Abstract
Enterobacter sakazakii is an opportunistic pathogen that causes systemic bacteremia and meningitis with high mortality, and powdered infant formula is a frequent source of this bacterium. However, the mechanisms that this organism uses to invade and translocate through the intestinal barrier are unknown. Using Caco-2 epithelial cells, we were able to demonstrate penetration of E. sakazakii and to determine invasion-associated properties. We found that E. sakazakii entry and invasion were dependent on the exposure time and multiplicity of infection and required bacterial de novo protein synthesis but was independent of cell polarity in the presence of tight junctions. Moreover, the presence of actin filaments and microtubule structures was required, and disruption of the tight junction significantly enhanced the initial association with Caco-2 cells and the efficiency of invasion, which provides a possible explanation for the preferential occurrence of this infection in babies and neonates. This is the first description of E. sakazakii invasion of host intestinal cells, and our findings suggest that this emerging pathogen employs a novel invasion mechanism for development of systemic infection.
Enterobacter sakazakii, a motile gram-negative rod, is an opportunistic pathogen that can cause local necrotizing enterocolitis, systemic bacteremia, and meningitis (7). Cases are rather sporadic, but epidemics are not unusual; the highest-risk group is neonates (<28 days old) that have low birth weights (<2,000 to 2,500 g) or are premature (<37 weeks of gestation) (7). The high mortality (40 to 80%) is a matter of concern (47), and recovery from E. sakazakii infection often leads to serious sequelae, including brain abscesses, developmental delays, and impaired sight and hearing. Thus, E. sakazakii, together with Listeria monocytogenes, Clostridium perfringens types A and B, and Cryptosporidium parvum, was categorized as “severe hazard for restricted populations, life threatening or substantial chronic sequelae or long duration” by the International Commission on Microbiological Specifications for Foods (16).
The natural habitat of E. sakazakii is unclear. It has been isolated from various environments, including different foods, such as spices (2, 32, 37), insects (14, 23), and the food production environment (20, 30). Powdered infant formula was implicated in several E. sakazakii outbreaks and is currently considered the major route of transmission (2, 30, 44, 46). The ability to grow at a wide range of temperatures (6 to 45°C) (17), efficient biofilm formation (17, 24), and relatively high resistance to heat (33) and osmotic stresses (3) have been suggested to be important for successful transmission of this bacterium. Because of the previous lack of in vitro (cell culture) or in vivo (animal) infection models, very little was known about the pathogenicity mechanisms and possible virulence determinants of E. sakazakii. Previous studies addressing cellular adhesion patterns (26), enterotoxin production (35), survival inside macrophages (43), and efficiency of penetration into the rat brain capillary endothelial cells (43) revealed great variability among different isolates. E. sakazakii can produce a mannose-sensitive hemagglutinin associated with type I fimbriae, which could act as an adhesion (1). It remains to be determined if E. sakazakii contains intrinsic and specific virulence factors or is a casual but dominant colonizer in very young children (35, 40).
As an oral pathogen causing systemic infection, E. sakazakii should possess the instrumentarium to penetrate the epithelial cell barrier in the intestines in order to access the blood circulation and spread. Availability of an in vitro cell culture model is essential to study the initial steps of entry of E. sakazakii into eukaryotic host cells and to identify potential virulence factors involved in such processes. We used gentamicin protection assays and confocal imaging of fluorescently tagged E. sakazakii cells to show that this bacterium actively invades human epithelial Caco-2 cells. Both f-actin and microtubule structures are required for invasion. Disruption of cellular tight junctions increased the initial association of E. sakazakii with Caco-2 cells and significantly enhanced the ability to invade.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Half-concentrated brain heart infusion medium (BHI/2) (Biolife, Milan, Italy) was used to cultivate bacteria at 37°C with constant shaking (21). We used three isolates of E. sakazakii (ATCC 29544, 732/03, and 966/04), three Enterobacter cloacae strains (SG36, SG38, and SG95) (laboratory stock), one Enterobacter agglomerans strain (SG71) (laboratory stock), two Salmonella enterica serovar Typhimurium strains (ATCC 14028 and DT104), Listeria monocytogenes strain ScottA, and Escherichia coli strain XL-1 Blue. When required, ampicillin was added to a final concentration of 100 μg/ml.
Cell culture.
Human enterocytelike Caco-2 cells (ATCC HTB-37; ATCC, Manassas, VA) (38) were maintained in RPMI 1640 medium (Sigma, St. Louis, MO) containing 10% fetal bovine serum (FBS) (Omnilab, Switzerland) (RPMI-FBS), unless indicated otherwise. Trypsin-treated cells that had been passaged six or fewer times were seeded into 24-well cell culture plates (approximately 5 × 104 cells per well) and grown at 37°C in the presence of 5% CO2. Cells took 1 to 2 days to form monolayers and were cultured for up to 20 days. If Caco-2 cells are incubated for more than 2 weeks, they can be polarized upon confluence to accommodate their different functions in different locations (apical or basolateral) (19). The medium was replaced every 2 days, and cell viability was determined by trypan blue staining (39).
Bacterial infection of Caco-2 cell monolayers.
In order to determine bacterial invasion of Caco-2 cells and the numbers of intracellular bacteria, a gentamicin protection assay was performed as described previously (18, 43), with some modifications. Briefly, bacteria were prepared by transferring a 2% inoculum from an overnight culture into fresh, prewarmed BHI/2, incubating the culture for 2 h, collecting cells by centrifugation, washing the cells with phosphate-buffered saline (PBS) (Sigma), and resuspending the cells in PBS. Caco-2 monolayer cells (in 950 μl of fresh RPMI-FBS per well) were infected with bacteria (50 μl per well) at a multiplicity of infection (MOI) of 50 and incubated for 1.5 h without centrifugation which may have physically promoted contact between bacteria and the cell monolayer. After four washes in PBS, fresh medium containing gentamicin (100 μg/ml; Sigma) was added, and the plates were incubated for 1.5 h and then washed five times with PBS. Then 1 ml of Triton X-100 (0.2% in PBS) was added, and the plates were incubated for another 30 min before the bacteria were collected and plated onto BHI agar using decimal dilutions. The Triton X-100 solution did not affect the viability of the bacteria (results not shown). In some experiments, the MOI and infection time were varied (see Fig. 3). The number of cell-associated bacteria was determined by incubating Caco-2 cell monolayers with the bacteria for the indicated times (see Fig. 3 and 8) and then washing the cells with PBS five times, lysing them with Triton X-100, and plating them as described above.
