherosclerosis (3, 21, 22). The second finding is consistent with studies demonstrating that C. pneumoniae infection may activate LFA-1 and/or Mac-1, the ligand of ICAM-1 (29). Therefore, the results of the ex vivo study with ICAM-1 knockout mice suggests that ICAM-1 is critical for C. pneumoniae-infected mononuclear phagocytes to infiltrate atherosclerotic lesions. This results in promoting a cascade of events, such as C. pneumoniae-induced production of proinflammatory cytokines and other proatherogenic factors by infiltrating macrophages induced by C. pneumoniae, which contribute to the progression of atherosclerosis.
In conclusion, by using GFP-macrophages, this study demonstrates that C. pneumoniae infection of macrophages enhances the adherence of macrophages to endothelial cells in vitro and the aortas of normolipidemic and hyperlipidemic mice ex vivo and that ICAM-1 is critical for the adherence of C. pneumoniae-infected macrophages to the aorta.
Acknowledgments
This study was supported by National Institutes of Health grants HL-56036 and AI-43060.
We thank Mark Berry and Angela Lam for technical support.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Arbiser, J. L., M. A. Moses, C. A. Fernandez, N. Ghiso, Y. Cao, N. Klauber, D. Frank, M. Brownlee, E. Flynn, S. Parangi, H. R. Byers, and J. Folkman. 1997. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc. Natl. Acad. Sci. USA 94861-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azenabor, A. A., G. Job, and O. O. Adedokun. 2005. Chlamydia pneumoniae infected macrophages exhibit enhanced plasma membrane fluidity and show increased adherence to endothelial cells. Mol. Cell. Biochem. 26969-84. [DOI] [PubMed] [Google Scholar]
- 3.Blessing, E., L. A. Campbell, M. E. Rosenfeld, N. Chough, and C.-C. Kuo. 2001. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis 15813-17. [DOI] [PubMed] [Google Scholar]
- 4.Ebnet, K., E. P. Kaldjian, A. O. Anderson, and S. Shaw. 1996. Orchestrated information transfer underlying leukocyte endothelial interactions. Annu. Rev. Immunol. 14155-177. [DOI] [PubMed] [Google Scholar]
- 5.Gieffers, J., G. van Zandbergen, J. Rupp, F. Sayk, S. Kruger, S. Ehlers, W. Solbach, and M. Maass. 2004. Phagocytes transmit Chlamydia pneumoniae from the lungs to the vasculature. Eur. Respir. J. 23506-510. [DOI] [PubMed] [Google Scholar]
- 6.Hogg, N., and C. Berlin. 1995. Structure and function of adhesion receptors in leukocyte trafficking. Immunol. Today 16327-334. [DOI] [PubMed] [Google Scholar]
- 7.Hu, H., G. N. Pierce, and G. Zhong. 1999. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J. Clin. Investig. 103747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iiyama, K., L. Hajra, M. Iiyama, H. Li, M. DiChiara, B. D. Medoff, and M. I. Cybulsky. 1999. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ. Res. 85199-207. [DOI] [PubMed] [Google Scholar]
- 9.Ishida, T., S. Y. Choi, R. K. Kundu, J. Spin, T. Yamashita, K. Hirata, Y. Kojima, M. Yokoyama, A. D. Cooper, and T. Quertermous. 2004. Endothelial lipase modulates susceptibility to atherosclerosis in apolipoprotein-E-deficient mice. J. Biol. Chem. 27945085-45092. [DOI] [PubMed] [Google Scholar]
- 10.Kalayoglu, M. V., B. N. Perkins, and G. I. Byrne. 2001. Chlamydia pneumoniae-infected monocytes exhibit increased adherence to human aortic endothelial cells. Microbes Infect. 3963-969. [DOI] [PubMed] [Google Scholar]
- 11.Kaukoranta-Tolvanen, S. S., T. Ronni, M. Leinonen, P. Saikku, and K. Laitinen. 1996. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb. Pathog. 21407-411. [DOI] [PubMed] [Google Scholar]
- 12.Kaul, R., and W. M. Wenman. 2001. Chlamydia pneumoniae facilitates monocyte adhesion to endothelial and smooth muscle cells. Microb. Pathog. 30149-155. [DOI] [PubMed] [Google Scholar]
