Abstract
In some patients, Escherichia coli strains establish significant bacteriuria without causing symptoms of urinary tract infection (UTI). These asymptomatic-bacteriuria (ABU) strains have been shown to express fewer virulence factors than the uropathogenic E. coli (UPEC) strains that cause severe, symptomatic UTI. Paradoxically, ABU strains carry many typical UPEC virulence genes, and the molecular basis of their low virulence therefore remains unclear. This study examined whether ABU strains might evolve from UPEC by genome loss and virulence gene attenuation. The presence of conserved E. coli K-12 genes was examined using an E. coli K-12 strain MG1655-specific DNA array and the distribution of UPEC virulence-related genes was examined with the E. coli pathoarray. Two groups of strains could be distinguished. Several ABU strains were shown by multilocus sequence typing and by comparative genomic analyses to be related to UPEC but to have smaller genome sizes. There were significant alterations in essential virulence genes, including reductive evolution by point mutations, DNA rearrangements, and deletions. Other strains were unrelated to UPEC and lacked most of the virulence-associated genes. The results suggest that some ABU strains arise from virulent strains by attenuation of virulence genes while others are nonvirulent and resemble commensal strains. We propose that virulence attenuation might constitute a general mechanism for mucosal pathogens to evolve toward commensalism.
Urinary tract infections (UTIs) remain a major cause of morbidity and mortality. Uropathogenic Escherichia coli (UPEC) strains cause 70 to 90% of community-acquired UTIs in an estimated 150 million individuals annually and about 40% of all nosocomial UTIs (39). These frequencies illustrate the magnitude of the problem but do not reflect the diversity of diseases in the urinary tract, as only symptomatic infections in the Western world are considered and third-world UTI has not been properly investigated. Furthermore, the frequency estimates do not include asymptomatic bacteriuria (ABU), which may be the most common form of UTI, occurring in 1% of schoolgirls, ≥2% of pregnant women, and about 20% of elderly individuals of both sexes (8). In ABU patients, E. coli establishes a carrier state, with more than 105 bacteria/ml of urine, but the patients do not develop symptoms (27). ABU thus resembles a state of commensalism, but mostly with a bacterial monoculture rather than a complex flora. This makes ABU a highly interesting model for the study of mechanisms of commensalism and the driving forces in the pathogen and the host. The ABU model may also provide information about the protective effects of the normal flora, as bacterial carriage has been shown to protect patients against symptomatic UTI.
The severity of UTI reflects the virulence of the infecting E. coli strain. Early studies observed that acute pyelonephritis isolates had properties that often were lacking in ABU strains (11, 28). Subsequent mechanistic studies made it possible to identify several of these properties as virulence factors. The acute pyelonephritis strains belong to a restricted set of serotypes, electrophoretic types, and genotypes. ABU strains, in contrast, are more diverse and lack essential virulence factors, like P fimbriae (6, 26). These studies suggested that ABU was caused by strains of low virulence, which do not provoke a host response and therefore cause no symptoms. Genotypic analyses contradicted this notion, however, as many ABU strains carry virulence genes but fail to express them (32). For example, 60% of ABU strains were pap DNA positive, but less than 20% of those strains expressed P fimbriae, suggesting that ABU strains may have arisen from virulent UPEC strains but achieved long-term persistence by attenuation of virulence factors that provoke a host response. There is increasing evidence that the extent of genome plasticity among E. coli isolates has been underestimated and that point mutations, gene loss, and insertion sequence (IS) element-mediated chromosomal rearrangements play important roles in adaptation.
The ABU strain E. coli 83972 was originally isolated from a girl with long-term ABU and has been used extensively to deliberately establish protective ABU in patients. E. coli 83972 belongs to the phylogenetic lineage B2 of E. coli, indicating a close relatedness to the UPEC strains, which cause symptomatic UTI. The strain does not express classical UPEC virulence factors, but genotypic analysis has revealed that E. coli 83972 possesses a large number of virulence-associated genes (9). A recent genotypic and phenotypic analysis of selected pathogenicity factors of strain 83972 suggested that the loss of functional type 1, F1C, and P fimbriae was due to deletions or multiple point mutations and proposed that this might be essential in order for E. coli 83972 to cause ABU (25, 35).
To extend our knowledge of the molecular mechanisms of ABU, we performed a detailed genotypic and phenotypic analysis of 11 ABU isolates. The results suggest that the concept of virulence attenuation can be generalized.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Eleven ABU isolates (ABU 5, ABU 20, ABU 21, ABU 27, ABU 37, ABU 38, ABU 57, ABU 62, ABU 63, ABU 64, and ABU 83972) isolated in 1974 in Göteborg, Sweden (28) were included in this study. For comparison, E. coli K-12 strain MG1655 (22) and UPEC strains 536 (O6:K15:H31) (2) and CFT073 (O6:K2:H1) (40), as well as fecal isolate Nissle 1917 (O6:K5:H1) (16), were analyzed. The E. coli strains were routinely grown in Luria-Bertani (LB) or M63B1 glucose medium (38) with or without 1.5% Bacto agar (Difco Laboratories, Detroit, MI). Where appropriate, kanamycin, ampicillin, or chloramphenicol was added to the growth medium at a concentration of 25 μg/ml, 50 μg/ml, or 30 μg/ml, respectively.
Genotypic characterization.
Further detailed genomic characterization included genomic fingerprinting by pulsed-field gel electrophoresis (PFGE) and detection of fitness- and virulence-associated genes of extraintestinal pathogenic E. coli (ExPEC) (afa and draBC, bmaE, cdtB, cnf1, clbA to clbQ, cvaC, fimH, fyuA, hlyA, ibeA, iroN and iroB, iutA, kpsMTI, kpsMTII, kpsMTII K1, kpsMTII K5, malX, papAH, papG, sfa and focDE, sfaS, focG, rfc, and traT) by PCR (10, 24, 30). Allocation of the ABU isolates to the major phylogenetic groups of E. coli was done according to the results of a triplex PCR (7).
