Abstract
The Mongolian gerbil model of Helicobacter pylori infection resembles human gastritis. In this study, we investigated the role of NF-κB activation in H. pylori-infected gerbils. Activated macrophages were significantly increased in H. pylori-infected gastric mucosa and were identified as being important cells with potent activation of NF-κB, which plays an important part in producing proinflammatory cytokines. Macrophage depletion by the administration of clodronate resulted in milder inflammation in gerbils infected with H. pylori. In macrophages, the inhibition of IκB kinase β (IKKβ), which is a critical kinase for NF-κB activation, resulted in lower proinflammatory cytokine expression caused by heat-killed H. pylori cells. Furthermore, treatment with IKKβ inhibitor resulted in milder inflammation in gerbils with H. pylori gastritis. Collectively, our data suggest that H. pylori-mediated gastric inflammation critically depends on the efficient recruitment and activation of macrophages, with sufficient NF-κB activation.
Helicobacter pylori, a gram-negative pathogen, induces chronic inflammation in the stomach (21). H. pylori infection is known to be causally related to chronic active gastritis and peptic ulcers. Epidemiological studies have also demonstrated its association with gastric malignant diseases, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma (6, 19, 26). H. pylori was defined as a definite carcinogen at the World Health Organization/International Agency for Research on Cancer meeting in 1994 (15). The association between H. pylori infection and gastric adenocarcinoma was demonstrated in a recent prospective study (25).
In the H. pylori-infected gastric mucosa, numerous inflammatory cells, such as neutrophils, macrophages, and even lymphocytes, are infiltrated (4). Among these, macrophages are thought to be critical cell types for the innate immune response against H. pylori and to express abundant proinflammatory cytokines. However, the function of macrophages in H. pylori-associated gastritis has not been determined in vivo.
NF-κB transcription factors are critical regulators of genes involved in inflammation, innate immunity, and suppression of apoptosis (7). In resting cells, NF-κB is retained in the cytoplasm by IκB inhibitors, which are rapidly degraded in response to stimuli such as tumor necrosis factor alpha (TNF-α) and bacterial lipopolysaccharide (LPS), resulting in NF-κB nuclear entry (5). This process requires the phosphorylation of IκBs by the IκB kinase (IKK) complex, which is composed of three subunits: IKKα, IKKβ, and IKKγ. IKKβ is critical for IκB degradation and the activation of NF-κB in response to proinflammatory stimuli and pathogen-associated molecular patterns. NF-κB dimers can translocate to the nucleus, in which they modulate the transcription of genes encoding cytokines, chemokines, and antiapoptotic factors (5, 7). IKKβ deficiency results in the absence of innate immunity, as well as a deficiency of p65, an NF-κB subunit (8, 9). Thus, selective inhibition of the IKK complex has been raised as a promising target to block aberrant NF-κB activity in inflammatory diseases such as H. pylori infection (12).
To determine whether the macrophages and the NF-κB activation in gastric mucosa infected with H. pylori are involved in gastric inflammation, we used the Mongolian gerbil model, which shows inflammation similar to that in the human stomach. Surprisingly, macrophage depletion markedly reduced H. pylori-induced gastric inflammation. In addition, the inhibition of IKKβ by its inhibitor also resulted in a marked decrease in gastric inflammation, suggesting that NF-κB signaling in the gastric epithelium, a critical regulator of its inflammation, has important implications for understanding the pathogenetic mechanisms of gastric inflammation and carcinogenesis.
MATERIALS AND METHODS
Bacterial strains and culture.
The clinical isolate H. pylori TN2 (a type I H. pylori) and its cagE mutant were used in this study (16). Bacteria were maintained under microaerophilic conditions in brucella broth culture medium (Becton Dickinson, Franklin Lakes, NJ) supplemented with 5% fetal bovine serum (Gibco Laboratories, Grand Island, NY). Heat-killed H. pylori cells were prepared from cultured H. pylori cells (107 CFU/ml). The number of the infecting bacteria in the stomach was measured as previously described (16).
