Abstract
Despite the widely held belief that gastric acid serves as a barrier to bacterial pathogens, there are almost no experimental data to support this hypothesis. We have developed a mouse model to quantify the effectiveness of gastric acid in mediating resistance to infection with ingested bacteria. Mice that were constitutively hypochlorhydric due to a mutation in a gastric H+/K+-ATPase (proton pump) gene were infected with Yersinia enterocolitica, Salmonella enterica serovar Typhimurium, Citrobacter rodentium, or Clostridium perfringens cells or spores. Significantly greater numbers of Yersinia, Salmonella, and Citrobacter cells (P ≤ 0.006) and Clostridium spores (P = 0.02) survived in hypochlorhydric mice, resulting in reduced median infectious doses. Experiments involving intraperitoneal infection or infection of mice treated with antacids indicated that the increased sensitivity of hypochlorhydric mice to infection was entirely due to the absence of stomach acid. Apart from establishing the role of gastric acid in nonspecific immunity to ingested bacterial pathogens, our model provides an excellent system with which to investigate the effects of hypochlorhydria on susceptibility to infection and to evaluate the in vivo susceptibility to gastric acid of orally administered therapies, such as vaccines and probiotics.
The ubiquitous distribution of gastric acid among fish, amphibians, reptiles, birds, and mammals implies that it is evolutionarily advantageous (14). Three main functions are ascribed to gastric acid: (i) it activates pepsinogen and denatures proteins, (ii) it augments absorption of dietary calcium and iron, and (iii) it inhibits infectious agents from reaching the intestine (12).
Hydrochloric acid is secreted by parietal cells in the stomach. Acidification of the gastric lumen occurs due to the activity of the gastric H+,K+-ATPase or “proton pump,” which exchanges luminal K+ for cytoplasmic H+ (6, 29, 36). This enzyme has an α subunit, which contains the catalytic site of the enzyme (1), and a highly glycosylated β subunit (2, 25, 32, 34, 35). Gastric acid secretion is stimulated primarily by histamine released from enterochromaffin-like cells in response to gastrin (17).
Gastric juice consists of HCl and pepsin and can kill bacteria within 15 min when the pH is less than 3.0 (8). If the pH is raised above 4.0, bacterial overgrowth may occur. Hypochlorhydria (4 < pH < 7) (3) and achlorhydria (pH > 7) can be acquired or iatrogenic. Acquired hypochlorhydria can result from atrophic gastritis or be induced by malnutrition (10, 15). Iatrogenic hypochlorhydria can be caused by gastric surgery or by drugs that inhibit acid secretion (18). Regardless of the cause, a number of studies have associated hypochlorhydria with an increased risk of infection (12, 22). However, as discussed by Martinsen et al. (18), gastrointestinal infections themselves reduce gastric acid secretion in humans and animals, and few systematic experimental and epidemiological studies have been performed to determine the contribution of gastric acid to infection resistance.
Since the H+,K+-ATPase β subunit is required for activity of the H+,K+-ATPase, H+,K+-ATPase β-subunit-deficient mice (31) have a gastric luminal pH of ∼7, whereas wild-type mice have a gastric luminal pH of ∼3.6. Moreover, H+,K+-ATPase β-subunit-deficient mice do not produce any gastric HCl in response to treatment with histamine (21). To investigate the contribution of gastric acid to infection resistance, we infected H+,K+-ATPase β-subunit-deficient mice perorally with the gram-negative bacterial pathogens Yersinia enterocolitica, Salmonella enterica serovar Typhimurium, and Citrobacter rodentium and the gram-positive pathogen Clostridium perfringens and compared the resistance of these mice to infection to that of control mice.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. Y. enterocolitica strains were grown on Luria or Yersinia selective (CIN) agar (Oxoid Ltd., Basingstoke, England) at 30°C. C. rodentium and S. enterica serovar Typhimurium strains were grown on Luria-Bertani agar at 37°C. C. perfringens was grown on nutrient agar (Oxoid) supplemented with 0.1% (wt/vol) sodium thioglycolate and 0.375% (wt/vol) glucose at 37°C in GasPak anaerobic jars (BD Diagnostics, Sparks, MD). When required, antibiotics (Sigma-Aldrich, St. Louis, MO) were used at the following final concentrations: 25 μg/ml streptomycin, 50 μg/ml nalidixic acid, 100 μg/ml kanamycin, and 25 μg/ml chloramphenicol. For mouse infection experiments, overnight cultures of all of the bacteria except C. perfringens that were grown in Luria-Bertani broth supplemented with the appropriate antibiotics were used. The number of bacteria administered to mice was determined by plating 10-fold serial dilutions of the inoculum on appropriate media.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristic(s) | Reference(s) |
|---|---|---|
| Y. enterocolitica 8081 | Biotype 1B, serotype O:8 | 23 |
| Y. enterocolitica 8081u− | Y. enterocolitica 8081 ureF::Kmr | 4 |
| S. enterica serovar Typhimurium SL1344 | Resistant to streptomycin | 11 |
| C. rodentium ICC169 | Resistant to nalidixic acid | 7, 37 |
| C. perfringens JIR4472 | SM101(pJIR1841), spore forming, resistant to chloramphenicol | V. Adams, D. Lyras, and J. I. Rood, unpublished |
To prepare C. perfringens vegetative cells, a log-phase culture of strain JIR4472 was prepared by inoculating a single colony into 6 ml of preboiled fluid thioglycolate broth (Difco, Detroit, MI). After overnight growth at 37°C, a 3-ml aliquot was used to inoculate 90 ml of preboiled fresh Trypticase-peptone-glucose medium (27), which was then incubated for 4 h at 37°C. The cells were collected by centrifugation, washed once with sterile phosphate-buffered saline (PBS), and resuspended in 2 ml of PBS prior to inoculation into mice. C. perfringens spores were prepared in a similar manner, except that a 3-ml aliquot of an overnight fluid thioglycolate broth culture was used to inoculate 90 ml of Duncan and Strong's medium (5) and grown at 37°C for 24 h. The cells were collected by centrifugation and washed once with sterile ice-cold water. The cell pellet was resuspended in 3 ml of water and heated at 80°C for 20 min to kill the vegetative cells.