Genome comparison by DNA-DNA hybridization and hybridization data analysis.
Genome comparison of the different ABU isolates by DNA-DNA hybridization to the Panorama E. coli gene arrays (Sigma-Genosys, Cambridge, United Kingdom) and a modified version of the “E. coli pathoarray” was performed as described before (9, 16, 20). Hierarchical cluster analysis of the hybridization data was performed with the CLUSTER software (12) based on the presence or absence of genes. The output was displayed with the software TREEVIEW (12). Virulence-associated genes of ExPEC were detected by multiplex PCR (24).
Genomic fingerprinting.
Genomic fingerprinting and rough genome size determination of the individual isolates was done by PFGE. Genomic DNA for the analysis by PFGE was prepared in agarose plugs as previously described (9), cleaved by I-CeuI or XbaI (New England Biolabs), separated on a CHEF-Dr III system (Bio-Rad) at 12°C in 0.5× Tris-borate-EDTA buffer with 6.5 kV/cm2 and pulse times increasing from 5 to 50 s over a period of 22 h and 30 to 80 s over a period of 18 h, and then finished with 80 to 80 s over a period of 3.5 h.
MLST.
The allocation of the ABU isolates to different clonal lineages was performed as described on the Max-Planck-Institute for Infection Biology, Berlin, Germany, website (http://web.mpiib-berlin.mpg.de/mlst/dbs/Ecoli/documents/primersColi_html). Sequence types (STs) were assigned using the E. coli multilocus sequence typing (MLST) database hosted at the Max-Planck-Institute for Infection Biology (http://web.mpiib-berlin.mpg.de/mlst/dbs/Ecoli). We used eBURST to visualize groups of related STs and the putative founder organism with so-called population snapshots (13). Information on all isolates was deposited at the Max-Planck-Institute for Infection Biology E. coli MLST database.
DNA techniques.
Qiagen (Hilden, Germany) products were used to isolate and purify genomic DNA. Primers were obtained from Sigma-Genosys (Taufkirchen, Germany), while restriction enzymes were purchased from New England Biolabs (Frankfurt am Main, Germany). For Southern blot hybridization, DNA was transferred to Nytran Supercharge nylon membranes (Schleicher & Schuell BioSciences, Dassel, Germany). Hybridization with and detection of horseradish peroxidase-labeled probes was performed with the ECL labeling and signal detection system (Amersham General Electric Healthcare, Freiburg, Germany). The size and sequence context of the large internal deletion of the fim operons of ABU isolates 21 and 38 was determined by inverse PCR. For this purpose, genomic DNA (4 μg) was digested with PstI and the individual restriction fragments were consecutively circularized upon religation using T4 DNA ligase (New England Biolabs). The resulting DNA circles were precipitated, PCR amplified, and used as a template for DNA sequence determination. The complete list of primers used for DNA amplification and sequence analysis is available in Table S1 in the supplemental material.
DNA sequence analysis.
The genomic regions of interest (fim, pap, foc, and hly determinants) were amplified by PCR, subcloned into pGEM T-easy (Promega), and consecutively sequenced by primer walking using an ABI-310 sequencer. Homology searches were performed with the BLAST programs of the National Center for Biotechnology Information (1) (http://www.ncbi.nlm.nih.gov/BLAST/). Putative ORFs were identified using Vector NTI (InforMax, Oxford, United Kingdom) and Artemis (37; http://www.sanger.ac.uk/Software/Artemis/).
Phenotypic assays.
Motility at 37°C was analyzed on LB agar plates containing 0.3% agar. The diameter of the migration zone around the inoculation site was measured in at least three independent experiments.
The capacity of bacterial strains to express a d-mannose binding phenotype was measured by the ability to agglutinate Saccharomyces cerevisiae cells on glass slides. Aliquots of bacterial overnight cultures were incubated with a yeast suspension (10 mg/ml [dry weight]). Agglutination, which is susceptible to inhibition by d-mannose (2%), was monitored visually by aggregation and precipitation of the cells.
Expression of P and F1C fimbriae was demonstrated by hemagglutination of defibrinated sheep and bovine erythrocytes, respectively.
A suspension of sheep and bovine blood (Elocin Laboratory, Munich, Germany) was mixed on a glass slide with a toothpicked colony of E. coli. After incubation for some minutes on ice, agglutination occurred. P and F1C fimbrial expression was detected by immunoagglutination with polyclonal sera raised against purified P and F1C fimbriae, respectively (29). Immunoagglutination was performed by mixing 10 μl of an overnight culture of the E. coli strains to be tested with 10 μl of anti-FocA antibody dissolved in phosphate-buffered saline on microscope slides, followed by incubation on ice until aggregation of bacterial cells was clearly observed. E. coli strain CFT073 was used as a positive control and E. coli strain HB101 as a negative control.
To test their hemolytic activity, cells from E. coli colonies were spread on sheep blood agar plates (Oxoid) with a toothpick and incubated overnight at 37°C. Lysis of the blood cells by alpha-hemolysin was detected by formation of clear halos around the colonies after incubation.
Microcin and colicin production was assessed by the presence of clear zones of growth inhibition of the indicator strain E. coli DH5α around colonies of the tested strains after overnight incubation at 37°C on M9 agar plates. Expression of the siderophore aerobactin was assessed by the presence of growth zones of the iron-deficient indicator strain E. coli LG1522 around colonies of the tested strains on medium supplied with dipyridyl (5).
Isolation of lipopolysaccharide (LPS) from the E. coli strains used in this study was performed as previously described (17).
Nucleotide sequence accession numbers.
