Abstract
The receptor activator of NF-κB ligand (RANKL) and the proinflammatory cytokines are believed to play important roles in osteoclastogenesis. We recently reported that the innate immune recognition receptor, Toll-like receptor 2 (TLR2), is crucial for inflammatory bone loss in response to infection by Porphyromonas gingivalis, the primary organism associated with chronic inflammatory periodontal disease. However, the contribution of macrophage-expressed TLRs to osteoclastogenesis has not been defined. In this study, we defined a requirement for TLR2 in tumor necrosis factor-alpha (TNF-α)-elicited osteoclastogenesis in response to exposure to P. gingivalis. Culture supernatant (CS) fluids from P. gingivalis-stimulated macrophages induced bone marrow macrophage-derived osteoclastogenesis. This activity was dependent on TNF-α and occurred independently of RANKL, interleukin-1β (IL-1β), and IL-6. CS fluids from P. gingivalis-stimulated TLR2−/− macrophages failed to express TNF-α, and these fluids induced significantly less osteoclast formation compared with that of the wild-type or the TLR4−/− macrophages. In addition, P. gingivalis exposure induced up-regulation of TLR2 expression on the cell surface of macrophages, which was demonstrated to functionally react to reexposure to P. gingivalis, as measured by a further increase in TNF-α production. These results demonstrate that macrophage-dependent TLR2 signaling is crucial for TNF-α-dependent/RANKL-independent osteoclastogenesis in response to P. gingivalis infection. Furthermore, the ability of P. gingivalis to induce the cell surface expression of TLR2 may contribute to the chronic inflammatory state induced by this pathogen.
Bone metabolism is a complex process, and in healthy individuals, a homeostatic balance is achieved between bone resorption and new bone formation (7). Osteoclasts play an important role in bone resorption and originate from the fusion of precursors belonging to the monocyte/macrophage lineage. The receptor activator of nuclear factor κB ligand (RANKL), a member of the tumor necrosis factor (TNF) ligand super family, is a major factor involved in osteoclast generation. Both the soluble and the transmembrane forms of RANKL induce osteoclast formation when these cells are cultured with macrophage colony-stimulating factor (M-CSF) (16). RANKL-induced osteoclast formation is inhibited by osteoprotegerin (OPG), a decoy receptor of RANKL (31, 38). Osteoclast differentiation also occurs through a mechanism that is independent of RANKL-RANK interaction and is dependent on TNF-alpha (TNF-α) (3, 20). It has been reported that TNF-α-dependent osteoclastogenesis occurs when permissive levels of RANKL exist (22). Recently, however, Kim et al. (19) reported that transforming growth factor-β (TGF-β)-treated bone marrow macrophages (BMM) could be differentiated into osteoclast-like cells when cultured with TNF-α by a mechanism that was independent of RANKL-RANK interaction, suggesting that TNF-α also plays an important role in osteoclastogenesis.
In chronic inflammatory bone diseases, including rheumatoid arthritis, RANKL and proinflammatory cytokines such as TNF-α, interleukin-1β (IL-1β), and IL-6 have been shown to be important for disease progression (14). Histological evaluations have revealed that the cellular composition of inflammatory bone lesions in rheumatoid arthritis consists primarily of T and B cells, as well as macrophages (27, 32). T cells and B cells have been reported to contribute to the acceleration of bone resorption by the production of cytokines and RANKL (12, 21, 37). Macrophages in inflamed tissues can differentiate not only to osteoclasts (1), but they also appear to accelerate bone resorption through the production of proinflammatory cytokines (8).
Toll-like receptors (TLRs) are a group of pathogen-associated pattern recognition receptors, which have been identified as key participants in the innate recognition of pathogens (23, 33). TLR2 is activated by bacterial lipoproteins, peptidoglycans, and Staphylococcus aureus lipoteichoic acid (SLTA) (2, 29, 30). TLR4 recognizes enteric lipopolysaccharide (LPS) (34). Monocytes and macrophages express both TLR2 and TLR4 (15). Following TLR engagement, macrophages produce proinflammatory cytokines such as IL-1β, IL-6, and TNF-α (17, 18, 29). A recent study reported that the peripheral blood mononuclear cells of rheumatoid arthritis patients expressed increased levels of TLR2 and TLR4 compared to that expressed in cells obtained from healthy subjects (28). Iwahashi et al. (18) have also shown that both blood monocytes and synovial tissue macrophages from rheumatoid arthritis patients expressed high levels of TLR2. While these studies have correlated macrophage TLR expression with inflammatory tissue damage associated with bone destruction, a requirement for the macrophage-specific TLR signaling influence on osteoclast formation has not been defined.
Periodontal disease is a well-characterized chronic inflammatory bone-destructive disease induced by bacterial infection. While periodontal disease and rheumatoid arthritis are different diseases initiated by distinct causes, it has been suggested that similar pathological processes are involved in the resulting inflammatory bone destruction (14). Histological analysis of local inflammatory lesions obtained from patients with severe periodontal disease shows expression levels of TLR2 and TLR4 from macrophages that are increased compared to those from lesions obtained from patients with mild periodontal disease (25). Other studies, using real-time PCR, on the other hand, have reported significant down-regulation of TLR2 mRNA in samples obtained from patients with chronic periodontitis (26). We and others (6, 11) have reported that TLR2 is crucial for inflammatory bone loss in response to infection by the gram-negative pathogen Porphyromonas gingivalis, the primary organism associated with chronic inflammatory periodontal disease. However, the contribution of the innate immune recognition system of the macrophage in inflammatory bone loss has not been defined. In this study, we examined the requirements for TLR2 and TLR4 in P. gingivalis-infected macrophage-induced osteoclastogenesis. Using murine macrophages, we detail a mechanism by which TLR2-mediated signaling to P. gingivalis stimulates a potent soluble proinflammatory response which stimulates BMM to undergo osteoclastogenesis via TNF-α induction. Furthermore, we demonstrate that P. gingivalis infection induces the up-regulation of TLR2 expression on the cell surface of macrophages, which were demonstrated to functionally react to reinfection with P. gingivalis, as measured by a further increase in TNF-α production. The ability of P. gingivalis to induce the cell surface expression of TLR2 may further contribute to chronic inflammation, which is characteristic of human periodontal disease.