Susceptibility to acid in vitro.
The susceptibility of bacteria to acid was tested as described previously (4, 9). Briefly, stationary-phase cultures were diluted 1:105 in PBS at pH 2.0, 2.5, 3.0, 3.5, 4.0, and 7.0. The buffer used for Y. enterocolitica also contained 3.4 mM urea (4). Following incubation for 2 h at 37°C, bacteria were enumerated by spreading them on appropriate media, and the viable counts were compared to those in the original inocula.
Mice.
H+,K+-ATPase β-subunit-deficient transgenic mice (genotype −/−) (31) and wild-type BALB/cCrSlc mice (genotype +/+) were housed under specific-pathogen-free conditions in the Department of Microbiology and Immunology Animal House, The University of Melbourne, Melbourne, Australia. The genotypes of the mice were confirmed by PCR analysis. Six-to-eight-week-old male and female mice were used throughout this study. In each experiment, the two strains of mice were age and sex matched as closely as possible. During the course of the experiments, mice were given nonacidified, autoclaved water and were housed in isolator cages with free access to food unless otherwise specified. All work with animals was approved by The University of Melbourne Animal Ethics Committee and was conducted in accordance with the guidelines for animal experimentation of the Australian National Health and Medical Research Council.
Oral infection of mice.
Two series of experiments were performed, in which mice were examined 3 days or 30 min after inoculation with bacteria. For the 3-day experiments, which were designed to measure bacterial colonization, various doses of stationary-phase cultures of Y. enterocolitica, S. enterica serovar Typhimurium, or C. rodentium were administered by gavage to between 5 and 10 BALB/cCrSlc and H+,K+-ATPase β-subunit-deficient mice using a 20-gauge needle (Cole-Palmer, Vernon Hills, IL). Three days later, the mice were killed by CO2 inhalation, and the small intestine of mice inoculated with Y. enterocolitica or S. enterica serovar Typhimurium and the cecum of mice inoculated with C. rodentium were removed aseptically and placed in 5 ml of PBS. Samples were weighed, homogenized with a Polytron homogenizer (Kinematica, Lucerne, Switzerland), and diluted in PBS, and the bacteria were enumerated on selective agar. The median infectious dose (ID50) was calculated using the method of Reed and Muench (24). For the 30-min experiments, which were designed to determine the short-term effects of gastric acid on bacterial viability, mice were inoculated by gavage with 108 CFU of Y. enterocolitica, S. enterica serovar Typhimurium, C. rodentium, or C. perfringens cells or spores in 200 μl of unbuffered saline. After 30 min, the mice were killed by CO2 inhalation, and the stomach, small intestine, and large intestine were removed and placed in 2, 5, and 5 ml of PBS, respectively, for homogenization and bacterial enumeration as described above.
Pretreatment of mice with gastric acid-altering agents.
Approximately 16 h prior to infection, a wire grid was placed on the base of each mouse cage and food was removed.
(i) Histamine.
Treatment procedures were performed as previously described (21). Briefly, 30 min before infection, BALB/cCrSlc and H+,K+-ATPase β-subunit-deficient mice were inoculated intraperitoneally (i.p.) with histamine (10 mg/kg; Sigma-Aldrich) dissolved in 100 μl of 0.15 M NaCl or with diluent alone (21). Mice were then inoculated with bacteria by gavage and killed 30 min later.
(ii) Sodium bicarbonate.
Thirty minutes before infection, five BALB/cCrSlc mice were inoculated by gavage with 100 μl of a sterile solution of 10% (wt/vol) sodium bicarbonate dissolved in saline. Another group of BALB/cCrSlc mice and a group of H+,K+-ATPase β-subunit-deficient mice were inoculated by gavage with saline alone. All mice were then inoculated by gavage with 200 μl of a bacterial suspension containing 108 CFU of C. rodentium and killed 30 min later by CO2 inhalation.
(iii) Omeprazole.
Five BALB/cCrSlc mice were treated with the proton pump inhibitor omeprazole as described previously (21). Mice were inoculated i.p. with 100 μl of omeprazole (400 μmol/kg) dissolved in dimethyl sulfoxide-polyethylene glycol (average Mr, 8,000) (9:1, vol/vol). Another group of BALB/cCrSlc mice and a group of H+,K+-ATPase β-subunit-deficient mice were inoculated with the vehicle alone. Mice were inoculated by gavage with C. rodentium as described above 40 min after the i.p. inoculation and killed 30 min later.
