Abstract
The ability of Pseudomonas aeruginosa to cause a broad range of infections in humans is due, at least in part, to its adaptability and its capacity to regulate the expression of key virulence genes in response to specific environmental conditions. Multiple two-component response regulators have been shown to facilitate rapid responses to these environmental conditions, including the coordinated expression of specific virulence determinants. RsmA is a posttranscriptional regulatory protein which controls the expression of a number of virulence-related genes with relevance for acute and chronic infections. Many membrane-bound sensors, including RetS, LadS, and GacS, are responsible for the reciprocal regulation of genes associated with acute infection and chronic persistence. In P. aeruginosa this is due to sensors influencing the expression of the regulatory RNA RsmZ, with subsequent effects on the level of free RsmA. While interactions between an rsmA mutant and human airway epithelial cells have been examined in vitro, the role of RsmA during infection in vivo has not been determined yet. Here the function of RsmA in both acute and chronic models of infection was examined. The results demonstrate that RsmA is involved in initial colonization and dissemination in a mouse model of acute pneumonia. Furthermore, while loss of RsmA results in reduced colonization during the initial stages of acute infection, the data show that mutation of rsmA ultimately favors chronic persistence and results in increased inflammation in the lungs of infected mice.
Pseudomomas aeruginosa is a metabolically versatile gram-negative bacterium that is capable of causing opportunistic infections in plants, animals, and humans. In humans, P. aeruginosa infections occur in the gastrointestinal tract and respiratory system and in patients with ocular infections, burn wounds, and cystic fibrosis (CF). The characteristics of acute and chronic infections caused by P. aeruginosa are quite distinct and are associated with selected expression of a certain subset of virulence factors. The pathogenesis of acute infections, such as ventilator-associated pneumonia, is thought to require the expression of a functional type III secretion system (T3SS), along with other toxins and proteases (37). These infections typically result in systemic infections and ultimately mortality. On the other hand, individuals with CF are usually colonized with P. aeruginosa early in life (1), and while P. aeruginosa can reach densities of 109 CFU/ml of sputum (33), P. aeruginosa infections in the lungs of CF patients are minimally invasive and rarely progress to septicemia. Rather, it is the inflammation and deterioration of pulmonary function resulting from chronic P. aeruginosa infection that is the main cause of mortality in CF patients. Therefore, investigating the mechanism(s) by which P. aeruginosa colonizes and becomes firmly established is critical for understanding the nature of life-long infections in CF patients.
The substantial proportion of the P. aeruginosa genome involved in regulating gene expression gives this opportunistic pathogen the ability to control a wide range of cellular processes, including the production of virulence factors in response to altered environmental conditions. One complex regulatory network consists of the posttranscriptional regulatory protein RsmA and its cognate regulatory RNA molecules, RsmY and RsmZ (2, 3, 16, 19, 32). These regulatory RNAs inactivate RsmA by directly binding to the protein and thereby altering the level of free RsmA available to bind other target RNAs. Signal transduction cascades involving GacS, LadS, and RetS modulate the expression levels of these regulatory RNAs. This results in increased or decreased levels of free RsmA, thus altering the production of virulence determinants (14, 21, 36).
Several investigators have studied RsmA-regulated gene expression. Work in our laboratory has demonstrated a key role for RsmA during the interaction with airway epithelial cells by positive regulation of the T3SS (28, 29). Additionally, RsmA has been shown to be a positive regulator of bacterial motility (3, 16) and Pseudomonas quinolone signal biosynthesis (3), while it negatively regulates N-acylhomoserine synthesis (16, 32), antibiotic resistance (3, 28), and extracellular enzyme production (32). Expression of the T3SS has been shown to be important in the initial stages of acute infection, while a lack of bacterial motility has been observed for isolates taken from chronically infected patients (12, 23). Together, these findings suggest that RsmA may play a critical role in determining what virulence factors are produced during the transition from the acute stage to the chronic stage of infection. Despite the large body of in vitro data regarding RsmA regulation of virulence factor production, it is not yet known whether P. aeruginosa RsmA is a key contributor to colonization, dissemination, virulence, and persistence in animal models of infection.
The main objective of this study was to investigate the role of RsmA in colonization, persistence, and virulence in mouse models of acute and chronic infections. First, we present in vitro data demonstrating that mutation of rsmA increased the adhesion of P. aeruginosa to plastic and glass, increased biofilm formation at the air-liquid interface (pellicle), and significantly reduced competitive growth relative to growth of the parent strain PAO1, both in vitro and when mice are coinfected in vivo. Mutation of rsmA also resulted in a significant defect in the ability of P. aeruginosa to colonize and disseminate in a mouse model of acute pneumonia when the bacterium was inoculated singly in vivo. Furthermore, loss of rsmA in a mouse model of chronic infection resulted in a significantly lower mortality rate in infected mice while favoring the persistence of P. aeruginosa, and this persistence resulted in increased inflammation in the murine lungs.