MATERIALS AND METHODS
Animals.
C57BL/6 wild-type (WT) (Jackson Laboratory, Bar Harbor, ME), TLR2 knockout (TLR2−/−), and TLR4 knockout (TLR4−/−) mice (originally provided by S. Akira, Osaka University) were cared for in accordance with Boston University institutional animal care and use committee procedures.
Bacteria and growth conditions.
P. gingivalis strain 381 was grown anaerobically on blood agar plates (BBL medium; Becton Dickinson Co.) and was used to seed brain heart infusion broth (pH 7.4; Difco) supplemented with yeast extract (Difco), hemin (1 μg/ml; Sigma), and menadione (1 μg/ml; Sigma). Organism numbers were standardized at an optical density at 660 nm of 1 (equivalent to 1 × 109 CFU/ml), using a spectrophotometer (model DU 7500; Beckman).
Peritoneal macrophage cultures.
Male mice (8 to 10 weeks of age) were injected intraperitoneally with 3.0 ml of a sterile thioglycolate solution (Remel). After 4 days, peritoneal exudate cells were harvested by lavage with RPMI 1640 medium (Gibco) containing 10% fetal bovine serum (FBS) (Gibco) and 50 μg/ml gentamicin (Sigma). After the cells were cultured in plate dishes for 24 h, nonadherent cells were removed. The adherent cells were cultured for 1 day with medium not containing antibiotics and then were used as peritoneal macrophages.
P. gingivalis-stimulated macrophage assays.
Peritoneal macrophages from WT, TLR2−/−, or TLR4−/− mice were cultured with P. gingivalis at various levels of multiplicity of infection (MOI) for 6 or 24 h in 12-well plate dishes (for fluorescence-activated cell sorter [FACS] analysis or osteoclast formation) or in 96-well plate dishes (for enzyme-linked immunosorbent assay [ELISA]). Cells were collected for FACS analysis, and culture supernatant (CS) fluids were centrifuged and stored at −70°C until used for ELISA or for osteoclast formation. Unstimulated macrophages were used as a control. In some experiments, cells were stimulated with 2 μg/ml Staphylococcus aureus lipoteichoic acid (SLTA) (InvivoGen) or with 100 ng/ml Escherichia coli LPS (InvivoGen). Some wells were incubated with either 20 μg/ml murine TLR2 blocking antibody (clone T2.5) or isotype-matched rat immunoglobulin G (IgG) (eBioscience) before the stimulation of cells.
TLR functional assay.
Peritoneal macrophages were cultured with P. gingivalis (MOI = 10) in 96-well culture plates for 1 h. Nonadherent bacteria were removed by washing, and these cells were cultured with medium for 5 h. After the medium was changed, these P. gingivalis-infected cells were reexposed to P. gingivalis (MOI = 10) for an additional 24 h. CS fluids and cells were collected for ELISA or for FACS analysis as described below.
Cytokines and RANKL assays.
Concentrations of IL-1β, IL-6, TNF-α (BD Biosciences), and RANKL (R&D Systems) in CS fluids were determined by using commercially available murine ELISA kits.
Flow cytometry analysis.
FACS analysis was performed with macrophage cultures by first washing cells in buffer (0.2% bovine serum albumin/phosphate-buffered saline), followed by incubation with Fc receptor blocker (eBioscience). The cells were then labeled with fluorescein isothiocyanate-conjugated anti-mouse TLR2 monoclonal antibody (clone 6C2; eBioscience), phycoerythrin-conjugated anti-mouse TLR4 monoclonal antibody (clone MTS510; eBioscience), phycoerythrin-conjugated anti-mouse RANKL monoclonal antibody (clone IK22/5; eBioscience), or with isotype controls. The cells were washed, and 10,000 events were analyzed by flow cytometry using a FACScan model flow cytometer (Becton Dickinson). The geometric means of three independent experiments were utilized for statistical analyses.
Osteoclast formation.
C57BL/6 mouse bone marrow cells were cultured in α-minimum essential medium (Gibco) containing 10% FBS, 100 μg/ml streptomycin, 100 IU of penicillin (Cellgro), and 5 ng/ml mouse recombinant M-CSF (R&D Systems) for 12 h in 100-mm-diameter dishes. Nonadherent cells were harvested and cultured with 30 ng/ml M-CSF in 100-mm-diameter dishes for an additional 24 h. Nonadherent cells were washed out, and adherent cells were collected and used as BMMs (3). BMMs (3 × 104 cells/0.5 ml/well) were cultured for 5 days in α-minimum essential medium containing 10% FBS, antibiotics, 30 ng/ml M-CSF, and 0.2 ml of CS fluids from macrophages of the WT, the TLR2−/−, or the TLR4−/− mice, challenged with P. gingivalis (MOI = 10) for 24 h in 8-well LabTek chamber slides (Nalge Nunc International). Wells received the addition of 300 ng/ml OPG or 5 μg/ml anti-mouse TNF-α, IL-1β, or IL-6 polyclonal antibody or normal goat IgG (R&D Systems). On day 3, the culture media and all reagents were replaced. To identify osteoclasts, cells were fixed in 4% paraformaldehyde and stained with tartrate-resistant acid phosphatase (TRAP; Sigma). TRAP-positive multinucleated (more than three unstained nuclei) cells were defined as osteoclasts, and the number was determined by microscopic counts. We also determined the ability of P. gingivalis cultures alone to stimulate osteoclast formation. For these experiments, C57BL/6 mouse BMMs (3 × 104 cells/0.5 ml/well) were cultured for 5 days in α-minimum essential medium containing 10% FBS, 30 ng/ml M-CSF, and P. gingivalis (MOI = 1:10) or 0.2 ml of culture medium from P. gingivalis, as described above.