Susceptibility of mice to infection by a parenteral route.
Five BALB/cCrSlc mice and five H+,K+-ATPase β-subunit-deficient mice were inoculated i.p. with 200 μl of saline containing 105 CFU of S. enterica serovar Typhimurium SL1344. Twenty-four hours later, the mice were killed, and the spleen and liver of each mouse were removed aseptically and placed in 2 and 5 ml of PBS, respectively. Samples were weighed and homogenized, the bacteria in each organ were enumerated, and the results were expressed in CFU/g of tissue.
Statistical analysis.
Data were analyzed with two-tailed Student's unpaired t test or two-tailed Fisher's exact test using the Prism or Instat programs (GraphPad Software, San Diego, CA). In all studies, a P value of <0.05 was considered statistically significant.
RESULTS
Susceptibility of bacteria to acid in vitro.
To gauge the acid resistance of the gram-negative bacterial strains used in this study, we tested their abilities to survive in PBS at various pHs (Table 2). Wild-type Y. enterocolitica was the most acid-resistant species, surviving at a pH as low as 2.0. This result was most likely due to its ability to produce urease, as the bacteria were killed by acid in the absence of urea (results not shown) and the urease mutant was acid susceptible. At pH 3.0, the viability of S. enterica serovar Typhimurium was reduced to around 4%, and C. rodentium was killed.
TABLE 2.
Ability of bacterial strains to survive for 2 h in phosphate buffer at various pHs
| Strain | % of surviving bacteria (mean ± SD) ata:
|
|||||
|---|---|---|---|---|---|---|
| pH 2.0 | pH 2.5 | pH 3.0 | pH 3.5 | pH 4.0 | pH 7.0 | |
| Y. enterocolitica 8081b | 36.6 ± 9.3 | 74.4 ± 4.6 | 72.5 ± 3.7 | 81.3 ± 5.3 | 66.4 ± 2.5 | 90.0 ± 10.2 |
| Y. enterocolitica 8081u−b | 0 | 0 | 0 | 12.3 ± 4.1 | 94.7 ± 38.5 | 114.9 ± 51.8 |
| S. enterica serovar Typhimurium SL1344 | 0 | 0 | 3.4 ± 5.5 | 80.0 ± 20.4 | 66.3 ± 19.2 | 78.3 ± 9.8 |
| C. rodentium ICC169 | 0 | 0 | 0 | 21.9 ± 23.4 | 54.3 ± 10.3 | 89.1 ± 15.7 |
Percentage of the original inoculum, determined in three independent experiments.
PBS contained 3.4 mM urea.
Susceptibility of H+,K+-ATPase β-subunit-deficient mice to colonization by bacterial pathogens.
To determine if H+,K+-ATPase β-subunit-deficient mice were more susceptible to infection with bacterial enteric pathogens, we orally infected BALB/cCrSlc and H+,K+-ATPase β-subunit-deficient mice using various doses of Y. enterocolitica, S. enterica serovar Typhimurium, and C. rodentium and 3 days later collected the tissue where initial colonization occurs. The small intestine was excised from mice infected with Y. enterocolitica or S. enterica serovar Typhimurium, while the cecum was removed from mice infected with C. rodentium. All of the mice that received the maximum dose (108 CFU) were colonized (Table 3). At lower doses of bacteria, greater numbers of the H+,K+-ATPase β-subunit-deficient mice than of the control mice were colonized. However, the difference was significant only for the mice infected with 106 and 107 CFU S. enterica serovar Typhimurium (P < 0.05, two-tailed Fisher's exact test). These results and the ID50s (Table 3) indicated that the H+,K+-ATPase β-subunit-deficient mice were more susceptible to bacterial colonization. They also indicated that a minimum dose of 108 CFU was required for reliable colonization of mice; therefore, this inoculum was used in all remaining experiments.
TABLE 3.
Numbers of BALB/cCrSlc wild-type mice (genotype +/+) and H+,K+-ATPase β-subunit-deficient transgenic mice (genotype −/−) colonized with Y. enterocolitica, S. enterica serovar Typhimurium, and C. rodentium 3 days after oral infection and ID50s
| Species | Dose (CFU)a | No. of mice containing >103 CFU per g of tissue/no. of mice tested (%)b
|
|
|---|---|---|---|
| Genotype +/+ mice | Genotype −/− mice | ||
| Y. enterocolitica | 108 | 7/7 (100) | 8/8 (100) |
| 107 | 4/5 (80) | 7/7 (100) | |
| 106 | 2/7 (29) | 4/7 (57) | |
| 105 | 0/8 (0) | 2/8 (25) | |
| S. enterica serovar Typhimurium | 108 | 9/10 (90)c | 9/9 (100) |
| 107 | 3/8 (38) | 9/9 (100)d,e | |
| 106 | 3/8 (38) | 8/8 (100)e | |
| 105 | 1/8 (13) | 4/8 (50) | |
| C. rodentium | 108 | 7/7 (100) | 7/7 (100) |
| 107 | 0/7 (0) | 4/8 (50) | |
| 106 | 0/9 (0) | 3/8 (38) | |
For BALB/cCrSlc wild-type mice (genotype +/+) the ID50s of Y. enterocolitica, S. enterica serovar Typhimurium, and C. rodentium were 106.41, 106.85, and 107.5 CFU, respectively; for H+,K+-ATPase β-subunit-deficient transgenic mice (genotype −/−) the ID50s of these organisms were 105.65, 105, and 107 CFU, respectively.