Cell lines.
Mouse macrophage cell line J774A.1 was maintained in RPMI medium containing 10% fetal bovine serum and 50 μg/ml kanamycin.
Reagents.
Human TNF-α was purchased from R&D Systems (Minneapolis, MN). Escherichia coli LPS was purchased from Sigma (St. Louis, MO). The polyclonal anti-phospho-IκBα (Ser32), anti-phospho-Jun N-terminal protein kinase (JNK) (Thr183/Tyr185), and anti-phospho-p38 (Thr180/Tyr182) were purchased from Cell Signaling Technology (Beverly, MA). Anti-IκBα, anti-α-tubulin, and anti-β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Clodronate liposomes were a gift from Roche GmbH (Mannheim, Germany) (1). IKK inhibitor IMD-0354 was molecularly designed and synthesized and provided by the Institute of Medicinal Molecular Design (Tokyo, Japan) (18, 23).
Animals.
Four-week-old male specific-pathogen-free Mongolian gerbils were purchased from Japan SLC (Shizuoka, Japan). They were maintained under standard laboratory conditions (at room temperature with relative humidity of 55% ± 5% [mean ± standard deviation] and a 12-/12-h light/dark cycle).
Systematic depletion of macrophages by clodronate liposomes.
Gerbils were given phosphate-buffered saline (PBS) or clodronate liposomes intravenously. After 48 h, LPS was injected intraperitoneally, and the animals were killed 10 h later. The liver, spleen, and colon were removed, and the F4/80-positive cells were counted as described below.
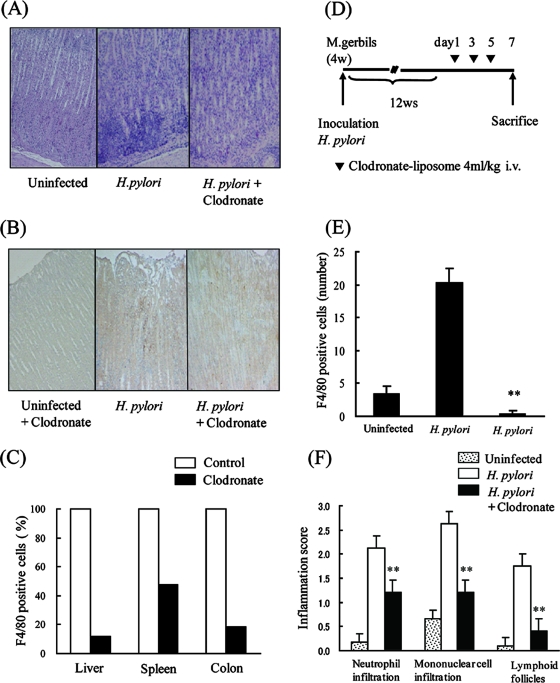
In the long-term infection experiment, Mongolian gerbils were inoculated with broth culture containing 5 × 107 CFU H. pylori. Twelve weeks after inoculation, as shown in Fig. 1, PBS (n = 4 gerbils) or clodronate liposomes (n = 5 gerbils) were injected intraperitoneally. Following death, one-half of the stomach was embedded in paraffin and stained with hematoxylin and eosin (H&E) or F4/80 for histopathological examination. The inflammation level was evaluated by using the updated Sydney system (3). The other half of the stomach was used for measuring the number of infecting bacteria in the stomach.
FIG. 1.