Pit formation assay.
BMMs (2 × 104 cells/0.25 ml/well) were plated on dentine disks (OsteoSite) in 96-well culture dishes and cultured in α-minimum essential medium containing 10% FBS, antibiotics, and 100 μl of CS fluids containing peritoneal macrophages of WT, TLR2−/−, or TLR4−/− mice infected with P. gingivalis in the presence of M-CSF (30 ng/ml) for 7 days. Media and all reagents were replaced every 3 days. After they were cultured, the dentine disks were cleaned by ultrasonication in 1 M of NH4OH to remove adherent cells. After they were rinsed, the dentine disks were stained with hematoxylin for visualizing resorption lacunae (20).
Statistical analysis.
Statistical analysis was carried out with Statview software (SAS Institute Inc.). An unpaired t test and one- or two-factor analysis of variance with the Tukey-Kramer test were performed to assess differences among groups. P values of <0.05 were considered statistically significant.
RESULTS
P. gingivalis-infected macrophages produce the proinflammatory cytokines TNF-α, IL-1β, and IL-6 but not RANKL.
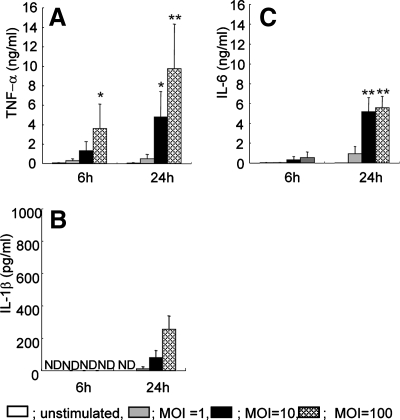
While a few studies have examined the proinflammatory response of mouse macrophages to P. gingivalis infection, little is known about the expression of proinflammatory cytokines believed to play a role in osteoclastogenesis following exposure to live P. gingivalis. Thus, in our initial studies, we examined the production of TNF-α, IL-1β, IL-6, and soluble RANKL in CS fluids from peritoneal macrophages of WT mice in response to infection by live P. gingivalis bacteria at different MOIs. Since the focus of this study was not to examine the role of specific bacterial components in the macrophage response but rather the response to live P. gingivalis, for these studies we choose not to use protease inhibitors. We also monitored macrophages via microscopy for all experiments in which macrophages were incubated with P. gingivalis and did not observe significant changes in the morphology of the cells over time, following macrophage stimulation with P. gingivalis exposure (data not shown). This was expected, since the MOI for these studies was fairly low (1:10). Significantly elevated levels of TNF-α, IL-1β, and IL-6 expression were detected 24 h postexposure to P. gingivalis at MOIs of 10 and 100 (Fig. 1A to C); however, RANKL production was not increased when macrophages were cultured with P. gingivalis (data not shown). We also examined macrophages that were challenged for membrane-bound RANKL expression levels by using FACS analysis. The expression levels of RANKL mRNA and those of membrane RANKL were similar between challenged and unchallenged macrophages (data not shown). Since it has been reported that TGF-β can influence osteoclastogenesis (8), we also measured TGF-β levels in CS fluids. TGF-β expression levels in CS fluids from live P. gingivalis-stimulated cells were not significantly different from those of unstimulated cells (P > 0.05; data not shown). These results indicate that macrophages cultured with P. gingivalis express proinflammatory cytokines but do not produce soluble or membrane-bound RANKL.
FIG. 1.
Comparison of proinflammatory cytokines and RANKL production from peritoneal macrophages cultured with P. gingivalis. Peritoneal macrophages from WT mice were cultured with P. gingivalis at the indicated MOIs for 6 or 24 h. CS fluids were then harvested for ELISA of TNF-α (A), IL-1β (B), and IL-6 (C). Control cultures were incubated with culture medium alone (unstimulated). Data are shown as the means and standard deviations of two independent experiments, each performed in triplicate. *, P < 0.05; **, P < 0.01 compared with uninfected controls at each time point. ND, not detectable.
TLR2 contributes to TNF-α production by murine macrophages in response to P. gingivalis infection.
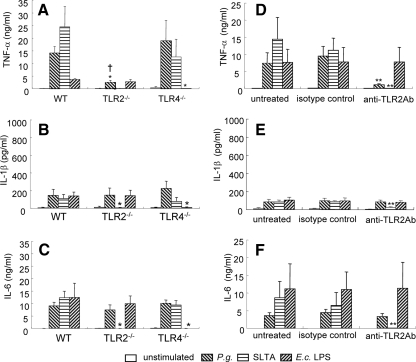
We and others (6, 11) recently reported that TLR2 is crucial for inflammatory bone loss in response to P. gingivalis infection in a mouse model of oral bone loss. To assess requirements for the TLR signaling pathways in proinflammatory cytokine production in response to P. gingivalis stimulation, we cultured macrophages from WT, TLR2−/−, and TLR4−/− mice with live P. gingivalis and assessed the expression of each cytokine in CS fluids. As expected, macrophages from the TLR2−/− and TLR4−/− mice did not produce cytokines in response to their cognate ligands (SLTA and E. coli LPS, respectively), while macrophages obtained from WT mice responded well to stimulation with both antigens (Fig. 2A to C). TNF-α production by the TLR2−/− mouse macrophages cultured with P. gingivalis was clearly reduced compared to that of the WT or the TLR4−/− mice (Fig. 2A). IL-1β and IL-6 production levels were similar among all strains of mouse macrophages cultured with P. gingivalis (Fig. 2B and C). To verify the requirement for TLR2 in eliciting proinflammatory cytokines from macrophages in response to P. gingivalis exposure, we incubated WT macrophages with anti-mouse TLR2 blocking antibody, followed by exposure to P. gingivalis. We observed that the blocking antibody treatment inhibited SLTA-induced cytokine production but failed to inhibit the stimulation of macrophages cultured with E. coli LPS. Macrophages cultured with P. gingivalis, after anti-TLR2 antibody treatment, demonstrated a marked reduction in TNF-α production but showed no changes in levels of IL-1β and IL-6 (Fig. 2D to F). Collectively, these results indicate that TLR2 signaling is involved in mediating the TNF-α response to live P. gingivalis and that the responses of IL-1β and IL-6 are mediated via TLR2-independent mechanisms.