MATERIALS AND METHODS
Bacterial strains.
The P. aeruginosa strains used in this study were wild-type strain PAO1 (Holloway collection), rsmA mutant PAZH13 (32), PAZH13Gm (lacZ-marked rsmA mutant) (H. Mulcahy, H. O'Connor, E. P. O'Grady, M. Cullinane, T. Zhexian, C. Adams, and F. O'Gara, unpublished data), and PAO1::p16Slux (lux-marked PAO1 strain) (34). Strains were routinely grown at 37°C in Luria-Bertani (LB) medium. Antibiotics were added to P. aeruginosa strains at the following concentrations: erythromycin, 800 μg ml−1; and gentamicin (Gm), 30 μg ml−1. PAZH13Gm was constructed as follows as part of a separate study. PAZH13Gm (also designated PAZH13 mexE-lacZ) contains a Tn7 chromosomal insertion of the promoter region of the mexE gene fused to the lacZ gene and a Gm cassette, originally from the vector pUC18miniTn7TGmlacZ (4). This strain is blue in the presence of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as mexE expression is high in PAZH13 (3). Integration of pUC18miniTn7TGmmexE-lacZ into the chromosome of PAZH13 had no effect on the growth or competitive ability of the strain.
Adhesion assays and pellicle formation.
Adherence assays were performed in 96-well polystyrene microtiter plates. Wells were filled with 150 μl of culture to obtain a starting optical density of 0.1. The cultures were incubated at 37°C for 8 h without shaking. The extent of adhesion was determined by staining with crystal violet. Following rinsing, cell-associated crystal violet was solubilized in ethanol, and the absorbance at 595 nm was measured. To monitor adhesion to glass, PAO1 and PAZH13 were inoculated at an initial optical density of 0.01 into sucrose asparagine medium supplemented with 30 μM iron in a sterile petri dish containing an ethanol-washed glass slide. After 16 h the slide was removed, gently rinsed, and stained with crystal violet prior to visualization by microscopy at a magnification of ×100. Pellicle formation was measured as described by Friedman and Kolter (11). Pellicle formation was visually assessed after 5 days of static incubation at room temperature in glass tubes. The robustness of the pellicle formed was also determined by evaluating its resistance to boiling and vortexing.
In vitro and in vivo competition experiments.
Competitive growth experiments with PAO1 and PAZH13Gm or with PAZH13 and PAZH13Gm (as controls) were carried out in vitro in LB medium for up to 30 generations (24 h). Strains were grown overnight and diluted to obtain a starting optical density of 0.01. The starting ratios of PAO1 to PAZH13Gm or of PAZH13 to PAZH13Gm were confirmed by plate counting on LB medium and LB medium containing Gm. Bacteria were subcultured every 8 h in fresh medium, and samples were taken every 4 h to enumerate the CFU on LB agar and selective LB agar containing Gm. In vivo competition experiments were carried out using a mouse model of acute pneumonia (see below) by using a 1:1 inoculum (6 × 105 CFU each) containing PAO1::p16Slux and PAZH13. At 18 h postinfection (p.i.) mice were sacrificed, and organs were removed and homogenized. The numbers of bacteria were determined by plating serial dilutions and comparing of the numbers of luminescent (PAO1::p16Slux) and nonluminescent (PAZH13) colonies.
Mouse model of acute pneumonia.
Acute mouse infection was performed using the mouse model described by Comolli et al. (6). Briefly, P. aeruginosa strains were grown in LB broth at 37°C overnight with shaking, after which bacteria were collected by centrifugation and resuspended in phosphate-buffered saline (PBS) (Sigma) to obtain 6 × 107 CFU/ml. The exact number of bacteria was determined by plating serial dilutions of each inoculum on LB agar plates. Female BALB/c mice (6 to 8 weeks old) were anesthetized by intraperitoneal administration of ketamine hydochloride (65 mg/kg) and xylazine (13 mg/kg). Anesthetized animals were infected intranasally with 20 μl of culture (10 μl in each nostril), using a final inoculum of 1.2 × 106 CFU per mouse. At 18 h p.i. mice were sacrificed, and the lungs, spleen, and liver were removed and homogenized in 5 ml of PBS. Serial 10-fold dilutions were prepared using PBS, and 10-μl portions of diluted bacterial suspensions were plated on LB agar plates. The number of CFU was calculated and was considered the level of bacterial infection in the entire lungs, spleen, or liver. All animal experiments were approved by the animal ethics committees of University College Cork or the University of the Balearic Islands.