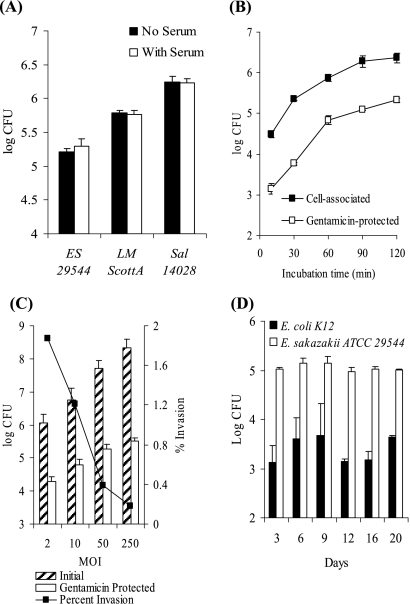
FIG. 3.
E. sakazakii invasion properties. (A) Gentamicin protection assay performed either with or without serum added to the cell culture medium during the 1.5 h of bacterial infection. (B) At various times after infection (10, 30, 60, 90, and 120 min), Caco-2 cell monolayers were either treated with Triton X-100 to obtain the total number of cell-associated E. sakazakii cells or supplemented with gentamicin to determine the number of intracellular E. sakazakii cells. (C) Dependence of the infection rate on the number of cells present initially. Caco-2 cells were challenged with various numbers of bacteria (MOI, 2 to 250), and the number of gentamicin-protected E. sakazakii cells was determined after 1.5 h. The results are expressed as percent invasion (number of gentamicin-protected bacteria/initial number of bacteria × 100). (D) Effect of cell age and differentiation state on invasion and persistence. A gentamicin protection assay with E. sakazakii ATCC 29544 and E. coli XL-1 (control) was performed using Caco-2 cell monolayers, and polarity was generated over time by prolonged incubation. Fresh medium was added every 2 days. The data are the means and standard deviations of three independent experiments.
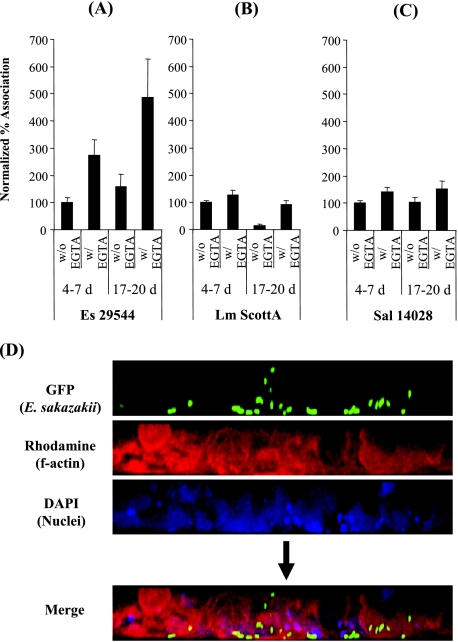
FIG. 8.
Initial association of bacterial cells with EGTA-pretreated Caco-2 cells. Monolayers were either 4 to 7 or 17 to 20 days old and were pretreated or not pretreated with 5 mM EGTA, followed by exposure to the bacteria for 30 min. (A to C) Unbound bacteria were removed by washing with PBS, and cells were disrupted by Triton X-100 treatment before plating for CFU counting. The percent association (normalized values) was determined by comparing association rates to the control EGTA rates. ES 29544, E. sakazakii ATCC 29544; Lm ScottA, L. monocytogenes ScottA; Sal 14028, Salmonella Typhimurium strain ATCC 14028. The data are the means and standard deviations of three independent experiments. (D) Polarized Caco-2 cells (18-day-old monolayer) were treated with 5 mM EGTA and infected with E. sakazakii ATCC 29544(pHGFP) for 30 min, followed by five washes with PBS. Actin structures and DNA were stained as described in the legend to Fig. 2. The preferred basolateral site of entry is clearly evident.
Confocal laser fluorescence microscopy.
For expression of green fluorescent protein (GFP), plasmid pHGFP (25) was transformed into E. sakazakii ATCC 29544 by electroporation. Electrocompetent bacteria were prepared by growing E. sakazakii ATCC 29544 to the mid-log phase, washed four times, and resuspended in 10% ice-cold glycerol. Cells were transformed by electroporation (Gene Pulser; 2.5 V; 200 Ω; 25-μF capacity; Bio-Rad, Hercules, CA) and plated on Luria-Bertani agar containing 100 μg/ml of ampicillin. We found that the plasmid was stable without drug selection, as prolonged incubation (3 days) in the absence of ampicillin and successive inoculation (2%) into fresh medium every 12 h did not result in loss of the bright green phenotype. Also, the presence of the plasmid and cytosolic GFP did not affect the invasion properties of E. sakazakii (data not shown).
For confocal laser scanning microscopy, round coverslips (diameter, 12 mm) were placed in the wells of a 24-well tissue culture plate, seeded with 104 Caco-2 cells, and incubated for 24 h. After infection with GFP-labeled E. sakazakii (pHGFP) (1.5 h) and gentamicin protection (1.5 h) as described above, the coverslips were removed, and cells were fixed with formaldehyde (3.7% in PBS, 15 min) and washed three times with PBS. Caco-2 cells were permeabilized for 3 min with 0.1% Triton X-100 and then sequentially incubated with rhodamine-labeled phalloidin (1:200 dilution of a 0.1-mg/ml stock solution; Sigma) and 4′,6′-diamidino-2-phenylindole (DAPI) (1:100 dilution of a 5-mg/ml solution) for 20 and 3 min, respectively, and extensively washed with PBS. Fluorescence microscopy was done with an inverted confocal laser scanning microscope (TCS-SPE; Leica Microsystems, Mannheim, Germany) equipped with a heated stage (temperature control) and a CO2 incubation chamber. Different series of images from 0.4-μm x-y-z sections were obtained and then analyzed and stacked by using the recommended software (LAS AF; Leica Microsystems).
Treatment with Cam and CyH.