The DNA sequences of nonfunctional fim, pap, foc, and hly determinants of ABU isolates were submitted to the EMBL database under the following accession numbers: hlyCABD ABU strain 83972, AM690759; hlyCABD ABU strain 27, AM690760; hlyCABD ABU strain 37, AM690761; foc determinant ABU strain 83972, AM690762; foc determinant ABU strain 27, AM690763; foc determinant ABU strain 37, AM690764; pap determinant ABU strain 27, AM690765; pap determinant ABU strain 63, AM690766; and fimGH genes ABU strain 21, AM701828.
RESULTS
Diverse genome contents in ABU E. coli isolates.
The genome contents of the ABU strains were examined by comparative genomic hybridization (CGH). Conserved E. coli K-12 genes were detected by an E. coli K-12-specific array. The ABU isolates showed considerable genetic diversity (Fig. 1 and Table 1; see Tables S2 and S3 in the supplemental material). On average, 11.4% (range, 8.5% to 16.4%) of the translatable ORFs present in the E. coli K-12 prototype strain were not detectable in the ABU isolates (Table 1). Most of the missing ORFs coded for hypothetical, unclassified, or unknown gene products, according to the functional classification of the chromosomally encoded genes and proteins in E. coli K-12 (GenProtEC database [http://genprotec.mbl.edu]). In addition, the 11 isolates differed in ORFs, which represent mobile genetic elements or code for structural components in the cell. The strains could be subgrouped according to their CGH barcodes into clusters, which generally correlated with the main phylogenetic lineages and the genome sizes of the individual isolates (Fig. 1 and Table 1).
FIG. 1.
Genomic alterations among ABU E. coli isolates. White and black denote the presence and absence, respectively, of genes detected by CGH. The dendrogram shows the estimated genomic relationships of the different strains obtained by hierarchical cluster analysis.
TABLE 1.
Genotypic characterization of ABU E. coli strains by comparative genomic hybridization
| Characteristic | Value in ABU strain:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 57 | 21 | 38 | 62 | 20 | 27 | 37 | 83972 | 63 | 64 | 5 | |
| No. (%) of E. coli strain MG1655 genes (n = 4,290) detected | 3,798 (88.5) | 3,670 (85.5) | 3,588 (83.6) | 3,919 (91.4) | 3,853 (89.8) | 3,796 (88.5) | 3,796 (88.5) | 3,925 (91.5) | 3,804 (88.7) | 3,866 (90.1) | 3,813 (88.9) |
| No. (%) of ExPEC genes (n = 274) detected | 21 (7.7) | 10 (3.6) | 8 (2.9) | 67 (24.5) | 132 (48.2) | 102 (37.2) | 111 (40.5) | 134 (48.9) | 136 (49.6) | 140 (51.1) | 52 (19) |
| No. (%) of IPEC genes (n = 101) detected | 2 (2) | 1 (1) | 2 (2) | 3 (3) | 5 (5) | 3 (3) | 4 (4) | 6 (6) | 2 (2) | 4 (4) | 18 (18) |
| ECOR groupa | A | B1 | B1 | B1 | B2 | B2 | B2 | B2 | B2 | B2 | D |
| STb | 554 | 553 | 553 | 53 | 73 | 73 | 73 | 73 | 555 | 12 | 405 |
| Genome size (Mb)c | 4.2 | 4.7 | 4.7 | 4.7 | 5.3 | 4.9 | 5.1 | 4.9 | 5.1 | 5.1 | 5.1 |
Affiliation with the main phylogenetic lineages of E. coli was determined according to the method of Clermont et al. (7).
MLST was performed as described previously (http://web.mpiib-berlin.mpg.de/mlst/dbs/Ecoli/documents/primersColi_html).
The genome size was assessed by PFGE of I-CeuI-digested genomic fragments. The genome size assessed by this approach may differ from the actual genome size by ± 0.2 Mb.
Phylogenetic lineages among the ABU isolates.
The relatedness of ABU and UPEC strains was examined by MLST (Table 1). Seven strains belonged to ECOR groups B2 and D, which typically include UPEC; four belonged to the ECOR groups A and B1, which are uncommon for extraintestinal pathogens. Interestingly, the majority of the ECOR group B2 isolates belonged to ST 73, which includes the well-characterized UPEC isolate CFT073, as well as the nonpathogenic fecal isolate Nissle 1917 (16, 17, 40). According to the E. coli MLST database, the STs 12 and 405 include ExPEC, as well as nonpathogenic isolates of ECOR groups B2 and D, respectively. ST 53 has so far only been shown to include nonpathogenic strains of ECOR group A or B1. Three ABU isolates belonged to previously unknown STs (ST553 to ST555).
Virulence genes in ABU strains.
Virulence-related UPEC genes were studied by E. coli pathoarray hybridization. The CGH results of the E. coli pathoarray were partially confirmed by PCR, allowing the detection of typical ExPEC-associated determinants coding for, e.g., different adhesins, toxins, the polyketide colibactin, siderophores, and capsules (Tables 1 and 2). The fimH gene coding for the type 1 fimbrial adhesin was present in all strains tested, but genes of the P and F1C fimbria-encoding gene clusters were present only in ABU isolates of ECOR group B2. The screening for toxin (alpha-hemolysin, cytolethal distending toxin 1, and cytotoxic necrotizing factor 1)-, siderophore system (aerobactin, salmochelin, and yersiniabactin)-, and group II capsule-encoding genes also indicated their preferential distribution among strains of ECOR groups B2 and D. With the exception of ABU strains 62 and 5, isolates of the ECOR groups A and B1 had fewer ExPEC-associated genes (mean, 9.7% of the detectable ExPEC genes based on their CGH patterns) than strains that belonged to the ECOR groups B2 and D (mean, 42.1% of the detectable ExPEC genes). Typical virulence-associated marker genes of intestinal pathogenic E. coli (IPEC) were rarely detectable in the ABU strains tested. On average, 4.5% of the strains had detectable IPEC genes, with 6% in the ECOR B2 and D strains and 2% in strains of ECOR groups A and B1 (Fig. 1 and Table 1).