FIG. 2.
P. gingivalis infection induces TNF-α production in macrophages via a TLR2-dependent pathway. Peritoneal macrophages from WT, TLR2−/−, and TLR4−/− mice were cultured with P. gingivalis (P.g.) (MOI = 10) for 24 h (A to C). In another experiment, peritoneal macrophages from WT mice were pretreated with anti-TLR2 blocking antibody, the isotype control antibody for 1 h or were left untreated, followed by culture with P. gingivalis (MOI = 10) for 24 h (D to F). Cells were also stimulated with SLTA (2 μg/ml) or with E. coli (E.c.) LPS (100 ng/ml) as a control. CS fluids were harvested for ELISA of TNF-α (A and D), IL-1β (B and E), or IL-6 (C and F). Control cultures were incubated with culture media only (unstimulated). Data are shown as the means and standard deviations of two independent experiments, each performed in triplicate. *, P < 0.01 compared with each of the WT macrophages; †, P < 0.01 compared with P. gingivalis culture with TLR4−/− macrophages; **, P < 0.01 compared with each of the isotype controls.
Live P. gingivalis stimulation sensitizes murine macrophages, making them hyperresponsive to subsequent exposure.
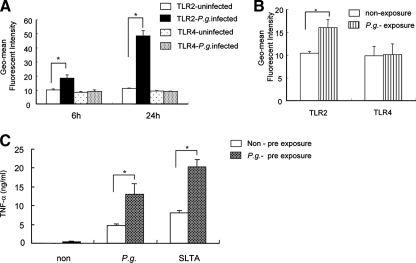
We have previously reported that P. gingivalis infection results in the up-regulation of TLR expression on the cell surface of endothelial cells (39). We postulated that the ability of P. gingivalis to stimulate the cell surface expression of TLRs on the endothelial cell could serve as a mechanism with which to sensitize these cells to other TLR ligands and could play a role in the chronic inflammation induced by this pathogen. To determine if a similar response occurred with macrophages, we cultured peritoneal macrophages with P. gingivalis and examined the temporal expression levels of TLR2 and TLR4. Macrophages cultured with P. gingivalis showed an increase in the cell surface expression of TLR2 compared to that of unstimulated macrophage cultures at both 6 and 24 h poststimulation. However, we did not observe changes in the levels of expression of TLR4 following incubation of macrophages with P. gingivalis at either time point (Fig. 3A).
FIG. 3.
P. gingivalis up-regulates TLR2 surface expression on murine macrophages. (A) Peritoneal macrophages from WT mice were cultured with P. gingivalis (MOI = 10) or with medium alone (uninfected), and the temporal expression levels of TLR2 and TLR4 were determined by FACS analysis at 6 and 24 h. Data are expressed as the geometric (Geo)-mean of fluorescence intensities and standard deviations (SDs) of three independent experiments. *, P < 0.01 by unpaired t test. P.g., P. gingivalis. (B) Peritoneal macrophages from WT mice were cultured with P. gingivalis (MOI = 10) or with medium alone for 1 h, and then nonadherent bacteria were removed by washing out cells. The cells were then incubated with medium alone for 5 h, and the temporal expression levels of TLR2 and TLR4 were determined by FACS analysis. Data are expressed as the geometric mean fluorescence intensities and SDs of three independent experiments. *, P < 0.01 by unpaired t test. (C) Non-preexposed (Non) peritoneal macrophages or macrophages preexposed to P. gingivalis (MOI = 10) for 1 h were washed and incubated with medium alone for 5 h. Following a change of the medium, cells were incubated with medium, with P. gingivalis (MOI = 10), or with SLTA (2 μg/ml) for 24 h, and TNF-α production in the CS fluids was assessed by ELISA. Data are shown as the means and SDs of two independent experiments, each performed in triplicate. *, P < 0.01 by unpaired t test.
We next determined if the increased expression level of TLR2 on the surface of macrophages, elicited by P. gingivalis exposure, could enhance TNF-α production upon subsequent exposure of the macrophages to this organism. We confirmed that the level of TLR2 expression, but not that of TLR4, was significantly up-regulated before reexposure (Fig. 3B). The exposure of macrophages to P. gingivalis prior to reexposure with the organism resulted in a significant increase in TNF-α production compared to that of macrophages that had not been exposed to P. gingivalis. Stimulation of SLTA, a TLR2 agonist, also increased TNF-α production when macrophages were exposed to P. gingivalis (Fig. 3C). Collectively, these results indicate that similar to observations for endothelial cells, the exposure of macrophages to live P. gingivalis can induce the cell surface expression of TLR2, which results in sensitization of the macrophage as measured by TNF-α production following reexposure with this organism.
Osteoclast formation induced by macrophages cultured with P. gingivalis is mediated by TNF-α but not by RANKL.