To test if bacterial de novo protein synthesis was required for invasion, a gentamicin protection assay was performed in the presence of chloramphenicol (Cam) (which inhibits bacterial protein synthesis) or cycloheximide (CyH) (which inhibits eukaryotic protein synthesis) during 1.5 h of the infection period and 1.5 h of the subsequent gentamicin treatment period. The final concentrations were 100 μg/ml (Cam) and 20 μg/ml (CyH).
Treatment with CyD and Co.
To test if the host cytoskeleton was required for bacterial invasion, Caco-2 cell monolayers were preincubated with various concentrations of cytochalasin D (CyD) (2-mg/ml stock solution in dimethyl sulfoxide) or colchicine (Co) (25-mg/ml stock solution in ethanol) (Sigma) for 1 h and were infected with bacteria as prepared described above. The drugs were included during bacterial infection as well as during gentamicin treatment (100 μg/ml).
EGTA treatment.
Eagle's minimum essential medium (MEM) (Sigma) was supplemented with l-glutamine (0.3 g/liter) and 10% FBS. For calcium supplementation, 1.8 mM Ca2+ (MEM/Ca2+) was added to the medium when necessary.
The Ca2+-sequestering agent EGTA (pH 7.5) specifically binds Ca2+ ions (36). For treatment with EGTA, Caco-2 cell monolayers were preincubated for 1 h with either RPMI-FBS, MEM/Ca2+, or MEM containing various concentrations of EGTA. Then cells were washed once, and fresh medium was added; this was followed by a gentamicin protection assay.
Statistical analysis.
All experiments described above were performed at least in triplicate, and the statistical significance of the data was analyzed and verified by using Student's t test and an α value of 0.05.
RESULTS
E. sakazakii can invade human enterocyte-like Caco-2 cells.
Preliminary experiments showed that all three E. sakazakii strains tested here were gentamicin sensitive and unable to grow in media containing as little as 0.5 μg/ml of the antibiotic (data not shown), which was in line with previous findings (9). The survival rate of E. sakazakii in RPMI-FBS containing 100 μg/ml of gentamicin was less than 0.001% after 90 min of exposure. These results indicated the usefulness of gentamicin for studying invasion properties, since it killed extracellular E. sakazakii not protected inside the host cell cytosol (gentamicin protection assay).
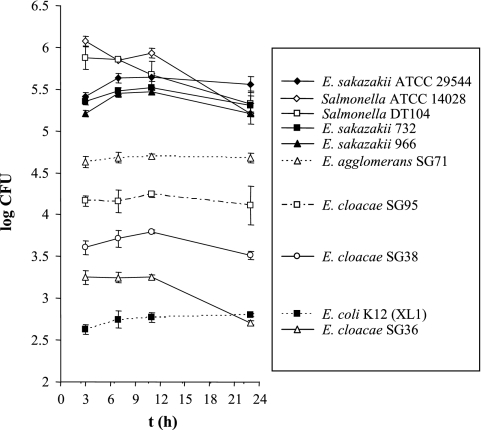
Caco-2 cell monolayers were infected with three different E. sakazakii strains (including type strain ATCC 29544), three strains of E. cloacae, and one E. agglomerans strain. Salmonella serovar Typhimurium (two strains) and one noninvasive E. coli strain were used as positive and negative controls, respectively. With an MOI of 50, 5.84 ± 0.05 and 5.75 ± 0.14 log CFU/ml of Salmonella serovar Typhimurium ATCC 14028 and DT104, respectively, were recovered after gentamicin treatment (3-h time point in Fig. 1). The level of recovery for the E. sakazakii strains was significantly higher than the for the other Enterobacter species (Fig. 1) and corresponded to approximately 0.5 to 1.0% of the initial counts of E. sakazakii in Caco-2 cells. Longer incubation (up to 20 h) in the presence of gentamicin did not significantly increase the number of bacteria recovered (Fig. 1), suggesting that there was no intracellular proliferation of the bacteria under these conditions.
FIG. 1.
Invasion and intracellular persistence of E. sakazakii and related enterobacteria. Caco-2 cell monolayers were infected at 0 h with either E. sakazakii strain ATCC 29544, 732, or 966, E. cloacae SG36, SG38, or SG95, E. agglomerans SG71, Salmonella serovar Typhimurium strain ATCC 14028 or DT104, or an E. coli K-12 strain and incubated for 1.5 h. After washing with PBS, gentamicin-containing RPMI-FBS was added, and the incubation was continued. At the indicated time points, bacteria were harvested from samples of the infected monolayers, and viable cell counts were determined by plate counting (see text). The data are the means and standard deviations of three independent experiments.
E. sakazakii ATCC 29544 and Salmonella serovar Typhimurium ATCC 14028 were used for further investigation, and L. monocytogenes ScottA was used in experiments investigating invasion properties.
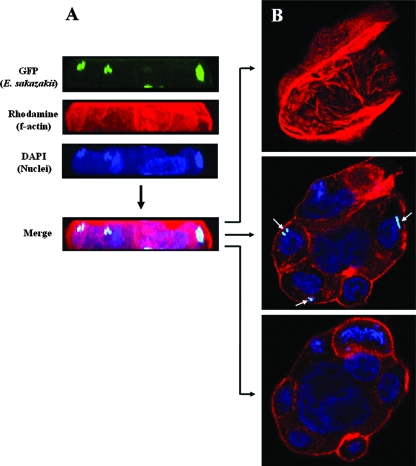
Using confocal fluorescence microscopy to study entry of E. sakazakii ATCC 29544(pHGFP) into Caco-2 cells (labeled with actin-specific rhodamine-labeled phalloidin), we were able to show that bacteria were present inside the host cells (Fig. 2). However, we did not observe any significant increase in the number of green fluorescent cells inside Caco-2 cells or bacterial lysis during the subsequent 20 h of incubation, suggesting that the bacteria had a “persistence without proliferation” phenotype inside the infected host cells. On the other hand, we cannot exclude the possibility that the rate of bacterial replication equaled the rate of bacterial killing, which would also have resulted in constant numbers of bacteria.
FIG. 2.
E. sakazakii invasion of Caco-2 cells. A gentamicin protection assay was performed as described in Materials and Methods. Cells and DNA were labeled with rhodamine-labeled phalloidin and DAPI, respectively. Fluorescence was analyzed by confocal laser scanning microscopy, using a series of images from 0.4-μm x-y-z sections. A merged image of the separate channels is shown in panel A, and images of upper, middle, and lower sections are shown in panel B. The intracellular locations of E. sakazakii cells are indicated by arrows.