TABLE 2.
Genotypic and phenotypic characterization of selected virulence traits of ABU E. coli strainsa
| ABU strain | ECOR group | Alpha-hemolysin
|
Type 1 fimbriae
|
P fimbriae
|
F1C fimbriae
|
Motility (mm) | Microcins/colicins | Aerobactin | LPS | Other detected virulence-associated genesc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hly | Hly | fim | Fim | pap | Pap | foc | F1C | |||||||
| 57 | A | − | − | fimBEAICDFGH+ | − | − | − | − | − | 0 | + | − | Rough | fyuA, kpsMTII |
| 21 | B1 | − | − | fimH+ | − | − | − | − | − | 13 | + | − | Smoothb | − |
| 38 | B1 | − | − | fimH + | − | − | − | − | − | 9 | + | − | Smoothb | − |
| 62 | B1 | − | − | fimBEAICDFGH+ | + | − | − | − | − | 24 | − | − | Smoothb | − |
| 20 | B2 | + | + | fimBEAICDFGH+ | + | − | − | + | + | 5 | + | − | Rough | cnf1, clbA to clbQ, fyuA, iroN to iroB, kpsMTI), malX |
| 27 | B2 | + | − | fimB′::′DFGH+ | − | + | − | + | − | 42 | − | − | Rough | cnf1, clbA to clbQ, fyuA, iroN to iroB, malX |
| 37 | B2 | + | + | fimB′::′DFGH+ | − | + | − | + | + | 0 | − | − | Rough | cnf1, clbA to clbQ, fyuA, iroN to iroB, kpsMTII, malX |
| 83972d | B2 | + | − | fimB′::′DFGH+ | − | + | − | + | − | 9 | − | + | Rough | cnf1, clbA to clbQ, fyuA, iutA, iroN to iroB, kpsMTII, malX |
| 63 | B2 | + | + | fimBEAICDFGH+ | + | + | − | − | − | 42 | − | − | Smooth | cnf1, fyuA, kpsMTII, ibeA, malX |
| 64 | B2 | + | + | fimBEAICDFGH+ | + | + | + | + | + | 42 | − | − | Smooth | cnf1, clbA to clbQ, fyuA, iroN to iroB, kpsMTII, rfc, malX |
| 5 | D | − | − | fimBEAICDFGH+ | − | − | − | − | − | 0 | + | − | Rough | afa and draBC, fyuA, kpsMTII, traT, malX |
+ indicates phenotypic expression, and − indicates absence of phenotypic expression of the individual traits tested.
The O side chains are shorter than those of smooth UPEC strain 536 (see Fig. S1 in the supplemental material).
These data indicate that ABU isolates differ considerably in their genome contents with regard to the presence of UPEC virulence-associated genes. The virulence-associated gene contents of about two-thirds of the ABU strains analyzed resembled that of typical UPEC, whereas in one-third of the ABU isolates, only a small number of such determinants existed.
ECOR group and genome size.
In general, isolates from ECOR groups B2 and D had genomes larger than 4.9 Mb and a higher percentage of ExPEC-associated genes, while isolates belonging to ECOR groups A and B1 had a smaller genome size (4.7 Mb or less) and a very low percentage of ExPEC-associated genes (Table 1). The genome sizes of ABU isolates of ECOR groups A and B1 resembled that of nonpathogenic E. coli K-12, which belongs to ECOR group A. In contrast, those of members of the ECOR group B2 and D were generally larger than that of E. coli K-12.
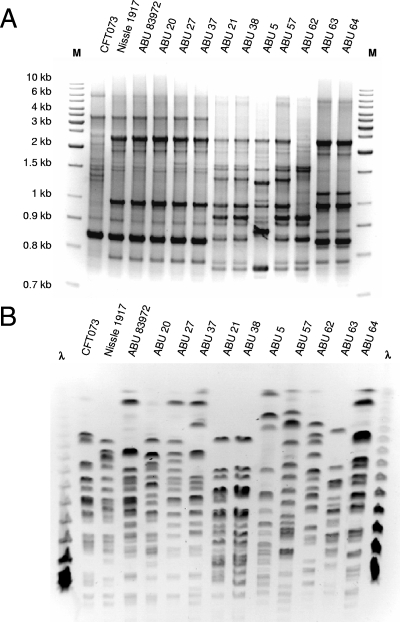
The genome structure of the ABU strains was further compared by PFGE and repetitive PCR. The genetic fingerprints were very similar among isolates belonging to the same ST (Fig. 2), but the genomic XbaI restriction fragment patterns indicated a marked diversity. Additionally, the genome size assessment by genomic I-CeuI restriction fragment patterns demonstrated that marked genome size differences exist even among strains of the same ST (Table 1). However, the genome sizes of ABU isolates belonging to ST 73 were, with one exception, always smaller than that of the closely related UPEC strain CFT073 (5.23 Mb), suggesting an overall reduction in genome size in ABU strains of ST 73 relative to UPEC strains of the same clonal lineage.
FIG. 2.
Genomic fingerprints of ABU E. coli isolates. The similarity of the genome structure was assessed by BOX-PCR (A) and PFGE (B) following XbaI digestion. To analyze the genome structure similarity among closely related ST 73 isolates, UPEC strain CFT073 and nonpathogenic strain Nissle 1917 were used as references.
These data indicate that ABU isolates differ considerably in their genome contents and in virulence-associated genes. About two-thirds of the analyzed ABU strains resembled UPEC and carried virulence-associated genes, but one-third of the ABU isolates were of a different origin and did not have such genes.