Effect of clodronate treatment on Helicobacter pylori-induced gastritis. (A) Mongolian gerbils were inoculated with H. pylori, and after 12 weeks, PBS or clodronate liposomes (4 ml/kg body weight) were injected intravenously. After 7 days, the gerbils were killed; their stomachs were removed, fixed, and stained with H&E (middle and right panels). Left panel shows uninfected control. Original magnification, ×100. (B) Gerbils were treated as described above. Paraffin-embedded stomach sections were prepared after PBS or clodronate injection and were immunostained for F4/80 (middle and right panel). Left panel shows stomach section of uninfected control. Original magnification, ×100. (C) Clodronate or PBS liposomes were injected intravenously into Mongolian gerbils. After 48 h, systemic inflammation was induced by intraperitoneal injection of LPS (5 mg/kg body weight). Ten hours later, the gerbils were killed; F4/80 expression in the colon, liver, and spleen was determined by immunohistochemistry. (D) Schematic representation of the experimental protocol for in vivo depletion of macrophages by clodronate. Gerbils were inoculated with H. pylori, and after 12 weeks (w), PBS or clodronate liposomes (4 ml/kg body weight) were injected intravenously. After 7 days, the gerbils were killed and their stomachs analyzed. (E) Numbers of F4/80-positive cells were compared for gerbils treated with PBS or clodronate as described above. (F) Inflammatory cell infiltration was evaluated by the updated Sydney system. The results shown in panels E and F are the means ± SEM. **, P < 0.05 by Student's t test.
ELISA.
For chemokine and cytokine analysis, supernatants from J774.1 cells cocultured with heat-killed H. pylori cells or E. coli LPS for 24 h were collected, and interleukin 6 (IL-6) and TNF-α production was analyzed using specific quantitative enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems), according to the manufacturer's protocols.
Immunohistochemistry.
Immunohistochemistry was performed by using anti-F4/80 or anti-phospho-IκBα in paraffin-embedded stomachs. Samples were incubated with anti-F4/80 (rat polyclonal antibody, 1:100; Caltag Laboratories, Burlingame, CA) followed by incubation with biotinylated goat anti-rat immunoglobulin G (IgG). For phospho-IκBα staining, samples were incubated with anti-phospho-IκBα (mouse monoclonal antibody, 1:50; Cell Signaling Technology) followed by incubation with biotinylated anti-mouse IgG. Sections were incubated with a horseradish peroxidase-conjugated streptavidin complex using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA), and the reaction was developed using diaminobenzidine substrate. The number of F4/80- or phospho-IκBα-positive cells was evaluated by taking the average of the results for three different tissue sections. For checking the specificity, we used only the secondary antibody without the first antibody on the same sections and found that the stainings were specific for F4/80 and phospho-IκBα.
Western blotting.
Equal amounts of cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidine difluoride membrane. The membrane was probed with the indicated primary antibody, followed by the horseradish peroxidase-conjugated secondary antibody, and developed by using an ECL plus kit (Amersham, Bucks, United Kingdom).
Treatment with IKK inhibitor.
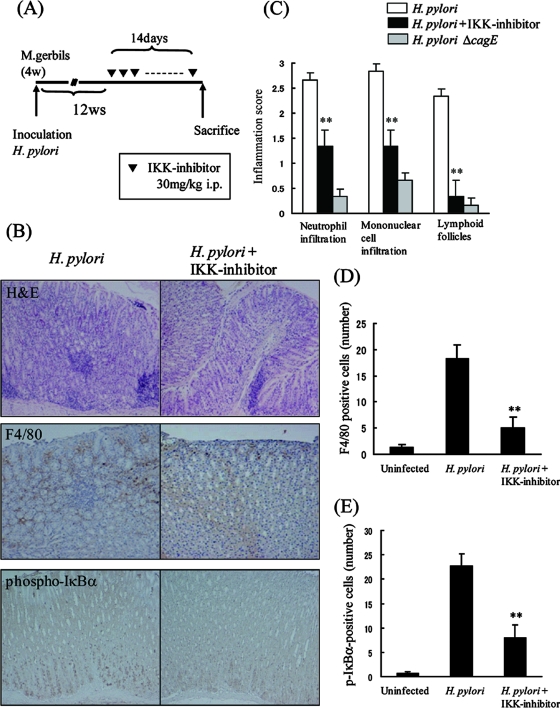
Mongolian gerbils were inoculated with broth culture containing 5 × 107 CFU H. pylori. Twelve weeks after inoculation, IKK inhibitor (n = 4 gerbils) or vehicle (n = 5 gerbils) was injected intraperitoneally, as shown in Fig. 4. Half of the stomach was fixed and stained with H&E, anti-F4/80 antibody, or anti-phospho-IκBα antibody. The number of phospho-IκBα-positive cells and the inflammation score were evaluated by using the updated Sydney system. The other half of the stomach was used for measuring the number of infecting bacteria in the stomach.