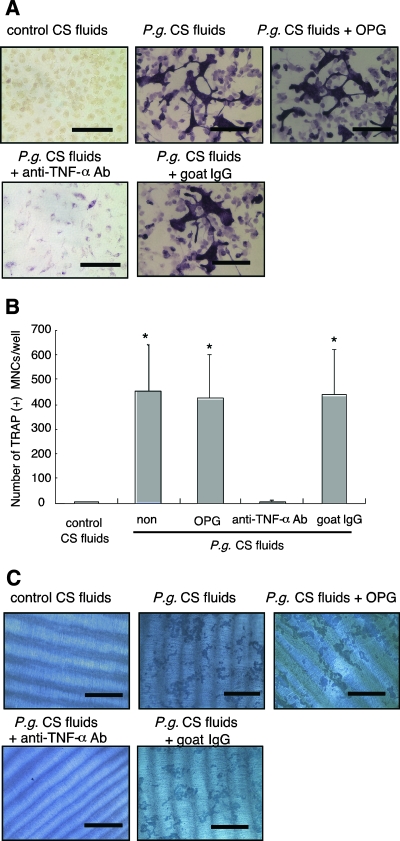
The differentiation of BMMs into osteoclasts has previously been demonstrated with RANKL and TNF-α, in conjunction with M-CSF (3, 20). To determine if CS fluids collected from peritoneal macrophages stimulated with P. gingivalis could induce osteoclast formation from BMMs, we stimulated BMMs with CS fluids from WT mouse peritoneal macrophages cultured with P. gingivalis for 24 h. As shown in Fig. 4, the formation of osteoclast-like cells was induced by the CS fluids from P. gingivalis-challenged macrophages but not by that of unchallenged macrophages. TNF-α blocking antibody, but not OPG or goat IgG control, completely blocked osteoclastogenesis induced by the CS fluids from peritoneal macrophages cultured with P. gingivalis (Fig. 4A and B). We confirmed that OPG or anti-TNF-α antibody completely blocked the osteoclast formation in response to their cognate ligands (RANKL or TNF-α, respectively) (P < 0.0001; data not shown). In addition, we demonstrated that neither IL-1β nor IL-6, in conjunction with M-CSF, could induce osteoclast formation from BMMs (P = 0.9955 and P = 0.9991, respectively, and data not shown). We also attempted to block osteoclastogenesis using anti-mouse IL-1β or anti-mouse IL-6 blocking antibodies; however, these reagents did not alter osteoclastogenesis elicited by CS fluids collected from P. gingivalis-challenged macrophages (P = 0.3152 and P = 0.1783, respectively, and data not shown). These results indicate that osteoclast-like cell formation is dependent upon TNF-α but not upon the IL-1β, IL-6, or soluble RANKL that was present in the CS fluids collected from WT macrophages cultured with P. gingivalis. We also examined the effect of bacteria alone or bacterial culture medium alone on osteoclastogenesis. Using either bacteria alone or bacterial culture medium alone, we did not observe osteoclastogenesis (data not shown). Thus, we conclude that P. gingivalis alone does not induce osteoclastogenesis.
FIG. 4.
CS fluids from P. gingivalis-infected peritoneal macrophages induce osteoclastogenesis. (A) Osteoclast formation. BMMs from WT mice were cultured with CS fluids from peritoneal macrophages that were previously infected with P. gingivalis CS fluids (P.g. CS fluids) or with CS fluids from uninfected macrophages (control CS fluids). Some CS fluids were supplemented with OPG (300 ng/ml) or anti-TNF-α blocking antibody (5 μg/ml) or control goat IgG (5 μg/ml), as indicated in panels A to C. After 5 days of culture, cells were stained with TRAP for the identification of osteoclasts. Representative photographs are shown. Bars = 100 μm. (B) Osteoclast numbers in cell cultures. BMMs were cultured under the same conditions as described above. TRAP-positive multinucleated cells were identified and counted. Data are shown as the means and standard deviations of two independent experiments, each performed in triplicate. *, P < 0.01 compared with control CS fluids. (C) Pit formation assay. BMMs were cultured on dentine disks under the same conditions as described above. After 7 days, cells were removed, and the dentine disks were stained with hematoxylin. Representative photographs are shown. Bars = 400 μm. All cultures in panels A to C were incubated in the presence of M-CSF (30 ng/ml). Every 3 days, the media and reagents were replaced.
We next assessed the functional activity of osteoclast-like cells to resorb dentine. CS fluids obtained from macrophages stimulated with P. gingivalis, but not that from unstimulated cells, also induced dentine resorption. The resorption of dentine by osteoclast-like cells elicited by using CS fluids collected from macrophages cultured with P. gingivalis was completely blocked by anti-TNF-α antibody, but not with OPG or control goat IgG (Fig. 4C). Taken together, these results indicate that the CS fluids of live P. gingivalis-stimulated peritoneal macrophages can promote functional osteoclast formation from BMMs.
The role of TLR2 in osteoclast formation induction by CS fluids from macrophages stimulated with P. gingivalis.
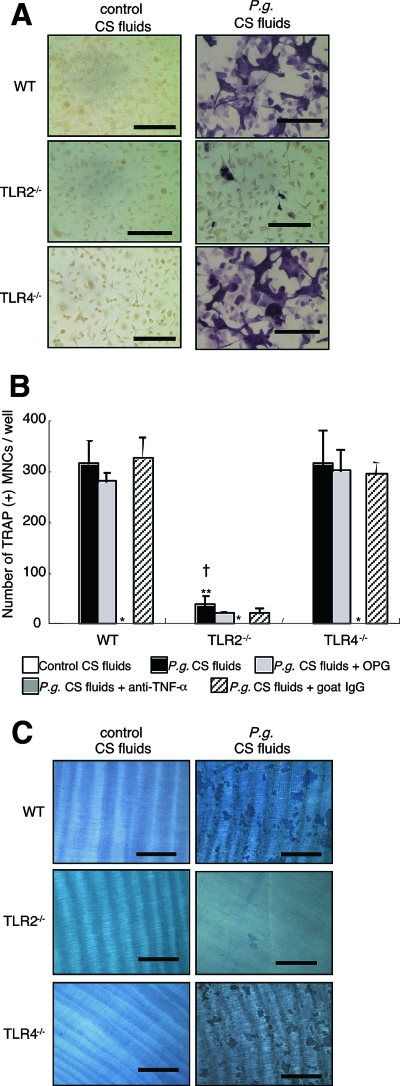
We next compared the ability of P. gingivalis-stimulated WT, TLR2−/−, and TLR4−/− peritoneal macrophages to induce osteoclastogenesis in vitro. CS fluids of live P. gingivalis-stimulated macrophages were able to induce osteoclast formation in all mouse strains examined; however, CS fluids obtained from the TLR2−/− macrophages induced differentiation of much fewer osteoclasts than the CS fluids obtained from WT or TLR4−/− macrophages stimulated by P. gingivalis (Fig. 5A and B). As expected, TNF-α levels in CS fluids obtained from P. gingivalis-stimulated TLR2−/− macrophages were lower (1.9 ± 0.8 ng/ml) than that observed for CS fluids obtained from P. gingivalis-infected WT (17.4 ± 2.5 ng/ml) and TLR4−/− macrophages (17.5 ± 8.0 ng/ml). These results indicate that the reduced formation of osteoclasts following incubation with CS fluids from TLR2−/− macrophages appears to result from low levels of TNF-α production. We also observed that anti-TNF-α blocking antibody, but not OPG and control goat IgG, added to the infected CS fluids completely inhibited osteoclast-like cell formation in all mouse strains tested, further confirming the importance of TNF-α in mediating osteoclast formation by CS fluids obtained from P. gingivalis-stimulated macrophages. (Fig. 5B).