Phenotypic comparison of different E. coli ABU isolates.
The virulence phenotype of the ABU strains was characterized after in vitro subculture, optimizing gene expression. The results showed that many of the virulence genes were nonfunctional. They included genes coding for the different fimbrial adhesins and the pore-forming toxin alpha-hemolysin. Furthermore, there were differences between individual strains in the expression of other virulence-associated characteristics, such as LPS, microcin, aerobactin, and motility (Table 2; see Fig. S1 in the supplemental material). The ECOR group B2 isolates expressed either long-chain LPS or no side chains. Interestingly, the ST 73 isolates had a rough LPS phenotype. The ECOR group B1 isolates had shorter O side chains than smooth strains. ABU isolates 5 (ECOR group D) and 57 (ECOR group A) did not express O side chains. Generally, most of the rough isolates were less motile than the smooth strains.
The nucleotide sequences of the hly operon among the closely related strains ABU27, ABU37, and ABU83972 were compared. The nonhemolytic phenotype of strains 27 and 83972 was attributed to an A-to-T transition at the hlyA nucleotide position 416, resulting in a premature stop codon and thus a truncated HlyA protoxin gene product.
Similarly, the three strains also differed in the ability to express functional F1C fimbriae. Whereas strain 37 expressed functional F1C fimbriae, these fimbrial adhesins were nonfunctional in strains 83972 and 27. Comparison of the DNA sequences of the encoding foc determinant in these strains demonstrated that the A-to-T transition at focD nucleotide position 1415 resulted in exchange of glutamine 472 for a leucine residue in the FocD fimbrial usher of the last two strains. Mutation of this amino acid alone results in a nonfunctional FocD usher protein (Table 3). The pap sequence analysis showed that ABU strains 27, 37, 63, and 83972 harbored identical papG alleles, which code for a nonfunctional P fimbrial adhesin. The fim sequence analysis showed that strains 27 and 37 carried a 4,253-bp deletion within the fim gene cluster, identical to the deletion in the fim determinant of strain 83972. Due to this deletion, a truncated fimB gene is fused with a truncated fimD gene, probably by recombination between a 7-bp DNA motif, GGCGTTT. Moreover, strains 21 and 38 carried a 29,349-bp deletion comprising large parts of the KpLE2 phage element and the fim operon. In these strains, most likely IS element-mediated deletion was responsible for the loss of a chromosomal region upstream of fecI to fimG (Fig. 3), as the 29-kb chromosomal region was replaced by a 1,347-bp DNA stretch that represents a nonfunctional allele of an IS element, ISEhe3, which is frequently found, e.g., in Shigella flexneri. A complete fim cluster was present in strains 5 and 57, which still lacked functional type 1 fimbriae, suggesting that the fim genes might have been inactivated by point mutations. The results show that fim is localized to an unstable genomic region and that frequent point mutations and deletions inactivate the gene cluster.
TABLE 3.
Identification of the Gln472 → Leu substitution critical for FocD function
| Strain | FocD amino acid substitution | Functional F1C fimbriaea |
|---|---|---|
| CFT073 | Gln 472, Ala 889 | + |
| Nissle 1917 | Gln 472, Ala 889 | + |
| ABU27 | Leu 472, Val 889 | − |
| ABU37 | Gln 472, Val 889 | + |
| ABU83972 | Leu 472, Val 889 | − |
+ indicates phenotypic expression, and − indicates absence of phenotypic expression of the individual traits tested.
FIG. 3.
Genetic structures of the fim determinant and the adjacent KpLE2 phage region in ABU E. coli isolates. The scheme is based on the E. coli K-12 chromosome. The filled (gray to white) arrows denote genes of the fim determinant, filled gray arrows denote ORFs of the KpLE2 prophage, filled white to black arrows denote the fec determinant located within KpLE2, and black arrows denote ORF A and nonfunctional ORF B of the ISEhe3-like element, which replaces large regions of KpLE2 in ABU strains 21 and 38.
The allelic variation in fimH indicated that there were FimH amino acid sequence differences between isolates of distinct phylogenetic groups (see Fig. S2 in the supplemental material). Strains of ECOR groups A and B1 differed from those of groups B2 and D by a valine instead of an alanine at position 27. The ABU isolates of group B2 had the highest number of amino acid exchanges relative to E. coli K-12 strain MG1655. Interestingly, marked differences in the FimH amino acid sequence were observed among the closely related B2 strains of ST 73.
The results demonstrate that ABU isolates are genotypically and phenotypically heterogeneous. Some ABU strains were related to UPEC by overall genotype and carried typical virulence genes but frequently lacked the ability to express these virulence factors due to genome reduction by deletions or accumulated point mutations. Consequently, and in contrast to UPEC strains that cause symptomatic disease, the closely related ABU isolates frequently lacked multiple typical virulence-associated phenotypes involved in the activation of local and systemic inflammatory response pathways in the urinary tract. Other strains lacked the virulence genes and had a smaller overall genome size.
DISCUSSION
The severity of acute and chronic infections depends on the virulence of the infecting strain. In UTI, molecular mechanisms of bacterial virulence and host resistance have been extensively characterized, but most of the information on bacterial virulence concerns UPEC and less is known about the molecular basis of “commensalism” in the urinary tract or ABU. Based on early phenotypic studies, most ABU strains were regarded as nonvirulent (11, 28), but later molecular studies showed that 60% of ABU strains carry virulence genes (6, 32). Here, we examined the genetic relatedness of ABU strains, as well as the molecular basis of their virulence attenuation. We showed that ABU not only depends on a specific set of bacterial traits, but results from different bacterial colonization strategies. ABU strains fall into two categories. Strains belonging to ECOR groups A and B1 resembled commensal E. coli with a smaller genome size and a lack of UPEC virulence genes. Strains belonging to ECOR groups B2 and D resembled prototypic UPEC but also showed a reduced genome size compared to fully virulent B2 strains. Important virulence genes in those strains were inactivated by deletions and point mutations. The results show for the first time that ABU is caused either by commensal-like E. coli strains or by attenuated pathogens. We speculate that some ABU strains may establish bacteriuria without provoking a host response while others persist by adjusting the expression of virulence factors. It has also been suggested that increased growth rates in urine present a selective advantage for ABU isolates (34, 36).