Mouse model of chronic infection.
The murine model of chronic lung infection using P. aeruginosa-laden agarose beads was established by following previously described protocols (24, 35). Briefly, for preparation of the agarose beads, bacteria (P. aeruginosa strain PAO1 or PAZH13) were grown to late log phase and mixed at a 1/10 ratio with 2% agarose in PBS (pH 7.4). The mixture was added to heavy mineral oil equilibrated at 55°C, stirred for 6 min at room temperature, and cooled for 10 min. The resulting agarose beads were washed with 0.5 and 0.25% deoxycholic acid (sodium salt) in PBS once and then with PBS alone three times. Serial 1/10 dilutions of homogenized bead slurry aliquots were plated on Mueller-Hinton agar for quantification of the bacterial content.
Female C57BL/6J mice were used. All animals weighed 20 to 25 g and were provided by Harlan Ibérica, S. L. The animals were specific pathogen free, and sterile water and food were provided ad libitum. Before inoculation, mice were anesthetized by intraperitoneal injection of 100 mg/kg of body weight ketamine (Pfizer) and 10 mg/kg xylazine (Sigma-Aldrich, Madrid, Spain). A vertical midline neck incision was then made to expose each mouse's trachea, and 20 μl containing approximately 2 × 104 agarose-embedded cells was transtracheally inoculated. Mouse survival was monitored over a 7-day period. At 7 days p.i. animals were sacrificed. Lungs were removed and, after samples were obtained for histopathology, were homogenized using an Ultra-Turrax T-25 disperser (IKA, Staufen, Germany) for enumeration of CFU.
For histopathological analysis, lungs were fixed in 10% formalin, embedded in paraffin, sectioned (4 to 6 μm), and stained with hematoxylin and eosin. Lung inflammation was then blindly scored by an expert pathologist using the criteria of Johansen et al. (18) (1, normal; 2, mild focal inflammation; 3, moderate to severe focal inflammation with areas of normal tissue; 4, severe inflammation to necrosis and severe inflammation throughout the lung).
Statistical analysis.
The statistical significance of data was analyzed using the Student's t test, Fischer's exact test, or a chi-square test, as appropriate. A P value of <0.05 was considered significant.
RESULTS
PAZH13 displays increased adhesion and pellicle formation.
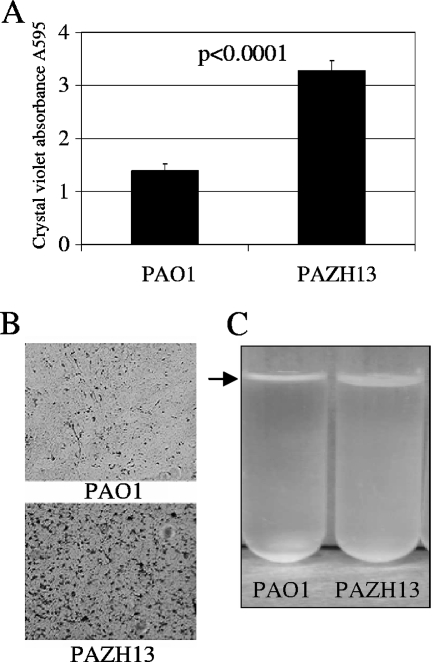
Several separate studies have investigated the effects of an rsmA mutation on a number of important phenotypes, some of which are associated with chronic infection. Increased cell-cell aggregation has been noted (16), while attachment to plastic has been shown to be reduced in an rsmA mutant (3). In order to further examine some of these phenotypes, investigations of adhesion and pellicle formation were performed. Increased adherence of PAZH13 compared to PAO1 was observed following crystal violet staining of cultures grown in rich medium in a 96-well plate that was incubated at 37°C without shaking for 8 h (Fig. 1A). Statistical analysis indicated that PAZH13 showed significantly increased adhesion to polystyrene at 8 h (P < 0.0001). The difference between PAO1 and PAZH13 was most pronounced at 8 h, and there was no detectable difference between the two strains at 24 h (data not shown). Furthermore, increased adherence to glass of PAZH13 compared to PAO1 was observed following 16 h of incubation in minimal medium at 37°C under static growth conditions (Fig. 1B). Increased pellicle formation by PAZH13 compared to PAO1 was also observed at the air-liquid interface in static cultures (Fig. 1C), which was not easily disrupted by boiling or vortexing of the samples (data not shown).