Invasion properties of E. sakazakii with Caco-2 cells.
The effects of several possibly important factors were evaluated. First, the presence of serum during bacterial infection did not significantly affect the number of gentamicin-protected E. sakazakii cells, similar to the results obtained with L. monocytogenes and Salmonella serovar Typhimurium (Fig. 3A).
Second, the number of host cell-associated and intracellular E. sakazakii cells increased as the incubation time increased (Fig. 3B). The counts of gentamicin-protected bacteria were generally approximately 1.5 orders of magnitude lower than the counts of cell-associated bacteria, confirming that the gentamicin protection assay is suitable for reliably measuring invasion capacity. The bacterial counts appeared to reach a plateau after 90 to 120 min of incubation. Therefore, we used 90 min of incubation in all further studies.
We also tested if E. sakazakii invasion is dependent on the MOI. We observed a gradual, linear increase in the number of intracellular E. sakazakii cells that was correlated with a higher initial bacterial challenge, but there was a corresponding decrease in the percentage of invading bacteria.
Finally, we determined if cell polarity with intact tight junctions has an effect on the invasion of Caco-2 cells by E. sakazakii by using nonpolarized and polarized Caco-2 cells. No significant difference in E. sakazakii invasion behavior was observed between Caco-2 cell monolayers that were infected after 3 days (nonpolarized) and Caco-2 cell monolayers that were grown for 20 days before infection (polarized) (Fig. 3D). Likewise, the number of gentamicin-protected E. coli cells used as a control did not change significantly as Caco-2 cells aged (Fig. 3D).
Invasion by E. sakazakii requires bacterial protein synthesis but not host cell protein synthesis.
Cam (or CyH) inhibits bacterial (or eukaryotic) protein biosynthesis by binding to the ribosomal subunits. The MICs of Cam for E. sakazakii ATCC 29544 were reported to be 4 to 8 μg/ml (9). Surprisingly, however, the working concentration used in this study (100 μg/ml) did not kill the bacteria tested after 3 h of incubation in RPMI-FBS (data not shown). When Cam was added, the rates of invasion of Caco-2 cells decreased considerably for E. sakazakii (6.9% ± 0.9% of the bacteria were recovered compared to the control without Cam), L. monocytogenes (0.1% ± 0.1%), and Salmonella serovar Typhimurium (2.1% ± 0.4%), whereas CyH had no effect (data not shown). This suggested that bacterial but not host cell de novo protein synthesis is required.
CyD treatment enhances invasion by E. sakazakii.
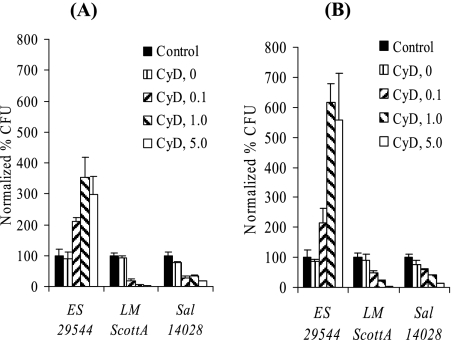
Infection of eukaryotic host cells pretreated with the actin polymerization inhibitor CyD indicates whether an actin cytoskeleton is required for invasion (7). When Caco-2 cells that were 4 to 7 days old and were pretreated with CyD were challenged with L. monocytogenes or Salmonella serovar Typhimurium, invasion was significantly inhibited. In contrast, invasion by E. sakazakii ATCC 29544 was enhanced by the f-actin inhibitor in a concentration-dependent manner (Fig. 4A).
FIG. 4.
E. sakazakii invasion of CyD-treated Caco-2 cells. Monolayers that were different ages (4 to 7 days old [A] and 17 to 20 days old [B]) were treated with different concentrations of CyD. Gentamicin protection assays were performed in the presence of CyD, and the normalized percentage of CFU was determined by comparison with the control (no drug). ES 29544, E. sakazakii ATCC 29544; LM ScottA, L. monocytogenes ScottA; Sal 14028, Salmonella serovar Typhimurium strain ATCC 14028. The data are the means and standard deviations of three independent experiments.
The behavior of L. monocytogenes and Salmonella serovar Typhimurium with polarized 17- to 20-day-old Caco-2 cell monolayers was similar to the behavior observed with nonpolarized cells. With E. sakazakii, however, we observed a significant increase (up to 700%) in the invasion efficiency (Fig. 4B). The increase in invasion by E. sakazakii after CyD treatment was most probably due to tight junction disruption (see below); this could also be observed by microscopy (data not shown). Addition of dimethyl sulfoxide alone (final dilution, 1:250) had no effect on bacterial invasion (Fig. 4).
E. sakazakii invasion is dependent on microtubules.
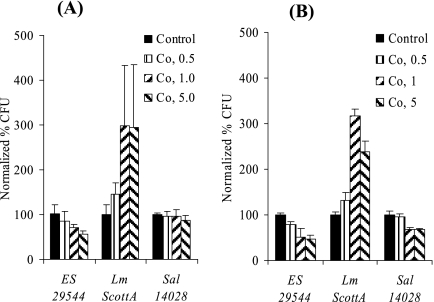
Several bacterial pathogens require the cellular microtubule structure for efficient invasion of their host cells (34). When we treated Caco-2 cell monolayers (that were 4 to 7 days old) with Co at different concentrations, a significant increase in the invasion by L. monocytogenes was observed (Fig. 5A). In contrast, invasion by E. sakazakii was inhibited (Fig. 5A). Similar results were obtained when 17- to 20-day-old Caco-2 cell monolayers were infected (Fig. 5B). Inhibition of Salmonella serovar Typhimurium invasion was not obvious (Fig. 5A) or was much less pronounced (Fig. 5B).
FIG. 5.
E. sakazakii invasion of Co-treated Caco-2 cells. Caco-2 cell monolayers that were in different differentiation states (4 to 7 days old [A] and 17 to 20 days old [B]) were treated with different concentrations of Co. Gentamicin protection assays were performed in the presence of Co, and the normalized percentage of CFU was determined by comparison with the control (no drug). ES 29544, E. sakazakii ATCC 29544; Lm ScottA, L. monocytogenes ScottA; Sal 14028, Salmonella serovar Typhimurium strain ATCC 14028. The data are the means and standard deviations of three independent experiments.