ABU is an interesting model for studying the evolution of commensalism rather than virulence. Classical studies by Haldane (19) proposed that microbes evolve to increase their virulence. The theory was based mainly on the observation that virulence increases pathogen transmission between hosts, thereby increasing the number of available multiplication sites for the microbe. Virulence for the urinary tract may partially fit this theory, but it does not serve mainly to increase the number of infected hosts, but rather the number of sites in a given host. By expressing fimbriae and other virulence factors, UPEC establishes a monoculture in the urinary tract with less competition than in the complex and competitive intestinal microflora. Unfortunately, virulence is only partially successful, due to the brief time window between the establishment of bacteriuria and the activation of a host defense, which in most cases eliminates the infection. The ABU strains, in contrast, avoid provoking a host response that leads to their elimination, and instead, they establish long-term persistence. The loss of virulence may therefore be a preferred evolutionary strategy, and there may be positive selection for variants that are adapted for growth in the urinary tract. The advantages include a rich source of nutrients and the potential for transmission to new hosts. This is in contrast to acute pyelonephritis, which is associated with mortality, premature delivery, and reduced fertility and thus with a potential loss of the ecological niche. Our results clearly demonstrate for the first time that reductive evolution is an attenuation mechanism converting virulent UPEC to asymptomatic carrier strains. While there was no common or specific set of genes that was inactivated or lost by all ABU isolates relative to virulent UPEC, the ECOR group B2 and D isolates showed distinct mutations in virulence-associated genes rather than a large overall genome loss, which is consistent with an ongoing host-bacterial coevolution.
The loss of virulence factors has been shown to reduce the host response to infection in animal models, and specifically, the loss of fimbriae decreases the innate host response and bacterial clearance from the urinary tract. More than 80% of UPEC strains express P fimbriae, 14 to 30% of UPEC strains express F1C fimbriae (31), and type 1 fimbrial expression is quite frequent. P fimbriae enhance the establishment of bacteriuria and trigger the innate defense by stimulating the production of cytokines, which orchestrate the subsequent recruitment of inflammatory cells. Type 1 fimbriae have a similar function in mice and have also been shown to enhance intracellular persistence in the mouse bladder mucosa, but these effects have not been reproduced in the human urinary tract (3, 4, 21). The weak host response to ABU is therefore consistent with the loss of adherence and functional fimbriae. Our results thus suggest that the host response may drive coevolution and that virulence-associated genes with proinflammatory effects may be targeted for inactivation. In this way, ABU isolates may succeed in persisting without inducing a bactericidal inflammatory response.
The pap gene cluster was attenuated by the acquisition of multiple point mutations, and as a consequence, the PapG adhesin was inactivated. The fim gene cluster was attenuated through different mechanisms. The fim genomic region was rather unstable, with partial deletions resulting in loss of a central 4.2-kb portion of the operon or in larger 29-kb deletions including adjacent DNA stretches. It is an interesting observation that in all cases of partial fim gene cluster deletion, the fimH gene, which is frequently used in screening tests as a marker for the presence of type 1 fimbrial genes (24), remains intact. This study suggests that a wider fim screening procedure is needed to understand the functionality of the fim gene cluster. Comparison of the foc determinants of the F1C fimbria-negative ABU strains 83972 and 27 relative to F1C fimbria-positive isolate 37 led to the discovery that one particular amino acid exchange in FocD (glutamine 472 to leucine) is responsible for the loss of the FocD usher activity and thus the absence of functional F1C fimbriae in these strains (Table 2). This glutamine residue is conserved among the related usher subunits FocD, FimD, and SfaF (see Fig. S3 in the supplemental material), and its exchange probably results in an altered conformation or stability of the usher protein. These findings exemplify the many different mechanisms of virulence attenuation that can lead to the ABU phenotype.
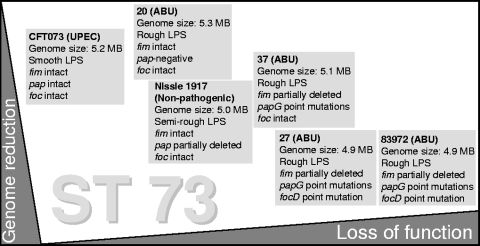
Our study suggests that ABU is caused by E. coli strains of different backgrounds, which share the ability to establish bacteriuria and to persist in the urinary tract, but the molecular details are poorly understood. ST 73 represents an important and successful phylogenetic lineage within ECOR group B2, which also includes the prototype UPEC strain E. coli CFT073 and the nonpathogenic E. coli strain Nissle 1917. The genomic and phenotypic diversity among members of ST 73 reflects the genome plasticity of E. coli. Although four ABU strains, as well as UPEC isolate CFT073 and the nonpathogenic strain Nissle 1917, belong to the same ST, they differ in the presence of functional fimbrial determinants, as well as in their LPS and hemolytic phenotypes (16, 17, 40). The DNA sequence diversity of their fim, pap, and foc genes is consistent with the phenotypic heterogeneity within this group of identical or very closely related organisms. Accordingly, the E. coli ST 73 includes highly virulent uropathogenic and ABU, as well as nonpathogenic, variants, which may have arisen from a common ancestor by reductive evolution (Fig. 4). Our results confirm recent findings (23, 41) that the current MLST schemes do not reliably predict the genotypes or phenotypes of individual isolates.