FIG. 1.
Attachment phenotypes of PAO1 and PAZH13. (A) Cells attached to polystyrene after 8 h of growth in LB medium were stained with crystal violet. The data are averages of two independent experiments, each with eight replicate wells. (B) Cells were grown in sucrose asparagine medium supplemented with iron and grown for 16 h in a petri dish containing an ethanol-washed glass slide. The slide was removed and stained with crystal violet prior to visualization by microscopy. (C) Standing cultures containing 6 ml of LB broth were grown at room temperature, and pellicle formation, indicated by an arrow, was visualized after 5 days of growth.
Competitive growth profiles of PAO1 and the rsmA mutant in vitro and in vivo.
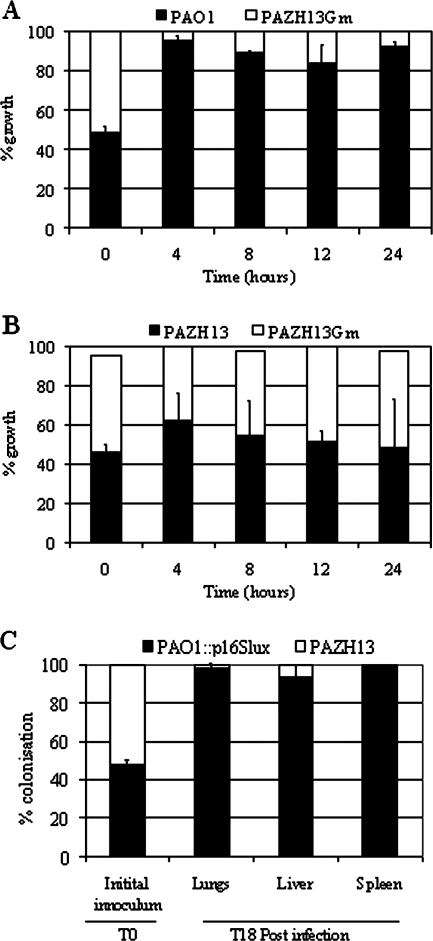
In vitro and in vivo competition experiments were carried out to determine whether loss of RsmA resulted in a difference in the fitness of the mutant population relative to the parent strain. Cultures containing PAO1 and PAZH13Gm at a ratio of 1:1 or PAZH13 and PAZH13Gm at a ratio of 1:1 were prepared and allowed to grow in LB medium, with subculturing every 8 h. The ratios of bacterial populations were determined at 4, 8, 12, and 24 h; the 24-h time point equaled approximately 30 generations of growth. The Gm resistance cassette enabled PAZH13Gm to be distinguished from PAO1 or the unlabeled PAZH13 cultures. No significant difference between PAZH13 and PAZH13Gm was observed, indicating that the competitive ability of the marked mutant strain relative to the unmarked mutant was not altered (Fig. 2B). In contrast, plate counts indicated that PAO1 outcompeted PAZH13Gm in LB medium as early as 4 h, indicating that loss of RsmA resulted in a mutant strain with significantly reduced fitness relative to PAO1 (Fig. 2A). While the doubling times of PAO1 and PAZH13 have previously been reported to be different (31 and 36 min, respectively) (16), this factor alone cannot account for the observed difference in the competitive growth profiles of PAO1 and PAZH13.
FIG. 2.
In vitro and in vivo competitive growth experiments. (A and B) In vitro competition experiments carried out using (A) PAO1 and PAZH13Gm or (B) PAZH13 and PAZH13Gm grown in LB medium. (C) In vivo competition experiments carried out using PAO1::p16Slux and PAZH13 in a mouse model of acute pneumonia. At 18 h (T18) p.i mice were sacrificed, and the lungs, liver, and spleen were removed and homogenized. The numbers of CFU of PAO1 and PAZH13 were calculated and considered the levels of bacterial infection in the entire lungs, liver, or spleen following comparison of the number of luminescent (PAO1::p16Slux) and nonluminescent (PAZH13) colonies.