FIG. 5.
Osteoclastogenesis induced by CS fluids of P. gingivalis-infected peritoneal macrophages from TLR2−/− and TLR4−/− mice. (A) Osteoclast formation. BMMs from WT mice were cultured with CS fluids from peritoneal macrophages obtained from WT, TLR2−/−, and TLR4−/− mice that were previously infected with P. gingivalis (P.g. CS fluids) or with uninfected macrophages (control CS fluids). After 5 days of culture, cells were stained with TRAP. Representative photographs are shown. Bars = 100 μm. (B) Osteoclast numbers. BMMs were cultured under the same conditions as described above. Some macrophages were also cultured in the presence of OPG (300 ng/ml), anti-TNF-α blocking antibody (5 μg/ml), or control goat IgG (5 μg/ml). TRAP-positive multinucleated cells were counted in each well. Data are shown as the means and standard deviations of two independent experiments, each performed in triplicate. *, P < 0.01 compared with P. gingivalis CS fluids of each mouse group; **, P < 0.01 compared with P. gingivalis CS fluids of the WT; †, P < 0.01 compared with P. gingivalis CS fluids of TLR4−/− mice. (C) Pit formation assay. BMMs were cultured on dentine disks under the same conditions as described above. After 7 days, cells were removed, and the dentine disks were stained with hematoxylin. Representative photographs are shown. Bars = 400 μm. All cultures in panels A to C were incubated in the presence of M-CSF. Every 3 days, the media and all reagents were replaced.
Resorption lacunae on the dentine were rarely observed for cells cultured with CS fluids from P. gingivalis-stimulated macrophages obtained from TLR2−/− mice. Conversely, dentine resorption induced by CS fluids from P. gingivalis-stimulated macrophages from TLR4−/− mice was similar to that observed for the CS fluids from WT mice macrophages (Fig. 5C). These results indicate that P. gingivalis-stimulated macrophages obtained from TLR4−/− mice also can induce functional osteoclast formation. Collectively, these results indicate that TLR2 signaling plays an important role in live P. gingivalis-induced osteoclastogenesis. Furthermore, TLR4 signaling does not appear to be critical for osteoclastogenesis and pit formation elicited by BMMs stimulated with CS fluids from macrophages in response to P. gingivalis exposure.
DISCUSSION
In this study, we have defined a requirement for TLR2 in TNF-α-elicited osteoclastogenesis in response to exposure to live P. gingivalis bacteria. Furthermore, we determined that osteoclastogenesis in response to P. gingivalis, while dependent on TNF-α, was independent of RANKL, IL-1β, and IL-6. In addition, we also demonstrate that the presence of P. gingivalis induced the up-regulation of TLR2 expression on the cell surface of macrophages, which were shown to react functionally to reinfection with P. gingivalis, as measured by a further increase in TNF-α production. The ability of P. gingivalis to induce the cell surface expression of TLR2 may thus contribute to the chronic inflammatory state induced by this pathogen.
While several reports have documented the roles that both T and B cells play in inflammatory oral bone loss (4, 12, 21, 35), it is clear that macrophages are also important immune cells in the initial innate immune recognition of bacteria. Our results demonstrate the contribution of the macrophage in response to infection by P. gingivalis and the resulting induction of osteoclastogenesis. Histological studies have reported that RANKL-bearing macrophages are present in diseased tissue obtained from patients with periodontal disease (9, 24). However, very little is known about the macrophage production of RANKL in vitro. One study demonstrated that low levels of RANKL could be detected in CS of unstimulated human macrophages differentiated from blood monocytes; however, the functional activity of RANKL was not examined (10). In our study, we found that the osteoclastogenesis and dentine pit formation induced by P. gingivalis-stimulated macrophage CS fluids was not affected by OPG. These results indicate that the induction of osteoclastogenesis by CS fluids was independent of soluble RANKL. RANKL exists not only as a soluble form but also as a membrane-bound form, and both the membrane form and the soluble RANKL form are capable of stimulating osteoclastogenesis. However, we did not observe differences in the expression levels of membrane bound RANKL on macrophages following stimulation with P. gingivalis. Collectively, these results suggest that RANKL does not play a role in osteoclast formation induced by live P. gingivalis-stimulated macrophages.
TNF-α has been previously reported to stimulate osteoclastogenesis by a RANKL-independent mechanism (3, 19, 20). Kim et al. (19) reported that TNF-α was capable of mediating BMM osteoclastogenesis; however, TGF-β was reported to also play a role in this process. While we did detect low levels of TGF-β in the fluids of unstimulated macrophages, we did not observe significant differences between the TGF-β production of unstimulated macrophages and that of P. gingivalis-stimulated macrophages. Since our BMMs were exposed to FBS prior to incubation with CS fluids and since it is known that TGF-β is present in FBS, we cannot definitively rule out a role for TGF-β in P. gingivalis-induced osteoclastogenesis in our macrophage system.