FIG. 4.
Genotypic and phenotypic diversity among closely related members of the E. coli clonal group (ST 73). The high E. coli genome plasticity results in marked phenotypic variability among individual members of the same ST, which thus includes pathogenic and nonpathogenic variants. Genome reduction/loss of function contributes to the evolution of these ABU variants from uropathogenic ancestors. fim, type 1 fimbrial determinant; pap, P fimbrial determinant; papG, P fimbrial adhesin-encoding gene; foc, F1C fimbrial determinant; focD, F1C fimbrial usher-encoding gene.
ABU development is also affected by the quality of the host response. In the murine UTI model, an ABU-like state is created when the innate immune response is disturbed. The innate response is controlled by Toll-like receptor 4 (TLR4), and mutations that disturb TLR4 signaling result in an asymptomatic carrier state resembling human ABU. The infected mice fail to recruit the inflammatory cells that are crucial for bacterial clearance (14, 15, 18), and as a result, bacteria persist in the urinary tract. The lack of inflammation is protective, however, as it prevents the symptoms and tissue damage that are associated with symptomatic UTI in a fully responsive host. Recently, children with ABU were shown to express smaller amounts of TLR4 than controls without UTI, and children with primary ABU had even lower levels than those who had an ABU recurrence after a prior symptomatic UTI episode (33). The TLR4 variations add an essential variable to the understanding of ABU. Regardless of host genetics, the “commensal” ABU strain would not be expected to cause symptomatic UTI, but a more virulent strain might cause symptoms and an attenuating host response in patients with normal TLR4 expression, eventually leading to ABU. Patients with low TLR4 levels would be expected to have a reduced innate response to infection and also to develop ABU with strains of higher virulence.
Our comparative analyses of different ABU isolates elucidate the remarkable genetic and phenotypic flexibility of E. coli isolates. Whereas the presence of certain bacterial virulence traits usually determines the type of infection, it has become clear that in cases of UTI in general, and especially of ABU, several successful bacterial strategies of infection exist. In this context, studies of the impact of the host response to bacterial genome plasticity, as well as large-scale comparative genomics of ABU strains and their closely related UPEC isolates causing symptomatic UTI, will be promising future approaches to understanding the driving forces and molecular mechanisms underlying the ABU phenomenon and the underlying molecular mechanisms that distinguish pathogens and commensals.
Supplementary Material
Acknowledgments
We thank B. Plaschke (Würzburg) for excellent technical assistance and H. Merkert (Würzburg) for helpful advice.
The Würzburg group was supported by the Deutsche Forschungsgemeinschaft (SFB479, TP A1) and the Bundesministerium für Bildung und Forschung (Kompetenznetz Pathogenomik, Projektgruppe 7). The Lund group was supported by the Swedish Medical Research Council; the Royal Physiographic Society; the Medical Faculty, Lund University; and the Österlund, Lundberg, Lundgren, Maggie Stephens, Söderberg, H. J. Forssman, Persson, and Wallenberg Foundations. C. Svanborg was the recipient of a Bristol-Myers Squibb unrestricted grant.
This work was carried out in the framework of the European Virtual Institute for Functional Genomics of Bacterial Pathogens (CEE LSHB-CT-2005-512061) and the ERA-NET project “Deciphering the intersection of commensal and extraintestinal pathogenic E. coli.”
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 26 November 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, H., J. Hacker, A. Juarez, C. Hughes, and W. Goebel. 1982. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J. Bacteriol. 1521241-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergsten, G., B. Wullt, M. A. Schembri, I. Leijonhufvud, and C. Svanborg. 2007. Do type 1 fimbriae promote inflammation in the human urinary tract? Cell Microbiol. 91766-1781. [DOI] [PubMed] [Google Scholar]
- 4.Bergsten, G., B. Wullt, and C. Svanborg. 2005. Escherichia coli, fimbriae, bacterial persistence and host response induction in the human urinary tract. Int. J. Med. Microbiol. 295487-502. [DOI] [PubMed] [Google Scholar]
- 5.Braun, V., R. Gross, W. Koster, and L. Zimmermann. 1983. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol. Gen. Genet. 192131-139. [DOI] [PubMed] [Google Scholar]
- 6.Caugant, D. A., B. R. Levin, G. Lidin-Janson, T. S. Whittam, C. Svanborg Eden, and R. K. Selander. 1983. Genetic diversity and relationships among strains of Escherichia coli in the intestine and those causing urinary tract infections. Prog. Allergy 33203-227. [DOI] [PubMed] [Google Scholar]
- 7.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 664555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colgan, R., L. E. Nicolle, A. McGlone, and T. M. Hooton. 2006. Asymptomatic bacteriuria in adults. Am. Fam. Physician 74985-990. [PubMed] [Google Scholar]
- 9.Dobrindt, U., F. Agerer, K. Michaelis, A. Janka, C. Buchrieser, M. Samuelson, C. Svanborg, G. Gottschalk, H. Karch, and J. Hacker. 2003. Analysis of genome plasticity in pathogenic and commensal Escherichia coli isolates by use of DNA arrays. J. Bacteriol. 1851831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrindt, U., G. Blum-Oehler, T. Hartsch, G. Gottschalk, E. Z. Ron, R. Fünfstück, and J. Hacker. 2001. S-fimbria-encoding determinant sfa(I) is located on pathogenicity island III(536) of uropathogenic Escherichia coli strain 536. Infect. Immun. 694248-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eden, C. S., L. A. Hanson, U. Jodal, U. Lindberg, and A. S. Akerlund. 1976. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet 308490-492. [PubMed] [Google Scholar]