Disruption of tight junctions enhances invasion.
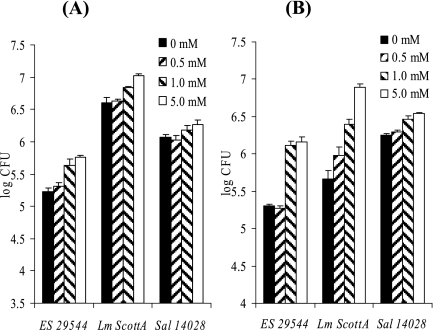
Opening of the epithelial tight junctions can be achieved by treatment with EGTA (28) and can be observed by microscopy (data not shown). When 4- to 7-day-old Caco-2 cell monolayers were pretreated with EGTA, the invasion capacity of E. sakazakii was slightly but significantly enhanced (3.3-fold with 5.0 mM EGTA treatment) (Fig. 6A). Likewise, invasion by L. monocytogenes (2.6-fold) and invasion by Salmonella serovar Typhimurium (1.5-fold) were also enhanced. When 17- to 20-day-old Caco-2 cells were used, the effects on the invasion abilities of E. sakazakii (7.1-fold) and L. monocytogenes (16.7-fold) were more pronounced (Fig. 6B), whereas Salmonella (1.9-fold) did not show a response to disruption of the epithelial cell tight junctions. Enhanced invasion of EGTA-treated Caco-2 cells by E. sakazakii was also observed when Ca2+-limited MEM was used after EGTA pretreatment (data not shown).
FIG. 6.
E. sakazakii invasion of EGTA-pretreated Caco-2 cells. Monolayer tissue cultures that were either 4 to 7 days old (A) or 17 to 20 days old (B) were pretreated with different concentrations of EGTA for 1 h, and then the gentamicin protection assay was performed in the absence of EGTA. Cells were released by Triton X-100 treatment, and viable cells counts were determined by plating. ES 29544, E. sakazakii ATCC 29544; Lm ScottA, L. monocytogenes ScottA; Sal 14028, Salmonella serovar Typhimurium strain ATCC 14028. The data are the means and standard deviations of three independent experiments.
CyD inhibits invasion of cells with disrupted tight junctions.
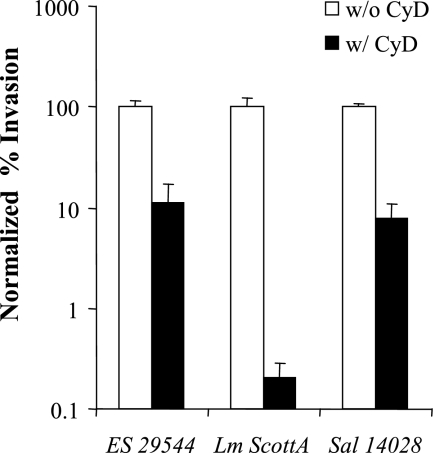
Based on the finding that tight junctions play a significant role in the E. sakazakii invasion process and the fact that CyD can disrupt these junctions (5, 6), we tested the effect of CyD on E. sakazakii invasion using Caco-2 cell monolayers with disrupted tight junctions (pretreated with EGTA). As shown in Fig. 7, the presence of CyD significantly inhibited invasion by E. sakazakii and also severely affected invasion by Salmonella serovar Typhimurium and, in particular, L. monocytogenes.
FIG. 7.
Effect of CyD on EGTA-pretreated Caco-2 cells (8 to 10 days old). Caco-2 cell monolayers were pretreated with 5 mM EGTA for 1 h, and then the gentamicin protection assay was performed, in which CyD was added or not added during infection and gentamicin treatment. ES 29544, E. sakazakii ATCC 29544; Lm ScottA, L. monocytogenes ScottA; Sal 14028, Salmonella Typhimurium strain ATCC 14028. The data are the means and standard deviations of three independent experiments.
Initial association of E. sakazakii with EGTA-pretreated host cells.
As the opening of tight junctions and the rounding of Caco-2 cells would have increased cell surface area, we tested whether the initial association (first 30 min) of bacteria with their host cells (intracellular and extracellular, attached cells) was also affected. Giemsa staining revealed that E. sakazakii adhered to a Caco-2 cell monolayer with a diffuse pattern (data not shown). However, E. sakazakii association increased significantly (2.7-fold) when 4- to 7-day-old Caco-2 cells were pretreated with EGTA and increased almost sixfold when 17- to 20-day-old Caco-2 cells were used (Fig. 8A). These results are different from those obtained with L. monocytogenes (Fig. 8B) and Salmonella serovar Typhimurium (Fig. 8C), for which no such increase was found. The preferred association of E. sakazakii with the basolateral region of 18-day-old Caco-2 cells with disrupted tight junctions was also visualized by confocal microscopy (Fig. 8D).
DISCUSSION
E. sakazakii was proposed as a new species in 1980 (9) and was first mentioned as a causative agent of human disease (meningoencephalitis) shortly thereafter (22). Since then, most studies have focused on biochemical and physiological characterization of this species in efforts to develop better suitable isolation protocols and to establish the transmission route. The data available today show that E. sakazakii causes a serious systemic infection mostly in neonates and infants and that powdered infant formula is the most frequently identified source of the organism. Recently, Townsend et al. (43) showed that E. sakazakii is able to penetrate rat brain endothelial cells and to survive inside macrophages. The circumstantial evidence also implies that E. sakazakii has to be able to translocate through the intestinal barrier and establish a systemic infection with symptoms such as bacteremia and meningitis. Surprisingly, however, the pathogenicity mechanisms of E. sakazakii were virtually unknown. We began to study this organism by investigating its invasion of human Caco-2 cells originating from the colon. This cell line readily differentiates upon confluence and is suitable for simulating in vivo conditions (38). Caco-2 cells have previously been used as a model system for other bacterial pathogens, including L. monocytogenes (15) and Salmonella (11).