TNF-α is frequently detected in inflamed tissues from patients with periodontal disease (36). TNF-α has been demonstrated to contribute to oral bone loss in a P. gingivalis-induced inflammatory oral bone loss mouse model (13). P. gingivalis has also been demonstrated by others to induce TNF-α following stimulation of monocytes/macrophages (5), and recent studies have documented a role for TLR2 in this response (40). Our results are in agreement with these studies and further demonstrate that TNF-α production in response to P. gingivalis exposure is independent of TLR4 signaling. Moreover, we also observed that the production of both IL-1β and IL-6 from P. gingivalis-stimulated macrophages occurred via a TLR2-independent signaling mechanism. We and others recently reported that TLR2-deficient mice were resistant to inflammatory bone loss following oral infection with P. gingivalis (6, 11). We also demonstrated that TLR4 is not absolutely required for P. gingivalis-induced oral inflammatory bone loss (11). Thus, it appears that TLR2 is an essential signaling receptor for P. gingivalis, as demonstrated in both in vitro and in vivo models of inflammation.
Another important observation from this study is that live P. gingivalis induced the up-regulation of TLR2 expression on the surface of macrophages. Furthermore, we demonstrated that macrophages which were exposed to P. gingivalis responded upon subsequent exposure to P. gingivalis or SLTA, as measured by an increase in TNF-α production. In contrast to the results presented here, Muthukuru et al. (26) have reported that restimulation with P. gingivalis LPS induced down-regulation of TLR2 and TLR4 on human monocytes, with a reduction in cytokine secretion (26). The differences observed with our study may be due to the effects observed with live bacteria as opposed to effects observed with specific bacterial components. Collectively, these results indicate that similar to observations with endothelial cells, the exposure of macrophages to live P. gingivalis can induce the cell surface expression of TLR2, which results in the sensitization of the macrophage as measured by TNF-α production following reexposure with this organism. The ability of P. gingivalis to induce cell surface expression of TLR2 may be one mechanism that contributes to the chronic inflammatory state induced by this pathogen.
In conclusion, we have demonstrated the importance of TLR2 signaling in macrophage-induced osteoclastogenesis following live bacterial stimulation in vitro. Furthermore, we observed that this response is mediated via TNF-α. In addition, we have confirmed that P. gingivalis stimulation can induce cell surface expression of TLR2, which results in sensitization of the macrophage as measured by TNF-α production. It is tempting to speculate that chronic inflammation in response to P. gingivalis infection of the macrophage results from the increased expression of TLR2, which in turn, leads to the increased expression of TNF-α.
Acknowledgments
We thank Xinyan Liu for a critical review of this work and Sulip Goswami for technical assistance.
This study was supported by a Public Health Service grant to C.A.G. from the NIH/NHLBI (HL080387).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 12 November 2007.
REFERENCES
- 1.Adamopoulos, I. E., A. Sabokbar, B. P. Wordsworth, A. Carr, D. J. Ferguson, and N. A. Athanasou. 2006. Synovial fluid macrophages are capable of osteoclast formation and resorption. J. Pathol. 20835-43. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285736-739. [DOI] [PubMed] [Google Scholar]
- 3.Azuma, Y., K. Kaji, R. Katogi, S. Takeshita, and A. Kudo. 2000. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2754858-4864. [DOI] [PubMed] [Google Scholar]
- 4.Baker, P. J., M. Dixon, R. T. Evans, L. Dufour, E. Johnson, and D. C. Roopenian. 1999. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 672804-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodet, C., F. Chandad, and D. Grenier. 2006. Porphyromonas gingivalis-induced inflammatory mediator profile in an ex vivo human whole blood model. Clin. Exp. Immunol. 14350-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, E., G. Brahrach, L. Shapira, and G. Nussbaum. 2006. TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 1778296-8300. [DOI] [PubMed] [Google Scholar]
- 7.Calvo, M. S., D. R. Eyre, and C. M. Gundberg. 1996. Molecular basis and clinical application of biological markers of bone turnover. Endocr. Rev. 17333-368. [DOI] [PubMed] [Google Scholar]
- 8.Chu, C. Q., M. Field, S. Allard, E. Abney, M. Feldmann, and R. N. Maini. 1992. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br. J. Rheumatol. 31653-661. [DOI] [PubMed] [Google Scholar]
- 9.Crotti, T. N., M. D. Smith, R. Hirsch, S. Soukoulis, H. Weedon, M. Capone, M. J. Ahern, and D. R. Haynes. 2003. Receptor activator NF-κB ligand (RANKL) and osteopeotegerin (OPG) protein expression in periodontitis. Ann. Rheum. Dis. 611047-1054. [Google Scholar]
- 10.Evans, C. E., S. Mylchreest, and J. G. Andrew. 2006. Age of donor alters the effect of cyclic hydrostatic pressure on production by human macrophages and osteoblasts of sRANKL, OPG and RANK. BMC Musculoskelet. Disord. 721-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson, F. C., III, T. Ukai, and C. A. Genco. 2007. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis. Front. Biosci. 132041-2059. [DOI] [PubMed] [Google Scholar]
- 12.Han, X., T. Kawai, J. W. Eastcott, and M. A. Taubman. 2006. Bacterial-responsive B-lymphocytes induce periodontal bone resorption. J. Immunol. 176625-631. [DOI] [PubMed] [Google Scholar]
- 13.Hart, G. T., D. J. Shaffer, S. Akilesh, A. C. Brown, L. Moran, D. C. Roopenian, and P. J. Baker. 2004. Quantitative gene expression profiling implicates genes for susceptibility and resistance to alveolar bone loss. Infect. Immun. 724471-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes, D. R. 2004. Bone lysis and inflammation. Inflamm. Res. 53596-600. [DOI] [PubMed] [Google Scholar]