Bacteria were retrieved from the small intestine of mice infected with Y. enterocolitica or S. enterica serovar Typhimurium and from the cecum of mice infected with C. rodentium.
Including one mouse that succumbed to the infection.
Including two mice that succumbed to the infection.
P < 0.05, as determined by two-tailed Fisher's exact test.
Effect of treating wild-type mice with histamine on the viability of orally administered bacteria.
Normally, mice have a higher gastric pH than humans (pH 3.1 to 4.5 versus pH 1.5 to 3.5) (13). Accordingly, to optimize our animal infection model, an increase in the acidity of the mouse stomach was required to mimic the human stomach. A previous study showed that histamine induces an increase in acid secretion that peaks after 45 min (21). To determine if activating acid secretion affected bacterial survival during passage through the stomach, we treated BALB/cCrSlc and H+,K+-ATPase β-subunit-deficient mice with histamine or diluent and infected mice 30 min later with C. rodentium by gavage. The bacteria in the stomach, small intestine, and large intestine were enumerated after another 30 min. The percentage of bacteria that were able to survive passage through BALB/cCrSlc mice pretreated with histamine (8.4% ± 15.7% [mean ± standard deviation]) was lower than the percentage of bacteria that were able to survive passage through BALB/cCrSlc mice pretreated with the diluent (37.2% ± 31.8%) (P = 0.02, two-tailed Student's unpaired t test) (Fig. 1). However, no difference was observed between the H+,K+-ATPase β-subunit-deficient mice pretreated with histamine (104.3% ± 26.1%) and the H+,K+-ATPase β-subunit-deficient mice pretreated with diluent (114.2% ± 33.1% (P = 0.47) because these mice lack the enzyme responsible for gastric acid production and therefore addition of histamine had no effect. As lowering the gastric pH influenced bacterial survival in a way that more accurately mimicked human physiology, in subsequent experiments we compared histamine-treated wild-type mice and untreated H+,K+-ATPase β-subunit-deficient mice.
FIG. 1.
Effect of treating wild-type (genotype +/+) and H+,K+-ATPase β-subunit-deficient (genotype −/−) mice with histamine on the survival of C. rodentium in the intestine. Both mouse strains were fasted, treated with either histamine or diluent, and infected with 108 CFU C. rodentium 30 min later by gavage. Mice were killed 30 min later, and the stomach, small intestine, and large intestine were removed. The samples were placed in PBS, homogenized, and spread on selective agar plates. The results are expressed as percent survival (number of C. rodentium CFU isolated from each mouse/number of CFU in the inoculum × 100). The horizontal bars indicate means. The data were analyzed using a two-tailed Student unpaired t test.
Susceptibility of hyperchlorhydric and hypochlorhydric mice to orally administered bacterial pathogens.
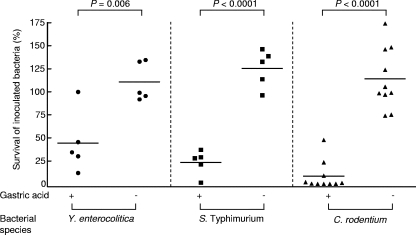
Next, we determined the susceptibility of hyperchlorhydric mice (acid-producing BALB/cCrSlc mice treated with histamine) and hypochlorhydric mice (H+,K+-ATPase β-subunit-deficient mice treated with diluent) to various bacterial pathogens. Greater numbers of Y. enterocolitica were found in the gastrointestinal tracts of hypochlorhydric mice (110.7% ± 21.1%) than in the gastrointestinal tracts of their hyperchlorhydric counterparts (44.4% ± 33.4%) (P = 0.006) (Fig. 2). Similar results were observed for S. enterica serovar Typhimurium (125.5% ± 20.4% versus 23.4% ± 13.5% [P < 0.0001]) and C. rodentium (114.2% ± 33.1% versus 8.4% ± 15.7% [P < 0.0001]).
FIG. 2.
Bacterial survival in hyperchlorhydric and hypochlorhydric mice. Fasted wild-type mice were treated with histamine (gastric acid +), and fasted H+,K+-ATPase β-subunit-deficient mice were treated with diluent (gastric acid −). Thirty minutes later mice were inoculated by gavage with 108 CFU Y. enterocolitica 8081, S. enterica serovar Typhimurium, or C. rodentium (see Fig. 1). Mice were killed 30 min later, and the stomach, small intestine, and large intestine were removed. The samples were placed in PBS, homogenized, and spread on selective agar plates. The results are expressed as percent survival (number of bacteria isolated from each mouse/number of bacteria in the inoculum × 100). The horizontal bars indicate means. The data were analyzed using a two-tailed Student unpaired t test.