Compared to the invasive potential of strains of other Enterobacter species (E. cloacae and E. agglomerans), all three E. sakazakii isolates tested here exhibited significantly higher invasiveness. With respect to clinical manifestations, E. cloacae and E. agglomerans have been responsible for a much higher percentage of bacteremia cases than E. sakazakii, possibly due to their more widespread presence in environments (40). On the other hand, although E. cloacae and E. agglomerans have also been more frequent contaminants of powdered infant formula (30), they were not shown to cause infection of infants by this transmission route. Apparently, other or more efficient pathogenicity mechanisms of E. sakazakii could be considered intrinsic virulence properties facilitating disease. With respect to enterotoxin production, large variations among different E. sakazakii isolates have been reported (35), similar to what was found for 50% lethal doses and adhesion properties (26). Thus, the uniform invasion rate and intracellular presence of the different E. sakazakii isolates studied here were quite intriguing.
In general, E. sakazakii invasion was directly correlated to cell contact time and MOI (Fig. 3). Bacterial association and invasion showed typical saturation kinetics, and an increase in the MOI resulted in a decrease in the level of invasion. Taken together, these data suggest that entry of E. sakazakii into Caco-2 cells may be receptor mediated.
Addition of Cam during the different steps of a bacterial infection cycle reveals a requirement for bacterial protein synthesis for continuation of the process. Using this approach, it was shown that Campylobacter jejuni, Citrobacter freundii, Salmonella serovar Typhi (34), and Actinobacillus actinomycetemcomitans (41) require de novo protein synthesis for invasion, whereas Yersinia enterocolitica does not (10). Our results demonstrate that E. sakazakii invasion is inhibited by this drug (similar to Listeria [6] and Salmonella [10]) but not by CyH, which specifically inhibits eukaryotic protein synthesis (34). These results suggest that E. sakazakii invasion is an active process requiring bacterial de novo protein synthesis.
Most, if not all, invasive bacteria are able to manipulate the host cell cytoskeleton. They do this either directly, by active secretion of bacterium-encoded effector molecules, or indirectly, by interaction with a host-specific receptor and subsequent stimulation of downstream signal transduction (4). Actin microfilaments are the most prominent molecules in this process. CyD prevents polymerization of g-actin and can even disrupt f-actin in a concentration-dependent manner, thereby inhibiting entry or movement of bacteria which require f-actin. Interestingly, E. sakazakii invasion was enhanced in CyD-treated Caco-2 cells. This effect was likely due to the other effect of CyD treatment, namely, opening of the tight junctions (see below). Our observation that invasion of L. monocytogenes and Salmonella serovar Typhimurium was impaired by CyD treatment is in line with previous reports (5, 12).
While actin microfilaments are frequently associated with the bacterial invasion process, microtubules may also be involved in penetration by microbial pathogens, such as C. jejuni and C. freundii (34). The manipulation of microtubules is important for formation and maintenance of Salmonella-containing vacuoles, as well as for invasion by some viruses (4). Treatment with Co interferes with tubulin polymerization and thereby inhibits entry of bacteria dependent on this process (34). Thus, our finding that Co-treated Caco-2 cells show decreased invasion by E. sakazakii suggests that microtubules are involved in the E. sakazakii entry process. Internalization of L. monocytogenes or Salmonella was not affected by Co treatment or was affected very little.
The tight junction is located in the apical end of the basolateral membrane, helps to establish cell polarity, and acts as a barrier to keep the molecules from crossing freely through the gaps between epithelial cells (28). Tight junctions are formed and maintained by the interaction between transmembrane proteins (occludin, claudins, and junctional adhesion molecules) which are connected to the actin cytoskeleton via cytoplasmic adaptor proteins (ZO). In addition, the adherens junction, composed of junction components such as cadherins (particularly E-cadherin in epithelial cells) and connected to actin cytoskeleton, facilitates formation and maintenance of a tight junction. The cadherin-mediated interaction is Ca2+ dependent, while other interactions are not Ca2+ dependent (28). EGTA is a Ca2+-selective chelating agent, and depletion of Ca2+ in a cell culture can open the tight junctions formed between confluent layers of eukaryotic cells. We found that EGTA-mediated disruption of the tight junction significantly increased the invasiveness of E. sakazakii and L. monocytogenes but did not affect that of Salmonella serovar Typhimurium. Enhanced invasion by L. monocytogenes of Caco-2 cells with disrupted tight junctions was very likely due to exposure of the basolateral eukaryotic receptor (E-cadherin) by EGTA treatment (27).
Enhanced entry of E. sakazakii upon disruption of the tight junction may be an important feature for systemic infection of neonates and infants. Newborns lack the normal microflora and fully established gut epithelial lining in their intestines. The epithelial lining contains more fingerlike villous cores (45) and might have increased mucosal permeability (8). In this context, it is noteworthy that premature birth and/or low birth weight are often mentioned as highest risk factors for E. sakazakii infection. Within a few days after birth, however, massive changes in the intestinal structure occur (48), and the microflora rapidly forms and becomes established (15), which also supports further development of intestinal epithelia. In addition, it should be noted that powdered infant formula is frequently contaminated with high levels of lipopolysaccharide (42) and that lipopolysaccharide is known to be able to disrupt tight junctions (29). Based on all the findings, it seems possible that when a sufficiently high number of E. sakazakii cells is ingested with reconstituted infant formula, this organism would be in a favorable environment to translocate to the intestinal barrier. Moreover, its ability to better associate with and more efficiently invade cells with disrupted tight junctions should facilitate the penetration in an immature barrier.
We also found that EGTA treatment increased the association of E. sakazakii with Caco-2 cell monolayers. In contrast, Salmonella did not show significantly elevated association or invasion levels under the same conditions, while entry of L. monocytogenes was enhanced upon disruption of tight junctions, due to the basolateral localization of the internalin A receptor E-cadherin (27). Currently, we can only speculate about the basis for the differences among these invasive pathogens, but it is possible that an unidentified host receptor(s) for an E. sakazakii adhesin(s) and/or invasin(s) might be present not only in apical areas but also in basolateral areas of Caco-2 cells.