Competition experiments were also performed using a mouse model of acute pneumonia. For these experiments PAO1::p16Slux was employed. This strain contains a lux (luminescence) reporter system generated by site-directed chromosomal integration and has been shown previously to have no significant effect on the ability of PAO1 to colonize or disseminate in the same in vivo mouse model. Additionally, this reporter has been shown to be stable in the absence of antibiotic selection for up to 50 generations of growth and in a mouse model of acute pneumonia (34). BALB/c mice were intranasally infected with 1.2 × 106 CFU of PAO1::p16Slux and PAZH13 at a 1:1 ratio (Fig. 2C). At 18 h p.i. mice were sacrificed, and the lungs, liver, and spleen were removed. All organs were homogenized, and samples were plated to enumerate the CFU. Comparison of the numbers of luminescent (PAO1::p16Slux) and nonluminescent (PAZH13) colonies indicated that PAO1::p16Slux significantly outcompeted PAZH13 in terms of the ability to colonize the lungs and the ability to disseminate to the liver and spleen (Fig. 2C).
RsmA is involved in initial colonization and dissemination in a mouse model of acute pneumonia.
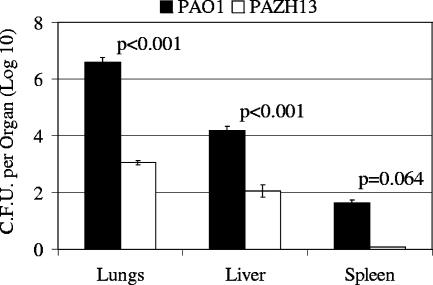
PAO1 and PAZH13 were also inoculated singly to assess and compare their abilities to colonize and disseminate in the mouse model of acute pneumonia. PAO1 was capable of colonizing the lungs of infected mice, and the number of cells increased 3.3-fold over the course of 18 h. Additionally, PAO1 was able to disseminate into the liver and spleen (Fig. 3). In contrast, PAZH13 exhibited significantly reduced colonization of the lungs, and the bacterial load was 3 orders of magnitude lower in PAZH13-infected mice than in PAO1-infected mice (Fig. 3). However, while significantly lower levels of PAZH13 were recovered from the lungs, PAZH13 was capable of disseminating to the liver and spleen, albeit at substantially reduced levels relative to PAO1 (Fig. 3).
FIG. 3.
Colonization and dissemination of PAO1 and PAZH13 in a mouse model of acute pneumonia. Mice were intranasally infected (singly) with 1.2 × 106 CFU of PAO1 or PAZH13 per mouse. At 18 h p.i. mice were sacrificed, and the lungs, spleen, and liver were removed and homogenized. The numbers of CFU were calculated and considered the levels of bacterial infection in the entire lungs, liver, or spleen.
Mutation of RsmA results in reduced mortality in a mouse model of chronic infection.
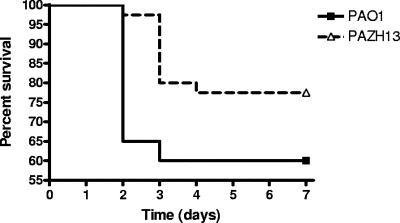
To examine the role of RsmA in virulence, we used the agarose bead murine model of chronic airway infection, which has been shown to produce bacterial growth conditions similar to those in CF airways (35). C57BL/6J mice were infected with 2 × 104 CFU of PAO1 (n = 20) or PAZH13 (n = 40), and the numbers of surviving animals were determined over a 7-day period. Mutation of rsmA attenuated virulence, as measured by mouse mortality, in this model of infection since the mortality of mice infected by PAZH13 was lower and occurred later than the mortality of mice infected by PAO1 (Fig. 4). For instance, whereas death of mice infected by PAO1 occurred mainly during the first 2 days (35% mortality at day 2 and 40% mortality at day 7), the mortality at day 2 was 2.5% for mice infected by PAZH13 (P = 0.001). The overall mortality (after 7 days) was also lower for PAZH13-infected mice (22.5% versus 40%), although the difference was not statistically significant (P = 0.13).
FIG. 4.
Survival of mice singly infected with PAO1 (n = 20) or PAZH13 (n = 40) in a chronic infection model. Transtracheal inoculation of PAO1 or PAZH13 using 2 × 104 CFU per mouse was performed, and the percentage of mice that survived was monitored daily for 7 days.
Loss of RsmA favors persistence and is associated with increased pulmonary inflammation in a mouse model of chronic infection.
Following the observation that loss of RsmA was associated with reduced mortality of infected mice, we examined the number of viable organisms that persisted in the lungs of chronically infected mice at 7 days p.i. The results indicated that PAO1 was cleared from 10 of 12 (83%) surviving mice at 7 days p.i. In the two mice from which PAO1 was recovered, the levels ranged from 4 to 16 CFU per lung. In contrast, PAZH13 was recovered from 10 of 31 surviving mice (32%), and the levels ranged from 3.6 × 101 to 3.4 × 104 CFU per lung. Thus, the median load of bacteria persisting in the lungs of PAO1-infected mice was 1.0 × 101 CFU per lung, in contrast to a median value of 1.9 × 103 CFU per lung for PAZH13-infected mice.