- 12.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 9514863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 1861518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frendeus, B., G. Godaly, L. Hang, D. Karpman, and C. Svanborg. 2001. Interleukin-8 receptor deficiency confers susceptibility to acute pyelonephritis. J. Infect. Dis. 183(Suppl. 1)S56-S60. [DOI] [PubMed] [Google Scholar]
- 15.Frendeus, B., C. Wachtler, M. Hedlund, H. Fischer, P. Samuelsson, M. Svensson, and C. Svanborg. 2001. Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol. Microbiol. 4037-51. [DOI] [PubMed] [Google Scholar]
- 16.Grozdanov, L., C. Raasch, J. Schulze, U. Sonnenborn, G. Gottschalk, J. Hacker, and U. Dobrindt. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 1865432-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grozdanov, L., U. Zähringer, G. Blum-Oehler, L. Brade, A. Henne, Y. A. Knirel, U. Schombel, J. Schulze, U. Sonnenborn, G. Gottschalk, J. Hacker, E. T. Rietschel, and U. Dobrindt. 2002. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J. Bacteriol. 1845912-5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagberg, L., R. Hull, S. Hull, J. R. McGhee, S. M. Michalek, and C. Svanborg Eden. 1984. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haldane, J. B. S. 1949. Disease and evolution. Ric. Sci. 192-11. [Google Scholar]
- 20.Hejnova, J., U. Dobrindt, R. Nemcova, C. Rusniok, A. Bomba, L. Frangeul, J. Hacker, P. Glaser, P. Šebo, and C. Buchrieser. 2005. Characterization of the flexible genome complement of the commensal Escherichia coli strain A0 34/86 (O83:K24:H31). Microbiology 151385-398. [DOI] [PubMed] [Google Scholar]
- 21.Hultgren, S. J., T. N. Porter, A. J. Schaeffer, and J. L. Duncan. 1985. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect. Immun. 50370-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen, K. F. 1993. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 1753401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, J. R., K. L. Owens, C. R. Clabots, S. J. Weissman, and S. B. Cannon. 2006. Phylogenetic relationships among clonal groups of extraintestinal pathogenic Escherichia coli as assessed by multi-locus sequence analysis. Microbes Infect. 81702-1713. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181261-272. [DOI] [PubMed] [Google Scholar]
- 25.Klemm, P., V. Roos, G. C. Ulett, C. Svanborg, and M. A. Schembri. 2006. Molecular characterization of the Escherichia coli asymptomatic bacteriuria strain 83972: the taming of a pathogen. Infect. Immun. 74781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leffler, H., and C. Svanborg-Eden. 1981. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect. Immun. 34920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindberg, U., I. Claesson, L. A. Hanson, and U. Jodal. 1978. Asymptomatic bacteriuria in schoolgirls. VIII. Clinical course during a 3-year follow-up. J. Pediatr. 92194-199. [DOI] [PubMed] [Google Scholar]
- 28.Lindberg, U., L. A. Hanson, U. Jodal, G. Lidin-Janson, K. Lincoln, and S. Olling. 1975. Asymptomatic bacteriuria in schoolgirls. II. Differences in Escherichia coli causing asymptomatic bacteriuria. Acta Paediatr. Scand. 64432-436. [DOI] [PubMed] [Google Scholar]
- 29.Nagy, G., U. Dobrindt, G. Schneider, A. S. Khan, J. Hacker, and L. Emödy. 2002. Loss of regulatory protein RfaH attenuates virulence of uropathogenic Escherichia coli. Infect. Immun. 704406-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nougayrède, J. P., S. Homburg, F. Taieb, M. Boury, E. Brzuszkiewicz, G. Gottschalk, C. Buchrieser, J. Hacker, U. Dobrindt, and E. Oswald. 2006. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313848-851. [DOI] [PubMed] [Google Scholar]
- 31.Pere, A., B. Nowicki, H. Saxen, A. Siitonen, and T. K. Korhonen. 1987. Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis. 156567-574. [DOI] [PubMed] [Google Scholar]
- 32.Plos, K., H. Connell, U. Jodal, B. I. Marklund, S. Marild, B. Wettergren, and C. Svanborg. 1995. Intestinal carriage of P fimbriated Escherichia coli and the susceptibility to urinary tract infection in young children. J. Infect. Dis. 171625-631. [DOI] [PubMed] [Google Scholar]
- 33.Ragnarsdottir, B., M. Samuelsson, M. C. Gustafsson, I. Leijonhufvud, D. Karpman, and C. Svanborg. 2007. Reduced toll-like receptor 4 expression in children with asymptomatic bacteriuria. J. Infect. Dis. 196475-484. [DOI] [PubMed] [Google Scholar]
- 34.Roos, V., E. M. Nielsen, and P. Klemm. 2006. Asymptomatic bacteriuria Escherichia coli strains: adhesins, growth and competition. FEMS Microbiol. Lett. 26222-30. [DOI] [PubMed] [Google Scholar]
- 35.Roos, V., M. A. Schembri, G. C. Ulett, and P. Klemm. 2006. Asymptomatic bacteriuria Escherichia coli strain 83972 carries mutations in the foc locus and is unable to express F1C fimbriae. Microbiology 1521799-1806. [DOI] [PubMed] [Google Scholar]
- 36.Roos, V., G. C. Ulett, M. A. Schembri, and P. Klemm. 2006. The asymptomatic bacteriuria Escherichia coli strain 83972 outcompetes uropathogenic E. coli strains in human urine. Infect. Immun. 74615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16944-945. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Struelens, M. J., O. Denis, and H. Rodriguez-Villalobos. 2004. Microbiology of nosocomial infections: progress and challenges. Microbes Infect. 61043-1048. [DOI] [PubMed] [Google Scholar]
- 40.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 9917020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirth, T., D. Falush, R. Lan, F. Colles, P. Mensa, L. H. Wieler, H. Karch, P. R. Reeves, M. C. Maiden, H. Ochman, and M. Achtman. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 601136-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.