FIG. 4.
Effect of IKK inhibitor on Mongolian gerbils infected with Helicobacter pylori. (A) Schematic representation of the experimental protocol. Mongolian gerbils were inoculated with H. pylori cells. After 12 weeks (w), IKK inhibitor (30 mg/kg body weight) or vehicle was injected intraperitoneally (i.p.). After 14 days, gerbils were killed and their stomachs analyzed. (B) Paraffin-embedded H. pylori-infected stomach sections with (right panel) or without (left panel) IKK inhibitor were prepared and stained with H&E. Immunohistochemistry for F4/80 or phospho-IκBα was also performed. Original magnification, ×100. (C) Inflammatory cell infiltration was evaluated by using the updated Sydney system. (D) The number of F4/80-positive cells was compared for gerbils treated with vehicle or IKK inhibitor. (E) The number of phospho-IκBα-positive (p-IκBα-positive) cells was compared for gerbils treated with vehicle or IKK inhibitor. The results shown in panels C to E are the means ± SEM. **, P < 0.05 by Student's t test.
Statistical analysis.
Data are expressed as the means ± standard errors of the means (SEM). Differences were analyzed by Student's t test. A P value of ≤0.05 was considered significant.
RESULTS
Helicobacter pylori induces chronic inflammation and macrophage accumulation in the stomach of Mongolian gerbils.
We initially investigated gastric inflammation in Mongolian gerbils inoculated with H. pylori. Twelve weeks after inoculation, severe gastritis characterized by neutrophil and mononuclear cell infiltration could be seen in the stomach. In contrast, inflammation was not observed in the stomach of uninfected gerbils (Fig. 1A). Infiltration of mature macrophages, one of the major cell types in the human gastric epithelium infected with H. pylori, was assessed by immunohistochemistry with monoclonal antibody against F4/80, a specific marker for tissue macrophages. Macrophage infiltration in gerbils infected with H. pylori was markedly elevated compared to that in uninfected gerbils, which showed only a few macrophages (Fig. 1B).
Improvement of chronic gastritis in Mongolian gerbils after depletion of macrophages.
Clodronate liposomes were used to specifically deplete macrophages. A single injection of clodronate into mice was reported to induce depletion of macrophages within 2 days (27). Mongolian gerbils were given clodronate liposomes, and the degree of macrophage depletion was assessed by immunostaining with F4/80, a specific marker for resident tissue macrophages, 2 days after administration. In Mongolian gerbils treated with clodronate, two- to fivefold reductions in F4/80-positive cells were observed in the colon, liver, and spleen (Fig. 1C).
To investigate the role of macrophages in chronic gastritis, we used a long-term H. pylori infection model. Mongolian gerbils were inoculated with H. pylori cells, and 12 weeks later, PBS or clodronate liposomes were injected intravenously three times a week (Fig. 1D). After 1 week, H. pylori cells were recovered from all animals in the challenged groups, and bacterial density was not different between the groups. The inflammation level in the stomach was evaluated by H&E staining or F4/80 immunostaining. Marked infiltration of macrophages was observed in gerbils treated with PBS liposomes. In contrast, gerbils injected with clodronate liposomes showed not only elimination of F4/80-positive macrophages, but also a decrease in infiltration with other inflammatory cells, such as neutrophils (Fig. 1A, B, E, and F). These results indicate that macrophage accumulation leads to full expression of gastric inflammation.
Macrophages are an important source of NF-κB-regulated proinflammatory cytokines in H. pylori-infected gastric mucosa.