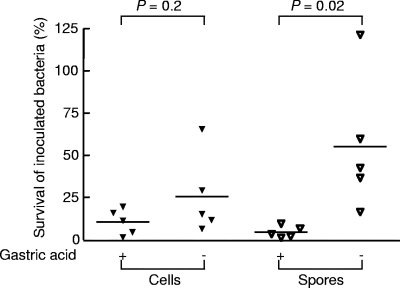
Since Y. enterocolitica, S. enterica serovar Typhimurium, and C. rodentium are all members of the Enterobacteriaceae, we wanted to investigate whether similar results would be observed for an entirely different type of intestinal pathogen. Histamine-treated BALB/cCrSlc and diluent-treated H+,K+-ATPase β-subunit-deficient mice were orally inoculated with vegetative cells of a derivative of a food poisoning strain of C. perfringens. Although slightly more bacteria survived passage through hypochlorhydric mice (25.6% ± 23.8%) than passage through hyperchlorhydric mice (10.6% ± 7.5%), the difference was not significant (P = 0.2, two-tailed Student's unpaired t test) (Fig. 3). By contrast, more C. perfringens spores were retrieved from hypochlorhydric mice (55.3% ± 40.0%) than from control animals (4.5% ± 3.5%) (P = 0.02) (Fig. 3).
FIG. 3.
Survival of C. perfringens vegetative cells and spores in hyperchlorhydric and hypochlorhydric mice. Fasted wild-type mice were treated with histamine (gastric acid +), and fasted H+,K+-ATPase β-subunit-deficient mice were treated with diluent (gastric acid −). Thirty minutes later all mice were inoculated by gavage with 108 CFU C. perfringens vegetative cells or spores. Mice were killed 30 min later, and the stomach, small intestine, and large intestine were removed. The samples were placed in PBS, homogenized, and spread on selective agar plates. The results are expressed as percent survival (number of bacteria isolated from each mouse/number of bacteria in the inoculum × 100). The horizontal bars indicate means. The data were analyzed using a two-tailed Student unpaired t test.
Susceptibility of H+,K+-ATPase β-subunit-deficient mice to parenteral infection with S. enterica serovar Typhimurium.
To ensure that the differences in bacterial colonization between the two mouse strains that we observed were due to differences in gastric acid production and not due to other factors, we performed two further experiments. First, mice were inoculated i.p. with S. enterica serovar Typhimurium and killed 1 day later, before they could succumb to the infection. There was no significant difference in bacterial numbers in either the spleen (3.28 × 106 ± 2.61 × 106 CFU/g versus 2.66 × 106 ± 2.16 × 106 CFU/g) (P > 0.5, two-tailed Student's unpaired t test) or the liver (6.43 × 106 ± 1.48 × 107 CFU/g versus 4.84 × 105 ± 3.97 × 105 CFU/g) (P = 0.4) between the BALB/cCrSlc and H+,K+-ATPase β-subunit-deficient mice.
Susceptibility of hypochlorhydric wild-type mice to orally administered C. rodentium.
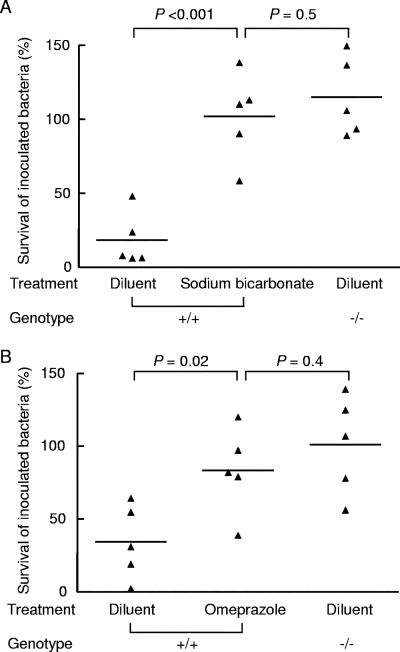
In a second series of experiments to determine if the observed differences in bacterial colonization were due to differences in gastric acid production, we rendered wild-type mice hypochlorhydric by pretreatment with sodium bicarbonate or omeprazole, a proton pump inhibitor, prior to challenge with C. rodentium. Similar numbers of bacteria survived passage through the stomachs of BALB/cCrSlc mice pretreated with sodium bicarbonate (102.0% ± 29.8%) and the stomachs of H+,K+-ATPase β-subunit-deficient mice pretreated with diluent alone (114.9% ± 26.8%) (P = 0.5) (Fig. 4A). Similar results were obtained for omeprazole-treated BALB/cCrSlc mice (83.4% ± 29.7%) and diluent-treated H+,K+-ATPase β-subunit-deficient mice (101.0% ± 34.0%) (P = 0.4) (Fig. 4B). In both experiments, significantly lower numbers of bacteria were recovered from BALB/cCrSlc mice pretreated with diluent (18.3% ± 18.2% and 34.2% ± 25.5% for the sodium bicarbonate and omeprazole experiments, respectively) (Fig. 4). These results indicate that the differences in susceptibility to bacterial colonization in the two groups of mice that we observed were due to differences in gastric acid production.
FIG. 4.