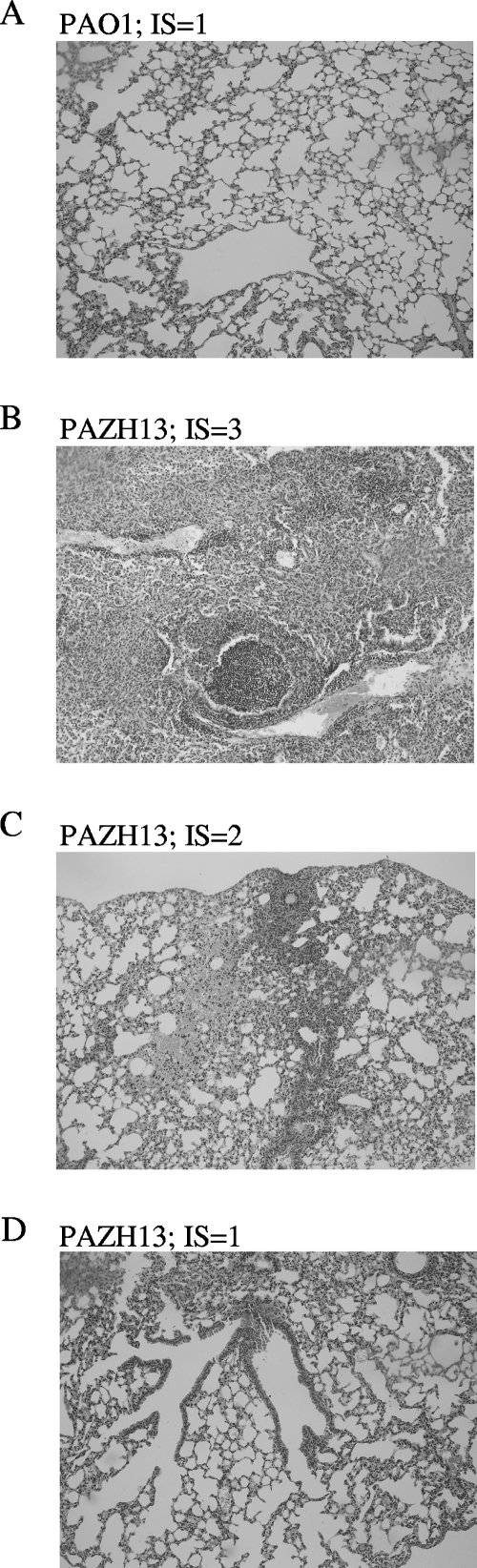
Inflammation in all surviving mice was scored by using the criteria of Johansen et al. (18), regardless of whether the infection persisted or was cleared. Overall, PAZH13 induced a higher level of inflammation in the lungs of infected mice than PAO1 induced (Fig. 5 and Table 1). All 12 PAO1-infected mice had a score of 1 (normal lungs), whereas 10 of the 31 (32%) PAZH13-infected mice had scores of 2 (mild focal inflammation) or 3 (moderate to severe focal inflammation). As expected, inflammation was significantly greater in murine lungs where PAZH13 infection persisted than in lungs where PAZH13 infection had been cleared (Table 1).
FIG. 5.
Histology of murine lungs after infection with P. aeruginosa: representative examples of the histology results obtained for murine lungs 7 days after infection with P. aeruginosa strain PAO1 (A) or PAZH13 (B, C, and D). IS, inflammatory score determined as described by Johansen et al. (18).
TABLE 1.
Comparative analysis of PAO1- and PAZH13-induced inflammation in a mouse model of chronic infection
| Strain | Infection | Inflammatory score for surviving mice (mean ± SD)a |
|---|---|---|
| PAZH13 | Persisting | 2.10 ± 0.74 |
| Cleared | 1.14 ± 0.49 | |
| PAO1 | Persisting | 1.0 ± 0 |
| Cleared | 1.0 ± 0 |
The mean inflammatory score was determined by using the criteria of Johansen et al. (18) (1, normal; 2, mild focal inflammation; 3, moderate to severe focal inflammation with areas of normal tissue; 4, severe inflammation to necrosis and severe inflammation throughout the lung). The values for strain PAZH13 were significantly different (P = 0.0004), whereas the values for strain PAO1 were not significantly different.