- 15.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 1684531-4537. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda, T., M. Kasai, M. Utsuyama, and K. Hirokawa. 2001. Determination of three isoforms of the receptor activator of nuclear factor-kappaB ligand and their differential expression in bone and thymus. Endocrinology 1421419-1426. [DOI] [PubMed] [Google Scholar]
- 17.Itoh, K., N. Udagawa, K. Kobayashi, K. Suda, X. Li, M. Takami, N. Okahashi, T. Nishihara, and N. Takahashi. 2003. Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J. Immunol. 1703688-3695. [DOI] [PubMed] [Google Scholar]
- 18.Iwahashi, M., M. Yamamura, T. Aita, A. Okamoto, A. Ueno, N. Ogawa, S. Akashi, K. Miyake, P. J. Godowski, and H. Makino. 2004. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 501457-1467. [DOI] [PubMed] [Google Scholar]
- 19.Kim, N., Y. Kadono, M. Takami, J. Lee, S. H. Lee, F. Okada, J. H. Kim, T. Kobayashi, P. R. Odgren, H. Nakano, W. C. Yeh, S. K. Lee, J. A. Lorenzo, and Y. Choi. 2005. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J. Exp. Med. 202589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi, K., N. Takahashi, E. Jimi, N. Udagawa, M. Takami, S. Kotake, N. Nakagawa, M. Kinosaki, K. Yamaguchi, N. Shima, H. Yasuda, T. Morinaga, K. Higashino, T. J. Martin, and T. Suda. 2000. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 191275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozuka, Y., Y. Ozaki, T. Ukai, T. Kaneko, and Y. Hara. 2006. B cells play an important role in lipopolysaccharide-induced bone resorption. Calcif. Tissue Int. 78125-132. [DOI] [PubMed] [Google Scholar]
- 22.Lam, J., S. Takeshita, J. E. Barker, O. Kanagawa, F. P. Ross, and S. L. Teitelbaum. 2000. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 1061481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauw, F. N., D. R. Caffrey, and D. T. Golenbock. 2005. Of mice and man: TLR11 (finally) finds profiling. Trends immunol. 26509-511. [DOI] [PubMed] [Google Scholar]
- 24.Liu, D., J. K. Xu, L. Figliomeni, L. Huang, N. J. Pavlos, M. Rogers, A. Tan, P. Price, and M. H. Zheng. 2003. Expression of RANKL and OPG mRNA in periodontal disease: possible involvement in bone destruction. Int. J. Mol. Med. 1117-21. [DOI] [PubMed] [Google Scholar]
- 25.Mori, Y., A. Yoshimura, T. Ukai, E. Lien, T. Espevik, and Y. Hara. 2003. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol. Immunol. 1854-58. [DOI] [PubMed] [Google Scholar]
- 26.Muthukuru, M., R. Jotwani, and C. W. Cutler. 2005. Oral mucosal endotoxin tolerance induction in chronic periodontitis. Infect. Immun. 73687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revell, P. A., V. Mayston, P. Lalor, and P. Mapp. 1988. The synovial membrane in osteoarthritis: a histological study including the characterization of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann. Rheum. Dis. 47300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rycke, L. D., B. Vandooren, E. Kruithof, F. D. Keyser, E. M. Veys, and D. Baeten. 2005. Tumor necrosis factor blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondyloarthropathy. Arthritis Rheum. 522146-2158. [DOI] [PubMed] [Google Scholar]
- 29.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 27815587-15594. [DOI] [PubMed] [Google Scholar]
- 30.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 27417406-17409. [DOI] [PubMed] [Google Scholar]
- 31.Simonet, W. S., D. L. Lacey, C. R. Dunstan, M. Kelley, M. S. Chang, R. Luthy, H. Q. Nguyen, S. Wooden, L. Bennett, T. Boone, G. Shimamoto, M. DeRose, R. Elliott, A. Colombero, H. L. Tan, G. Trail, J. Sullivan, E. Davy, N. Bucay, L. RenshawGegg, T. M. Hughes, D. Hill, W. Pattison, P. Campbell, S. Sander, G. Van, J. Tarpley, P. Derby, R. Lee, and W. J. Boyle. 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89309-319. [DOI] [PubMed] [Google Scholar]
- 32.Smith, M. D., J. O'Donnell, J. Highton, D. G. Palmer, M. Rozenbilds, and P. J. Roberts-Thomson. 1992. Immunohistochemical analysis of synovial membranes from inflammatory and non-inflammatory arthritides: scarcity of CD5 positive B cells and IL2 receptor bearing T cells. Pathology 2419-26. [DOI] [PubMed] [Google Scholar]
- 33.Takeda, K., and S. Akira. 2004. Microbial recognition by toll-like receptors. J. Dermatol. Sci. 3473-82. [DOI] [PubMed] [Google Scholar]
- 34.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 1655780-5787. [DOI] [PubMed] [Google Scholar]
- 35.Teng, Y.-T., H. Nguyen, X. Gao, Y.-Y. Kong, R. M. Gorczynski, B. Singh, R. P. Ellen, and J. M. Penninger. 2000. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 106R59-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tervahartiala, T., H. Koski, J. W. Xu, R. Hayrinen-Immonen, J. Hietanen, T. Sorsa, and Y. T. Konttinen. 2001. Tumor necrosis factor-alpha and its receptors, p55 and p75, in gingiva of adult periodontitis. J. Dent. Res. 801535-1539. [DOI] [PubMed] [Google Scholar]
- 37.Toraldo, G., C. Roggia, W. P. Qian, R. Pacifici, and M. N. Weitzmann. 2002. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kB ligand and tumor necrosis factor α from T cells. Proc. Natl. Acad. Sci. USA 100125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuda, H., N. Shima, N. Nakagawa, S. I. Mochizuki, K. Yano, N. Fujise, Y. Sato, M. Goto, K. Yamaguchi, M. Kuriyama, T. Kanno, A. Murakami, T. Morinaga, and K. Higashio. 1998. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1391329-1337. [DOI] [PubMed] [Google Scholar]
- 39.Yumoto, H., H.-H. Chou, Y. Takahashi, M. Davey, F. C. Gibson III, and C. A. Genco. 2005. Sensitization of human aortic endothelial cells to lipopolysaccharide via regulation of Toll-like receptor 4 by bacterial fimbria-dependent invasion. Infect. Immun. 738050-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, Q., T. Desta, M. Fenton, D. T. Graves, and S. Amar. 2005. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect. Immun. 73935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]