Treatment of mice with acid-suppressing agents. (A) Fasted wild-type mice (genotype +/+) were treated with 10% sodium bicarbonate or diluent, and fasted H+,K+-ATPase β-subunit-deficient mice (genotype −/−) were treated with only diluent. All mice were inoculated by gavage 30 min later with 108 CFU C. rodentium. Mice were killed after 30 min, and the stomach, small intestine, and large intestine were removed for enumeration of C. rodentium. (B) Fasted wild-type mice (genotype +/+) were treated with omeprazole or diluent, and fasted H+,K+-ATPase β-subunit-deficient mice (genotype −/−) were treated with only diluent and infected 30 min later with 108 CFU C. rodentium by gavage. Mice were killed after 30 min, and the stomach, small intestine, and large intestine were removed. In both experiments, the samples were placed in PBS, homogenized, and spread on selective agar plates. The results are expressed as percent survival (number of C. rodentium CFU isolated from each mouse/number of CFU in the inoculum × 100). The horizontal bars indicate means. The data were analyzed using a two-tailed Student unpaired t test.
DISCUSSION
It has been postulated that one of the main functions of gastric acid is to prevent ingested microorganisms from reaching the small intestine, where they have the potential to cause disease or to gain access to other parts of the body (12). Here, we systematically addressed the issue of the effectiveness of gastric acid as an antibacterial barrier by testing the survival of various bacterial pathogens in H+,K+-ATPase β-subunit-deficient and mice with a gastric pH similar to that found in humans. The bacteria that we used for this study were selected on the basis of differences in pathogenesis and intrinsic resistance to acid. Y. enterocolitica is primarily a gastrointestinal pathogen that produces diarrhea and fever but can also cause septicemia and postinfection autoimmunity (26). Y. enterocolitica serotype O:8 strains are highly pathogenic for mice and humans. S. enterica serovar Typhimurium causes enteritis in humans and in mice produces a disease that resembles typhoid fever (30). C. rodentium is a mouse pathogen that produces characteristic attaching and effacing lesions indistinguishable from those produced by enteropathogenic and enterohemorrhagic strains of Escherichia coli, which also cause diarrhea in humans (19, 20). C. perfringens, an anaerobic, spore-forming, gram-positive rod, is a common cause of food poisoning in humans, causing stomach cramps and diarrhea (28). The gastrointestinal symptoms are caused by type A isolates that produce CPE, a sporulation-specific enterotoxin. The bacteria are ingested as vegetative cells in contaminated food and then sporulate in the gut, producing CPE.
Before performing animal experiments, we investigated the survival of Y. enterocolitica 8081, Y. enterocolitica 8081u−, S. enterica serovar Typhimurium, and C. rodentium in PBS at various pHs. Our findings supported previous findings showing that Y. enterocolitica is highly acid resistant and that this phenotype is mediated by the ability of this organism to produce urease (4, 38). Our results for S. enterica serovar Typhimurium support those of Gorden and Small (9), who found that Salmonella species are unable to survive in Luria-Bertani broth at pH 2.5 for 2 h. The acid resistance of C. rodentium has not been tested previously, and we found that this species is slightly more acid sensitive than S. enterica serovar Typhimurium.
Zavros et al. (39) have reported that transgenic gastrin-deficient mice are susceptible to bacterial overgrowth when they are housed in conventional mouse facilities. While these authors isolated aerobic, facultative, and anaerobic bacteria from the stomachs of these mice, they did not quantify the susceptibility of individual mice to infection or the ability of specific pathogens to traverse the gastric environment and establish infection. Interestingly, the H+,K+-ATPase β-subunit-deficient mice used in this study do not exhibit bacterial overgrowth when they are maintained under specific-pathogen-free conditions (data not shown).
Sun et al. (33) performed controlled experiments which showed that nonpathogenic E. coli survives better in hypochlorhydric gastrin-deficient mice than in wild-type mice. We expanded these studies by performing systematic experiments to test the survival of several bacterial pathogens in a different type of hypochlorhydric mouse strain, which does not display the other effects of gastrin insufficiency, such as impaired gastric motility and delayed emptying. By infecting H+,K+-ATPase β-subunit-deficient and wild-type mice with various doses of Y. enterocolitica, S. enterica serovar Typhimurium, and C. rodentium and measuring colonization 3 days later, we showed that the hypochlorhydric mice were more susceptible to colonization than the wild-type mice except at the highest dose (108 CFU), at which wild-type mice were also colonized.
One of the disadvantages of using animals to model human infections is the inherent difference between humans and the animals used. In our case, the main problem was that mice have a higher resting gastric luminal pH than humans. We addressed this issue by increasing the gastric acid output of wild-type mice by treating them with histamine, thus making them hyperchlorhydric relative to untreated mice. We found that 2.5-fold more Y. enterocolitica, 5.4-fold more S. enterica serovar Typhimurium, and 13.6-fold more C. rodentium survived passage through the stomachs of hypochlorhydric mice than survived passage through the stomachs of hyperchlorhydric mice. This phenomenon was not restricted to gram-negative microorganisms, as C. perfringens spores also showed greater survival in hypochlorhydric mice. Very few studies examining the survival of vegetative cells and spores of C. perfringens at low pH have been carried out, although Li and McClane (16) found that in vitro the lowest pH supporting vegetative cell growth or spore outgrowth of food poisoning isolates was pH 5.1.