Our data revealed increased invasion by E. sakazakii when Caco-2 cells were treated with CyD. In contrast, when EGTA-pretreated Caco-2 cells were used to evaluate invasion in the presence of CyD, less invasion was observed. It should be noted that CyD treatment of cell monolayers disrupts tight junctions (5, 6). Thus, it is plausible that the increased E. sakazakii invasion in CyD-treated Caco-2 cell monolayers was due to disruption of tight junctions; the actin filament indeed plays a critical role in E. sakazakii entry into Caco-2 cells. These data again emphasize the importance of enhanced E. sakazakii invasion in epithelial structures with disrupted tight junctions. On the other hand, one cannot exclude the possibility that E. sakazakii may remodel the actin cytoskeleton for entry, using the basolateral surface; this presents an intriguing question for further study.
The dual requirement (actin filaments and microtubules) observed for E. sakazakii invasion is not unprecedented. Oelschlaeger et al. (34) showed that C. freundii invasion of T24 bladder cells was dependent on microtubules but not on microfilaments, while in Int-407 cells and another bladder cell line, 5637, the invasion was dependent on both cytoskeleton components. However, whether both cytoskeleton structures are also needed in other cell types remains to be elucidated.
The invasion potential of E. sakazakii with EGTA- or CyD-pretreated Caco-2 cells was dependent upon the age of the cells (4 to 7 days versus 17 to 20 days). This should not have been due to the various extents of tight junction disruption in Caco-2 cells that were different ages and in different parts of the differentiation cycle, because treatment with 1 and 5 mM CyD (Fig. 4) or EGTA (Fig. 6) did not make a significant difference regardless of the cultivation time. A possible explanation for the age dependence is the presence of a potential receptor recognized by Enterobacter cells, whose abundance might increase with time. Several observations supported this hypothesis: (i) not only E. sakazakii invasion but also the initial association was dependent on the culture time of Caco-2 cells (Fig. 5, 6, and 8); and (ii) use of 1- to 2-day-old Caco-2 cell monolayers, seeded after trypsin treatment and not having fully established tight junctions, did not support a significant increase in E. sakazakii invasion (data not shown). In addition, it was shown previously that the total protein content of Caco-2 cells increased even after confluence was reached (19).
In conclusion, our study demonstrates for the first time that E. sakazakii is able to invade human intestinal epithelial cells. Penetration appears to be receptor mediated and requires actin filaments as well as microtubules. In addition, disruption of tight junctions increases the initial association of E. sakazakii with Caco-2 cells, which might result in an enhanced invasion capacity. Our data also show that E. sakazakii has an invasion mechanism different from those employed by L. monocytogenes and Salmonella serovar Typhimurium. Further work is required to address the many open questions, such as identification of bacterial and host cell invasion-associated molecules, entry into other cell types, such as fibroblasts and endothelial cells, and the mechanisms of spreading in the infected host.
Acknowledgments
We thank Roger Stephan for providing E. sakazakii strains, Jochen Klumpp and Wolf-Dietrich Hardt for critical reading of the manuscript, and Simone Dell'Era for help with confocal fluorescence microscopy imaging.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Adegbola, R. A., and D. C. Old. 1983. Fimbrial haemagglutinins in Enterobacter species. J. Gen. Microbiol. 1292175-2180. [DOI] [PubMed] [Google Scholar]
- 2.Biering, G., S. Karlsson, N. C. Clark, K. E. Jonsdottir, P. Ludvigsson, and O. Steingrimsson. 1989. Three cases of neonatal meningitis caused by Enterobacter sakazakii in powdered milk. J. Clin. Microbiol. 272054-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breeuwer, P., A. Lardeau, M. Peterz, and H. M. Joosten. 2003. Desiccation and heat tolerance of Enterobacter sakazakii. J. Appl. Microbiol. 95967-973. [DOI] [PubMed] [Google Scholar]
- 4.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasie pathogens. Science 304242-248. [DOI] [PubMed] [Google Scholar]
- 5.Criss, A. K., and J. E. Casanova. 2003. Coordinate regulation of Salmonella enterica serovar Typhimurium invasion of epithelial cells by the Arp2/3 complex and Rho GTPases. Infect. Immun. 712885-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz, N., X. Alvarez, R. D. Berg, and E. A. Deitch. 1994. Bacterial translocation across enterocytes: results of a study of bacterial-enterocyte interactions utilizing Caco-2 cells. Shock 167-72. [PubMed] [Google Scholar]
- 7.Drudy, D., N. R. Mullane, T. Yuinn, P. G. Wall, and S. Fanning. 2006. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin. Infect. Dis. 42996-1002. [DOI] [PubMed] [Google Scholar]
- 8.Faix, R. G., and J. T. Adams. 1994. Neonatal necrotizing enterocolitis: current concepts and controversies. Adv. Pediatr. Infect. Dis. 91-36. [PubMed] [Google Scholar]
- 9.Farmer, J. J., III, M. A. Asbury, F. W. Hickman, D. J. Brenner, and the Enterobacteriaceae Study Group. 1980. Enterobacter sakazakii: a new species of “Enterobacteriaceae” isolated from clinical specimens. Int. J. Syst. Bacteriol. 30569-584. [Google Scholar]
- 10.Finlay, B. B., and S. Falkow. 1989. Common themes in microbial pathogenicity. Microbiol. Rev. 53210-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gahring, L. C., F. Heffron, B. B. Finlay, and S. Falkow. 1990. Invasion and replication of Salmonella typhimurium in animal cells. Infect. Immun. 58443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 552822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Hamilton, J. V., M. J. Lehane, and H. R. Braig. 2003. Isolation of Enterobacter sakazakii from midgut of Stomoxys calcitrans. Emerg. Infect. Dis. 91355-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmsen, H. J., A. C. Wildeboer-Veloo, G. C. Raangs, A. A. Wagendorp, N. Klijn, J. G. Bindels, and G. W. Wellings. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 3061-67. [DOI] [PubMed] [Google Scholar]
- 16.International Commission on Microbiological Specifications for Foods (ICMSF). 2002. Microorganisms in food 7. Microbiological testing in food safety management. Kluwer Academic/Plenum Publishers, New York, NY.