- 13.Kelly, K. J., W. W. Williams, Jr., R. B. Colvin, S. M. Meehan, T. A. Springer, J. C. Gutierrez-Ramos, and J. V. Bonventre. 1996. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Investig. 971056-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krüll, M., A. C. Klucken, F. N. Wuppermann, O. Fuhrmann, C. Magerl, J. Seybold, S. Hippenstiel, J. H. Hegemann, C. A. Jantos, and N. Suttorp. 1999. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J. Immunol. 1624834-4841. [PubMed] [Google Scholar]
- 15.Kuo, C.-C., and J. T. Grayston. 1976. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect. Immun. 131103-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo, C.-C., and J. T. Grayston. 1990. A sensitive cell line, HL cells, for isolation and propagation of Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 162755-758. [DOI] [PubMed] [Google Scholar]
- 17.Kuo, C.-C., A. Shor, L. A. Campbell, H. Fukushi, D. L. Patton, and J. T. Grayston. 1993. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J. Infect. Dis. 167841-849. [DOI] [PubMed] [Google Scholar]
- 18.Libby, P. 2002. Inflammation in atherosclerosis. Nature 420868-874. [DOI] [PubMed] [Google Scholar]
- 19.MacIntyre, A., R. Abramov, C. J. Hammond, A. P. Hudson, E. J. Arking, C. S. Little, D. M. Appelt, and B. J. Balin. 2003. Chlamydia pneumoniae infection promotes the transmigration of monocytes through human brain endothelial cells. J. Neurosci. Res. 71740-750. [DOI] [PubMed] [Google Scholar]
- 20.May, A. E., V. Redecke, S. Gruner, R. Schmidt, S. Massberg, T. Miethke, B. Ryba, C. Prazeres da Costa, A. Schomig, and F. J. Neumann. 2003. Recruitment of Chlamydia pneumoniae-infected macrophages to the carotid artery wall in noninfected, nonatherosclerotic mice. Arterioscler. Thromb. Vasc. Biol. 23789-794. [DOI] [PubMed] [Google Scholar]
- 21.Moazed, T. C., L. A. Campbell, M. E. Rosenfeld, J. T. Grayston, and C.-C. Kuo. 1999. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J. Infect. Dis. 180238-241. [DOI] [PubMed] [Google Scholar]
- 22.Moazed, T. C., C.-C. Kuo, J. T. Grayston, and L. A. Campbell. 1998. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J. Infect. Dis. 1771322-1325. [DOI] [PubMed] [Google Scholar]
- 23.Molestina, R. E., R. D. Miller, J. A. Ramirez, and J. T. Summersgill. 1999. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect. Immun. 671323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okabe, M., M. Ikawa, K. Kominami, T. Nakanishi, and Y. Nishimune. 1997. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407313-319. [DOI] [PubMed] [Google Scholar]
- 25.Piedrahita, J. A., S. H. Zhang, J. R. Hagaman, P. M. Oliver, and N. Maeda. 1992. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 894471-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez, J. A., and the Chlamydia pneumoniae/Atherosclerosis Study Group. 1996. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann. Intern. Med. 125979-982. [DOI] [PubMed] [Google Scholar]
- 27.Rothfuchs, A. G., C. Trumstedt, H. Wigzell, and M. E. Rottenberg. 2004. Intracellular bacterial infection-induced IFN-γ is critically but not solely dependent on Toll-like receptor 4-myeloid differentiation factor 88-IFN-αβ-STAT1 signaling. J. Immunol. 1726345-6353. [DOI] [PubMed] [Google Scholar]
- 28.Saikku, P., M. Leinonen, K. Mattila, M. R. Ekman, M. S. Nieminen, P. H. Mäkelä, J. K. Huttunen, and V. Valtonen. 1988. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet ii983-986. [DOI] [PubMed] [Google Scholar]
- 29.van de Stolpe, A., and P. T. van der Saag. 1996. Intercellular adhesion molecule-1. J. Mol. Med. 7413-33. [DOI] [PubMed] [Google Scholar]
- 30.Wang, J., H. Beekhuizen, and R. van Furth. 1994. Surface molecules involved in the adherence of recombinant interferon-gamma (rIFN-gamma)-stimulated human monocytes to vascular endothelial cells. Clin. Exp. Immunol. 95263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]