Our in vivo results also correlate with our in vitro results, which showed that Y. enterocolitica was the most acid-resistant bacterium, followed by S. enterica serovar Typhimurium and C. rodentium. The reductions in bacterial load that were observed were lower than that reported by Sun et al. (33), who found that E. coli survival in gastrin-deficient mice was increased more than 20-fold at 10 min after gavage compared to E. coli survival in control mice. These authors also reported much lower levels of bacterial survival, approximately 2.5% in gastrin-deficient mice compared to 0.1% in control mice. This low level of survival may have been due to the fact that the gastrin-deficient mouse strain had a stomach pH of 4.2, which is much lower than the stomach pH of the H+,K+-ATPase β-subunit-deficient mouse strain used here and previously (13, 31).
We used two approaches to verify that the only phenotype which had an impact on bacterial survival in H+,K+-ATPase β-subunit-deficient mice was the ability of the mice to produce gastric acid. First, we infected mice by a route that bypassed the stomach and found similar numbers of S. enterica serovar Typhimurium in the spleens and livers of wild-type and H+,K+-ATPase β-subunit-deficient mice that had been inoculated i.p. with bacteria 1 day earlier. Second, we treated mice with 10% sodium bicarbonate to neutralize stomach acid or with omeprazole, a proton pump inhibitor. Both of these treatments facilitated bacterial survival during passage through the stomach that was similar to the survival in the H+,K+-ATPase β-subunit-deficient mice. Together, these results indicate that undefined factors other than stomach acid were not responsible for the greater survival of S. enterica serovar Typhimurium in hypochlorhydric mice.
This study established a clear role for gastric acid in reducing susceptibility to infection with ingested bacterial pathogens. In addition, the hypochlorhydric/hyperchlorhydric animal model that we have developed provides an excellent system for evaluating treatment of hypochlorhydric patients with agents such as vaccines and probiotics.
Acknowledgments
We thank Danijela Krmek and Kim Huett for assistance with mouse experiments and Briony Gliddon for genotyping the mice. We also thank Gad Frankel for the gift of C. rodentium ICC169 and Richard Strugnell for the gift of S. enterica serovar Typhimurium SL1344. In addition, we thank Nhung Nguyen (Department of Biochemistry and Molecular Biology, The University of Melbourne) for advice on treating mice with omeprazole.
This study was supported by an Australian Bacterial Pathogenesis Program grant from the National Health and Medical Research Council of Australia. S. M. Tennant was the recipient of a J. N. Peters Bequest Fellowship from The University of Melbourne.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 19 November 2007.
REFERENCES
- 1.Besancon, M., J. M. Shin, F. Mercier, K. Munson, M. Miller, S. Hersey, and G. Sachs. 1993. Membrane topology and omeprazole labeling of the gastric H+,K+-adenosinetriphosphatase. Biochemistry 322345-2355. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan, J. M., B. H. Toh, J. M. Pettitt, D. C. Humphris, and P. A. Gleeson. 1990. Poly-N-acetyllactosamine-specific tomato lectin interacts with gastric parietal cells. Identification of a tomato-lectin binding 60-90 × 103 Mr membrane glycoprotein of tubulovesicles. J. Cell Sci. 95563-576. [DOI] [PubMed] [Google Scholar]
- 3.Cederberg, C., K. Rohss, P. Lundborg, and L. Olbe. 1993. Effect of once daily intravenous and oral omeprazole on 24-hour intragastric acidity in healthy subjects. Scand. J. Gastroenterol. 28179-184. [DOI] [PubMed] [Google Scholar]
- 4.De Koning-Ward, T. F., and R. M. Robins-Browne. 1995. Contribution of urease to acid tolerance in Yersinia enterocolitica. Infect. Immun. 633790-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan, C. L., and D. H. Strong. 1968. Improved medium for sporulation of Clostridium perfringens. Appl. Microbiol. 1682-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forte, J. G., and X. Yao. 1996. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol. 645-48. [DOI] [PubMed] [Google Scholar]
- 7.Ghaem-Maghami, M., C. P. Simmons, S. Daniell, M. Pizza, D. Lewis, G. Frankel, and G. Dougan. 2001. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect. Immun. 695597-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannella, R. A., S. A. Broitman, and N. Zamcheck. 1972. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 13251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorden, J., and P. L. Small. 1993. Acid resistance in enteric bacteria. Infect. Immun. 61364-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gracey, M., G. J. Cullity, Suharjono, and Sunoto. 1977. The stomach in malnutrition. Arch. Dis. Child. 52325-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291238-239. [DOI] [PubMed] [Google Scholar]
- 12.Howden, C. W., and R. H. Hunt. 1987. Relationship between gastric secretion and infection. Gut. 2896-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kararli, T. T. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16351-380. [DOI] [PubMed] [Google Scholar]
- 14.Koelz, H. R. 1992. Gastric acid in vertebrates. Scand. J. Gastroenterol. Suppl. 1932-6. [DOI] [PubMed] [Google Scholar]
- 15.Larner, A. J., and M. I. Hamilton. 1994. Infective complications of therapeutic gastric acid inhibition. Aliment. Pharmacol. Ther. 8579-584. [DOI] [PubMed] [Google Scholar]
- 16.Li, J., and B. A. McClane. 2006. Comparative effects of osmotic, sodium nitrite-induced, and pH-induced stress on growth and survival of Clostridium perfringens type A isolates carrying chromosomal or plasmid-borne enterotoxin genes. Appl. Environ. Microbiol. 727620-7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd, K. C. K., and J. H. Walsh. 1993. Regulation of acid secretion in vivo, p. 221-243. In J. H. Walsh (ed.), Gastrin. Raven Press, New York, NY.