- 17.Iversen, C., M. Lane, and S. J. Forsythe. 2004. The growth profile, thermotolerance and bilfilm formation of Enterobacter sakazakii grown in infant formula milk. Lett. Appl. Microbiol. 38378-382. [DOI] [PubMed] [Google Scholar]
- 18.Jones, B. D., H. F. Paterson, A. Hall, and S. Falkow. 1993. Salmonella typhimurium induces membrane ruffling by a growth factor-receptor-independent mechanism. Proc. Natl. Acad. Sci. USA 9010390-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jumarie, C., and C. Malo. 1991. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J. Cell. Physiol. 14924-33. [DOI] [PubMed] [Google Scholar]
- 20.Kandhai, M. C., M. W. Reij, L. G. Gorris, O. Guillaume-Gentil, and M. van Schothorst. 2004. Occurrence of Enterobacter sakazakii in food production environments and households. Lancet 36339-40. [DOI] [PubMed] [Google Scholar]
- 21.Kim, K.-P., J. Klumpp, and M. J. Loessner. 2007. Enterobacter sakazakii bacteriophages can prevent bacterial growth in reconstituted infant formula. Int. J. Food Microbiol. 115195-203. [DOI] [PubMed] [Google Scholar]
- 22.Kleiman, M. B., S. D. Allen, P. Neal, and J. Reynolds. 1981. Meningoencephalitis and compartmentalization of the cerebral ventricles caused by Enterobacter sakazakii. J. Clin. Microbiol. 14352-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuzina, L. V., J. J. Peloquin, D. C. Vacek, and T. A. Miller. 2001. Isolation and identification of bacteria associated with adult laboratory Mexican fruit flies, Anastrepa ludens (Diptera: Tephritidae). Curr. Microbiol. 42290-294. [DOI] [PubMed] [Google Scholar]
- 24.Lehner, A., R. Riedel, L. Eberl, P. Breeuwer, B. Diep, and R. Stephan. 2005. Biofilm formation, extracellular polysaccharide production, and cell-to-cell signaling in various Enterobacter sakazakii strains: aspects promoting environmental persistence. J. Food Prot. 682287-2294. [DOI] [PubMed] [Google Scholar]
- 25.Loessner, M. J., K. Kramer, F. Ebel, and S. Scherer. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44335-349. [DOI] [PubMed] [Google Scholar]
- 26.Mange, J. P., R. Stephan, N. Borel, P. Wild, K. S. Kim, A. Pospichil, and A. Lehner. 2006. Adhesive properties of Enterobacter sakazakii to human epithelial and brain endothelial cells. BMC Microbiol. 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mengaud, J., H. Ohayon, P. Gounon, R.-M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84923-932. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi, J., and Y. Takai. 2005. Molecular perspective on tight-junction assembly and epithelial polarity. Adv. Drug Deliv. Rev. 57815-855. [DOI] [PubMed] [Google Scholar]
- 29.Moriez, R., C. Salvador-Cartier, V. Theodorou, J. Fioramonti, H. Eutamene, and L. Bueno. 2005. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am. J. Pathol. 2671071-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muytjens, H. L., H. C. Zanen, H. J. Sonderkamp, L. A. Kollée, I. K. Wachsmuth, and J. J. Farmer III. 1983. Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J. Clin. Microbiol. 18115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Nazarowec-White, M., and J. M. Farber. 1997. Incidence, survival and growth of Enterobacter sakazakii in infant formula. J. Food Prot. 60226-230. [DOI] [PubMed] [Google Scholar]
- 33.Nazarowec-White, M., and J. M. Farber. 1997. Thermal resistance of Enterobacter sakazakii in reconstituted dried-infant formula. Lett. Appl. Microbiol. 249-13. [DOI] [PubMed] [Google Scholar]
- 34.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 906884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagotto, F. J., M. Nazarowec-White, S. Bidawid, and J. M. Farber. 2003. Enterobacter sakazakii: infectivity and enterotoxin production in vitro and in vivo. J. Food Protect. 66370-375. [DOI] [PubMed] [Google Scholar]
- 36.Portzehl, H., P. C. Caldwell, and J. C. Rueegg. 1964. The dependence of contraction and relaxation of muscle fibres from the crab Maia squinado on the internal concentration of free calcium ions. Biochim. Biophys. Acta 79581-591. [DOI] [PubMed] [Google Scholar]
- 37.Postupa, R., and E. Aldova. 1984. Enterobacter sakazakii: a Tween 80 esterase-positive representative of the genus Enterobacter isolated from powdered milk specimens. J. Hyg. Epidemiol. Microbiol. Immunol. 28435-440. [PubMed] [Google Scholar]
- 38.Rousset, M. 1986. The human colon carcinoma cell lines HT-29 and Caco-2: Two in vitro models for the study of intestinal differentiation. Biochimie 681035-1040. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 40.Sanders, W. E., Jr., and C. C. Sanders. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sreenivasan, P. K., D. H. Meyer, and P. M. Fives-Taylor. 1993. Requirements for invasion of epithelial cells by Actinobacillus actinomycetemcomitans. Infect. Immun. 611239-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Townsend, S., J. C. Barron, C. Loc-Carrillo, and S. Forsythe. 2007. The presence of endotoxin in powdered infant formula milk and the influence of endotoxin and Enterobacter sakazakii on bacterial translocation in the infant rat. Food Microbiol. 2467-74. [DOI] [PubMed] [Google Scholar]
- 43.Townsend, S. M., E. Hurrell, I. Gonzlaez-Gomez, J. Lowe, J. G. Frye, S. Forsythe, and J. L. Badger. 2007. Enterobacter sakazakii invades brain capillary endothelial cells, persists in human macrophages influencing cytokine secretion and induces severe brain pathology in the neonatal rat. Microbiology 1533538-3547. [DOI] [PubMed] [Google Scholar]
- 44.van Acker, J., F. de Smet, G. Muyldermans, A. Bougatef, A. Naessens, and S. Lauwers. 2001. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J. Clin. Microbiol. 39293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker-Smith, J. 1972. Variation of small intestinal morphology with age. Arch. Dis. Child. 4780-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weir, E. 2002. Powdered infant formula and fatal infection with Enterobacter sakazakii. CMAJ 1661570. [PMC free article] [PubMed] [Google Scholar]
- 47.Willis, J., and J. E. Robinson. 1988. Enterobacter sakazakii meningitis in neonates. Pediatr. Infect. Dis. J. 7196-199. [DOI] [PubMed] [Google Scholar]
- 48.Wooding, F. B. P., M. W. Smith, and H. Craig. 1978. The ultrastructure of the neonatal pig colon. Am. J. Anat. 152269-286. [DOI] [PubMed] [Google Scholar]