DISCUSSION
The complex mechanisms governing the ability of P. aeruginosa to regulate the transition between acute infection and chronic infection are not completely understood. Isolates from chronically infected patients typically lack motility (9, 23, 25), have increased antibiotic resistance (15), lack a functional T3SS (7, 17), and exhibit increased production of N-acylhomoserines (8, 13). Interestingly these phenotypes are similar to those observed when the gene encoding the regulatory protein RsmA is mutated. Therefore, because of the role of RsmA in regulating virulence factors and the number of similar phenotypic traits observed in the rsmA mutant PAZH13 and P. aeruginosa strains isolated from chronic infections, we investigated the hypothesis that RsmA plays a crucial role in virulence and in the establishment of acute or chronic infections in vivo.
In this study we highlighted an additional role of RsmA in biofilm formation and showed that an rsmA mutant displays increased or premature adhesion to polystyrene and glass at 8 and 16 h, respectively, as well as increased biofilm formation at the air-liquid interface (pellicle). This appears to be inconsistent with previously published data, which showed that an rsmA mutant exhibited reduced adhesion to polyvinyl chloride at 12 h (3). However, variations in the adhesion surfaces used (polyvinyl chloride versus polystyrene or glass) and the time points chosen can explain the differences in the results observed. Previously, studies have indicated that differences in the adhesion surfaces used and hence the surface properties can account for differences in the adherence phenotypes of bacteria (10).
Initial competition experiments indicated that the rsmA mutant was significantly outcompeted by PAO1 both in vitro and in vivo in the mouse model of acute pneumonia (Fig. 2). Additional experiments which employed inoculation of PAO1 or PAZH13 alone in the acute infection model (Fig. 3) also showed that PAO1 colonizes and disseminates to a substantially greater degree than PAZH13. Thus, even in the absence of competition, PAZH13 does not perform as well as PAO1 in the acute infection model (Fig. 3). These sets of data (coinoculation and single inoculation) suggest that an rsmA mutant strain is not as successful as PAO1 at initiating an acute infection. As a number of acute infection-related traits are also RsmA regulated (3, 14, 16, 28, 32), it is likely that loss of these virulence factors contributes to the reduced colonization and dissemination of PAZH13 in an acute infection model. However, we acknowledge that the reduced fitness of this mutant may also contribute, at least in part, to attenuation in the acute infection model.
However, despite the apparent lack of fitness the rsmA mutant demonstrated significantly enhanced infection in a model of chronic infection. When the effect of rsmA mutation on long-term persistence in a murine model of chronic infection was evaluated, PAO1-infected mice readily cleared the infection in the majority of cases, while, in contrast, PAZH13-infected mice showed a significantly higher level of persistence after 7 days. Additionally, the median bacterial load persisting in PAZH13-infected mice was significantly higher than that in PAO1-infected mice. Furthermore, this increased persistence was associated with a measurably higher level of inflammation in the murine lungs. Therefore, loss of RsmA favored the development of chronic infection with associated immunopathology.
While the precise mechanisms governing persistence and the development of inflammation in our model are currently unclear, our work clearly demonstrates that in the context of chronic infection, the rsmA mutant exhibited an enhanced ability to establish infection and cause disease. This raises a question regarding how we define virulence and the ability of an organism to cause disease in terms of acute and chronic infections. The pathogenesis of acute infections is thought to require expression of a functional T3SS, along with other cell-associated and secreted factors (37). However, the pathogenesis associated with chronic infection differs from that associated with acute infection. While it is clear that loss of rsmA results in reduced mortality in a mouse model of chronic infection, the rsmA mutant persists, elicits increased airway inflammation, and ultimately colonizes the lungs better than the wild-type strain in a chronic model of infection. As persistence and inflammation are considered hallmarks of a chronic infection, one could argue that an rsmA mutant is in fact more virulent than PAO1 in the context of a chronic infection.
Previously, a number of studies have highlighted genes that are thought to be involved in regulating the transition from acute infection to chronic infection. These genes include the RetS and LadS genes, which encode hybrid sensor kinases, as well as the gene encoding MutS, which is involved in mismatch repair (26). Comparative studies of wild-type strain PA103 and a retS mutant in a rabbit corneal infection model indicated that the retS mutant showed reduced ocular colonization 48 h p.i. However, the ocular colonization levels of the retS mutant were twofold higher than those observed in PA103-infected corneas at 7 days p.i., indicating that the retS mutant was compromised during initial infection but favored persistence over time (39). Both RetS and LadS have been shown to control the expression of virulence genes by acting through the well-characterized GacS/GacA two-component system (14, 36, 38). Although identification of specific environmental triggers for the sensors remains elusive, it is now clear that these regulators act by altering the expression of RsmZ (14, 36) (and possibly RsmY), which in turn binds to and sequesters RsmA. Thus, whether it is LadS, RetS, or GacS which perceives an environmental trigger, one key component of the resulting gene expression pattern involves a complex signal transduction pathway that appears to converge at the level of the global regulator RsmA.