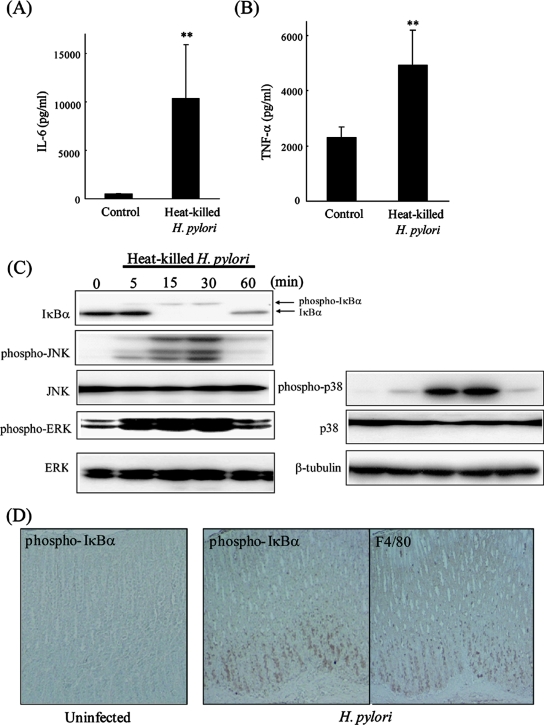
To investigate the response to H. pylori in macrophages, J774A.1 cells were cultured with heat-killed H. pylori cells for 24 h, and cytokine production was analyzed by ELISA. Inflammatory cytokines, such as IL-6 and TNF-α, in macrophage cells were significantly increased by heat-killed H. pylori cells (Fig. 2A and B). We also analyzed intracellular signaling pathways that regulate proinflammatory cytokine expression. IκBα was phosphorylated and degraded in J774A.1 cells cultured with heat-killed H. pylori cells (Fig. 2C), indicating that the NF-κB pathway was activated. Other signaling molecules—phospho-JNK, phospho-p38, and phospho-extracellular signal-regulated kinase (ERK)—were also activated in macrophage cells stimulated with heat-killed H. pylori cells (Fig. 2C). In addition, to investigate the intracellular signaling status in vivo, we performed immunohistochemistry for phospho-IκBα in the stomach of infected gerbils. The number of phospho-IκBα-positive cells, which were likely F4/80-positive macrophages, increased markedly in gerbils infected with H. pylori (Fig. 2D).
FIG. 2.
Effect of Helicobacter pylori on NF-κB signaling pathway in macrophages. (A) J774A.1 cells were cultured with heat-killed H. pylori cells for 24 h. IL-6 production levels were analyzed by ELISA. (B) Cells were treated as described above, and TNF-α production was analyzed by ELISA. The results shown in panels A and B are the means ± SEM. **, P < 0.05 by Student's t test. (C) J774A.1 cells were incubated with heat-killed H. pylori cells. Cell lysates were prepared at the indicated times, and the levels of phospho-IκBα, IκBα, phospho-JNK, JNK, phospho-p38, p38, phospho-ERK, and ERK were measured by Western blotting. (D) Mongolian gerbils were inoculated with H. pylori, and their stomachs were removed, fixed, and sectioned 12 weeks later. Serial sections were immunostained with phospho-IκBα (left and middle panels) and F4/80 (right panel). Original magnification, ×100.
Improvement of gastritis by inhibition of IKK in gerbil stomach.