- 18.Martinsen, T. C., K. Bergh, and H. L. Waldum. 2005. Gastric juice: a barrier against infectious diseases. Basic Clin. Pharmacol. Toxicol. 9694-102. [DOI] [PubMed] [Google Scholar]
- 19.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell. Microbiol. 71697-1706. [DOI] [PubMed] [Google Scholar]
- 20.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen, N. V., P. A. Gleeson, N. Courtois-Coutry, M. J. Caplan, and I. R. van Driel. 2004. Gastric parietal cell acid secretion in mice can be regulated independently of H/K ATPase endocytosis. Gastroenterology 127145-154. [DOI] [PubMed] [Google Scholar]
- 22.Nwokolo, C. U., D. E. Loft, R. Holder, and M. J. S. Langman. 1994. Increased incidence of bacterial diarrhoea in patients taking gastric acid antisecretory drugs. Eur. J. Gastroenterol. Hepatol. 6697-699. [Google Scholar]
- 23.Portnoy, D. A., and S. Falkow. 1981. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J. Bacteriol. 148877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed, L. J., and H. Muench. 1935. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 25.Reuben, M. A., L. S. Lasater, and G. Sachs. 1990. Characterization of a beta subunit of the gastric H+/K+-transporting ATPase. Proc. Natl. Acad. Sci. USA 876767-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robins-Browne, R. M. 2007. Yersinia enterocolitica, p. 293-322. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. American Society for Microbiology, Washington, DC.
- 27.Rood, J. I., E. A. Maher, E. B. Somers, E. Campos, and C. L. Duncan. 1978. Isolation and characterization of multiply antibiotic-resistant Clostridum perfringens strains from porcine feces. Antimicrob. Agents Chemother. 13871-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rood, J. I., and B. A. McClane. 2002. Clostridium perfringens: gastrointestinal diseases, p. 1117-1139. In M. Sussman (ed.), Molecular medical microbiology. Academic Press, London, United Kingdom.
- 29.Sachs, G. 1994. The gastric H,K ATPase—regulation and structure/function of the acid pump of the stomach, p. 1119-1138. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract. Raven Press, New York, NY.
- 30.Santos, R. L., S. Zhang, R. M. Tsolis, R. A. Kingsley, L. G. Adams, and A. J. Baumler. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 31335-1344. [DOI] [PubMed] [Google Scholar]
- 31.Scarff, K. L., L. M. Judd, B. H. Toh, P. A. Gleeson, and I. R. van Driel. 1999. Gastric H+,K+-adenosine triphosphatase beta subunit is required for normal function, development, and membrane structure of mouse parietal cells. Gastroenterology 117605-618. [DOI] [PubMed] [Google Scholar]
- 32.Shull, G. E. 1990. cDNA cloning of the beta-subunit of the rat gastric H,K-ATPase. J. Biol. Chem. 26512123-12126. [PubMed] [Google Scholar]
- 33.Sun, F. J., S. Kaur, D. Ziemer, S. Banerjee, L. C. Samuelson, and R. C. De Lisle. 2003. Decreased gastric bacterial killing and up-regulation of protective genes in small intestine in gastrin-deficient mouse. Dig. Dis. Sci. 48976-985. [DOI] [PubMed] [Google Scholar]
- 34.Toh, B. H., P. A. Gleeson, R. J. Simpson, R. L. Moritz, J. M. Callaghan, I. Goldkorn, C. M. Jones, T. M. Martinelli, F. T. Mu, D. C. Humphris, and. 1990. The 60- to 90-kDa parietal cell autoantigen associated with autoimmune gastritis is a beta subunit of the gastric H+/K+-ATPase (proton pump). Proc. Natl. Acad. Sci. USA 876418-6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyagarajan, K., R. R. Townsend, and J. G. Forte. 1996. The beta-subunit of the rabbit H,K-ATPase: a glycoprotein with all terminal lactosamine units capped with alpha-linked galactose residues. Biochemistry 353238-3246. [DOI] [PubMed] [Google Scholar]
- 36.van Driel, I, and J. M. Callaghan. 1995. Proton and potassium transport by H+/K+-ATPases. Clin. Exp. Pharmacol. Physiol. 22952-960. [DOI] [PubMed] [Google Scholar]
- 37.Wiles, S., S. Clare, J. Harker, A. Huett, D. Young, G. Dougan, and G. Frankel. 2004. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell. Microbiol. 6963-972. [DOI] [PubMed] [Google Scholar]
- 38.Young, G. M., D. Amid, and V. L. Miller. 1996. A bifunctional urease enhances survival of pathogenic Yersinia enterocolitica and Morganella morganii at low pH. J. Bacteriol. 1786487-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zavros, Y., G. Rieder, A. Ferguson, L. C. Samuelson, and J. L. Merchant. 2002. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology 122119-133. [DOI] [PubMed] [Google Scholar]