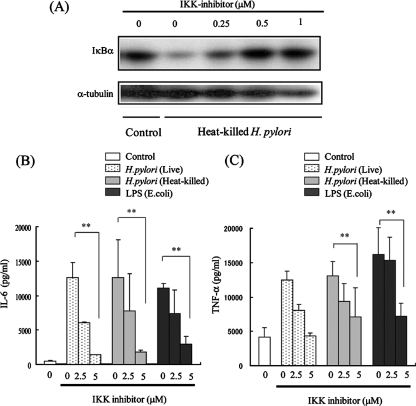
To investigate the relationship between gastric inflammation induced by H. pylori and NF-κB activation, we used IMD-0354 to inhibit IKKβ, a critical kinase for NF-κB activation (18, 23). In J774A.1 cells, IκBα degradation mediated by heat-killed H. pylori cells was strongly suppressed by treatment with IMD-0354 (Fig. 3A). Furthermore, the levels of production of IL-6 and TNF-α by heat-killed H. pylori cells and E. coli LPS were significantly suppressed, in a dose-dependent manner, by treatment with IMD-0354 (Fig. 3B and C). We also found that IL-6 and TNF-α production by live H. pylori cells was significantly suppressed by the treatment with IMD-0354. In contrast, NF-κB activation by live H. pylori cells was not significantly suppressed in gastric epithelial cells (data not shown). We investigated the role of NF-κB activation in the inflammatory response to H. pylori infection in Mongolian gerbils (Fig. 4A). Infiltration of neutrophils and mononuclear cells decreased markedly in the animals injected with IKKβ inhibitor (Fig. 4B and C). H. pylori cells were recovered from all animals in the challenged groups, and bacterial density was not different between the groups. Infiltration of F4/80- or phospho-IκBα-positive cells was also significantly lower in the gerbils injected with IKKβ inhibitor (Fig. 4B, D, and E). These results indicate that NF-κB activation is a critical regulator for H. pylori-mediated gastritis.
FIG. 3.
Effect of IKK inhibitor on Helicobacter pylori-mediated NF-κB activation and cytokine expression. (A) J774A.1 cells were treated with IKK inhibitor. After 1 h, cells were incubated with or without heat-killed H. pylori for 15 min. Cell lysates were prepared, and the degradation of IκBα was evaluated by Western blotting. Tubulin was used as a control. (B) J774A.1 cells were pretreated with or without IKK inhibitor for 1 h, and cells were incubated with or without live H. pylori cells, heat-killed H. pylori cells, or LPS (25 ng/ml). After 24 h, supernatants were collected, and IL-6 production was analyzed by ELISA. (C) Cells were treated as described above, and TNF-α production was analyzed by ELISA. The results shown in panels B and C are the means ± SEM. **, P < 0.05 by Student's t test.
DISCUSSION
Helicobacter pylori infection and macrophage recruitment.
In this study, we showed that H. pylori induced chronic inflammation and macrophage infiltration in gerbils, and that treatment with clodronate for depletion of macrophages caused milder inflammation. The infiltration of neutrophils and lymphocytes was also significantly suppressed in gerbils with macrophage depletion. H. pylori may make contact with epithelial cells and simultaneously stimulate tissue-localized macrophages by soluble factors to produce proinflammatory cytokines, which accumulate macrophages or neutrophils to the locus of inflammation. It has been reported that H. pylori infection induces monocyte chemoattractant protein 1 gene transcription by activating NF-κB via the NF-κB-inducing kinase-IKK signaling complex in gastric epithelial cells (14). This might be one of the reasons why the inhibition of IKK/NF-κB reduced macrophage accumulation induced by H. pylori. In addition, soluble factors, such as LPS, stimulate tissue-localized macrophages to produce proinflammatory cytokines and promote more macrophage accumulation. It is suggested that not only gastric epithelial cells, but also activated macrophages, lead to the accumulation of more macrophages and characterize H. pylori gastritis.
Macrophage accumulation and carcinogenesis.
One common tumor-promoting mechanism may involve inflammation (20). It is estimated that inflammation plays a role in the etiology of 15% of human cancers, mostly acting as a tumor promoter (2). However, precise details of the mechanism are still unknown. Tumor-associated macrophages are involved in tumor progression and metastasis via the release of matrix metalloproteinase, TNF-α, vascular endothelial growth factor, and other molecules. Recently, it was reported that decreasing the number of tumor-associated macrophages in the tumor stroma effectively suppresses tumor growth and metastasis by altering the tumor microenvironment (10). In an H. pylori-related cancer model, H. pylori increased the incidence of carcinogen-induced adenocarcinoma in the glandular stomach, suggesting that H. pylori infection may have tumor-promoting activity (22, 24). It is suggested that macrophage accumulation controlled by NF-κB activation at inflammatory sites has an important role in the induction of chronic inflammation, cell proliferation, and tumor promotion in chronically inflamed mucosa.
NF-κB activation and gastric inflammation.
In this study, we showed that most of the cells with phosphorylated IκBα showed F4/80-positive macrophages. This observation suggests that NF-κB was mainly and strongly activated in macrophages and may play an important role in the inflammatory response. However, we also detected phosphorylated IκBα in the epithelial cells in other areas.
We showed that the cag pathogenicity island (cagPAI) is responsible for various gastric lesions induced in the gerbil model (Fig. 4C) (16). It has also been suggested that cagPAI plays an important role in the pathogenesis of gastric diseases related to H. pylori infection in humans. In in vitro studies, signal transduction pathways, such as NF-κB or mitogen-activated protein kinase, are activated by cagPAI-positive, but not -negative, strains in gastric epithelial cells (11, 13). In contrast, several studies have reported that cagPAI does not contribute to the activation of NF-κB activation in monocytes or lymphocytes (11, 17). These in vivo and in vitro results suggest that H. pylori triggers gastric inflammation through gastric epithelial cells in a cagPAI-dependent manner, following amplification of inflammation due to inflammatory cells such as cagPAI-independent macrophages.
NF-κB transcription factors are critical regulators of genes involved in inflammation, innate immunity, and suppression of apoptosis (7). In this study, we used an inhibitor of IKKβ, a critical kinase for NF-κB activation, and found that the inhibitor was effective for regression of the inflammatory response to H. pylori infection. Although numerous reports suggest the importance of NF-κB activation by H. pylori in vitro, this is, in fact, the first report to show that NF-κB activation is important for H. pylori-mediated gastritis in vivo. The same concentration of inhibitor used in macrophages was not effective for the inhibition of H. pylori-mediated NF-κB activity in gastric epithelial cells (data not shown). We assume that the concentration or delivery of the inhibitor we used in the gastric epithelial cells was not sufficient for inhibition. As the inhibitor may also act mainly on mononuclear cells in vivo, our results indicate that NF-κB-mediated inflammatory cytokine expression from mononuclear cells, such as macrophages, might be the main avenue of H. pylori-mediated gastritis. This result is consistent with the result of macrophage depletion shown in this study.
In conclusion, depletion of macrophages and inhibition of IKKβ, which is mainly activated in macrophages, resulted in a marked decrease in gastric inflammation. These results suggest that NF-κB signaling, a critical regulator of inflammation in the gastric epithelium, has important implications for understanding the mechanisms involved in the pathogenesis of gastric inflammation and cancer.
Acknowledgments
This study was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant 18890244).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Claassen, I., N. Van Rooijen, and E. Claassen. 1990. A new method for removal of mononuclear phagocytes from heterogeneous cell populations in vitro, using the liposome-mediated macrophage “suicide” technique. J. Immunol. Methods 134153-161. [DOI] [PubMed] [Google Scholar]
- 2.Coussens, L. M., and Z. Werb. 2002. Inflammation and cancer. Nature 420860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 201161-1181. [DOI] [PubMed] [Google Scholar]
- 4.Ernst, P. 1999. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment. Pharmacol. Ther. 13(Suppl. 1)13-18. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl.)S81-S96. [DOI] [PubMed] [Google Scholar]
- 6.Graham, D. Y., G. M. Lew, P. D. Klein, D. G. Evans, D. J. Evans, Jr., Z. A. Saeed, and H. M. Malaty. 1992. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann. Intern. Med. 116705-708. [DOI] [PubMed] [Google Scholar]
- 7.Karin, M., and A. Lin. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3221-227. [DOI] [PubMed] [Google Scholar]
- 8.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science 284321-325. [DOI] [PubMed] [Google Scholar]
- 9.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J. Exp. Med. 1891839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo, Y., H. Zhou, J. Krueger, C. Kaplan, S. H. Lee, C. Dolman, D. Markowitz, W. Wu, C. Liu, R. A. Reisfeld, and R. Xiang. 2006. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J. Clin. Investig. 1162132-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda, S., M. Akanuma, Y. Mitsuno, Y. Hirata, K. Ogura, H. Yoshida, Y. Shiratori, and M. Omata. 2001. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 27644856-44864. [DOI] [PubMed] [Google Scholar]
- 12.Maeda, S., H. Yoshida, K. Ogura, Y. Mitsuno, Y. Hirata, Y. Yamaji, M. Akanuma, Y. Shiratori, and M. Omata. 2000. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology 11997-108. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuno, Y., H. Yoshida, S. Maeda, K. Ogura, Y. Hirata, T. Kawabe, Y. Shiratori, and M. Omata. 2001. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 4918-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori, N., A. Ueda, R. Geleziunas, A. Wada, T. Hirayama, T. Yoshimura, and N. Yamamoto. 2001. Induction of monocyte chemoattractant protein 1 by Helicobacter pylori involves NF-κB. Infect. Immun. 691280-1286. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.NIH. 1994. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on helicobacter pylori in peptic ulcer disease. JAMA 27265-69. [PubMed] [Google Scholar]
- 16.Ogura, K., S. Maeda, M. Nakao, T. Watanabe, M. Tada, T. Kyutoku, H. Yoshida, Y. Shiratori, and M. Omata. 2000. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 1921601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohmae, T., Y. Hirata, S. Maeda, W. Shibata, A. Yanai, K. Ogura, H. Yoshida, T. Kawabe, and M. Omata. 2005. Helicobacter pylori activates NF-kappaB via the alternative pathway in B lymphocytes. J. Immunol. 1757162-7169. [DOI] [PubMed] [Google Scholar]
- 18.Onai, Y., J. Suzuki, T. Kakuta, Y. Maejima, G. Haraguchi, H. Fukasawa, S. Muto, A. Itai, and M. Isobe. 2004. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc. Res. 6351-59. [DOI] [PubMed] [Google Scholar]
- 19.Parsonnet, J., D. Vandersteen, J. Goates, R. K. Sibley, J. Pritikin, and Y. Chang. 1991. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J. Natl. Cancer Inst. 83640-643. [DOI] [PubMed] [Google Scholar]
- 20.Philip, M., D. A. Rowley, and H. Schreiber. 2004. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 14433-439. [DOI] [PubMed] [Google Scholar]
- 21.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 3471175-1186. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama, A., F. Maruta, T. Ikeno, K. Ishida, S. Kawasaki, T. Katsuyama, N. Shimizu, and M. Tatematsu. 1998. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 582067-2069. [PubMed] [Google Scholar]
- 23.Tanaka, A., M. Konno, S. Muto, N. Kambe, E. Morii, T. Nakahata, A. Itai, and H. Matsuda. 2005. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood 1052324-2331. [DOI] [PubMed] [Google Scholar]
- 24.Tatematsu, M., M. Yamamoto, N. Shimizu, A. Yoshikawa, H. Fukami, M. Kaminishi, T. Oohara, A. Sugiyama, and T. Ikeno. 1998. Induction of glandular stomach cancers in Helicobacter pylori-sensitive Mongolian gerbils treated with N-methyl-N-nitrosourea and N-methyl-N′-nitro-N-nitrosoguanidine in drinking water. Jpn. J. Cancer Res. 8997-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uemura, N., S. Okamoto, and S. Yamamoto. 2002. H. pylori infection and the development of gastric cancer. Keio J. Med. 51(Suppl. 2)63-68. [DOI] [PubMed] [Google Scholar]
- 26.Wotherspoon, A. C., C. Ortiz-Hidalgo, M. R. Falzon, and P. G. Isaacson. 1991. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 3381175-1176. [DOI] [PubMed] [Google Scholar]
- 27.Yi, S., W. J. Hawthorne, A. M. Lehnert, H. Ha, J. K. Wong, N. van Rooijen, K. Davey, A. T. Patel, S. N. Walters, A. Chandra, and P. J. O'Connell. 2003. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J. Immunol. 1702750-2758. [DOI] [PubMed] [Google Scholar]