In a study performed by Mena et al. (26) it was shown that inactivation of the DNA mismatch repair system in P. aeruginosa attenuated mortality but led to increased persistence in CF mice. Furthermore, inactivation of the same system in Escherichia coli has also been shown to favor persistence of urinary tract infections (20). Indeed, hypermutatable strains or strains deficient in mismatch repair are highly prevalent among chronic P. aeruginosa strains, including those isolated from CF patients. Previously, the presence of hypermutatable strains has been associated with increased antibiotic resistance (5, 31) and also more recently with inactivation of LasR and selection of mucoid and small-colony variants (22). However, there is now direct evidence suggesting that the high prevalence of these hypermutatable strains in chronic P. aeruginosa infections is due to their ability to persist in the lungs of infected animals (26). One possible explanation for this may involve RsmA regulation.
The hypermutatable strains observed in chronic CF infections have been shown to be stable mutators which are produced mainly by alteration of the mutS gene (30, 31). Interestingly, in Erwinia carotovora subsp. carotovora strain Ecc71, HexA, the homologue of MutS, has been shown to negatively regulate the production of RsmB (RsmZ in P. aeruginosa) (27). This links hypermutatable strains with the RsmA/RsmZ regulatory network and suggests that RsmZ and RsmA may be downstream of mutS and the mismatch repair system in P. aeruginosa. Increased expression of RsmZ in a mutS mutant may result in reduced activity of RsmA and is suggestive of a mechanism by which the persistence of hypermutatable strains in chronic CF infection may be due, at least in part, to altered activity of RsmA.
In conclusion, findings of this study demonstrate that RsmA is involved in the ability of P. aeruginosa to cause acute infection in a mouse model of acute pneumonia and contributes significantly to the pathogenesis of P. aeruginosa in a chronic model of infection. However, while the ability of PAZH13 to cause acute (reduced colonization and dissemination) and chronic (reduced mortality) infections is initially compromised, this strain is eventually able to cause infections, leading to increased persistence of PAZH13 and airway inflammation in a chronic model of infection. The mechanism by which this persistence is achieved is not currently understood. Studies of RetS, LadS, and GacS identified a role for these sensor proteins in the regulation of genes associated with chronic persistence by alteration of the expression of RsmZ. This study clearly indicates that RsmA is a point of convergence for multiple sensory inputs with an important effect on the outcome of the infection. Studies focusing on the environmental inputs which ultimately influence the level of free RsmA, as well as additional studies focusing on the mechanisms of RsmA-mediated regulation of target genes, should significantly improve our understanding of the nature of chronic P. aeruginosa infections.
Acknowledgments
This research was supported in part by grants awarded by the Higher Education Authority of Ireland (grants PRTLI 3 E and PRTLI 3 H to F.O.), the Science Foundation of Ireland (grant SFI 02/IN.1/B1261 to F.O.), the European Commission (grant to F.O. and A.O.), and the Health Research Board (grants RP/2004/145 and RP/2006/271 to F.O.). P.G.C., C.H., and C.G.M.G. were supported by the Irish Government under the National Development Plan (2000-2006) and by Science Foundation Ireland through a Centre for Science Engineering and Technology award to the Alimentary Pharmabiotic Centre. M.D.M., N.B., C.G., and A.O. were supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, through the Spanish Network for Research in Infectious Diseases (grants REIPI C03/14 and RD06/0008).
We thank Christian U. Riedel, Institute of Microbiology and Biotechnology, University of Ulm, Ulm, Germany, for supplying p16Slux prior to publication, Paul Williams and Herbert P. Schweizer for providing strains, and Pat Higgins for excellent technical assistance.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 19 November 2007.
REFERENCES
- 1.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183444-452. [DOI] [PubMed] [Google Scholar]
- 2.Burrowes, E., A. Abbas, A. O'Neill, C. Adams, and F. O'Gara. 2005. Characterisation of the regulatory RNA RsmB from Pseudomonas aeruginosa PAO1. Res. Microbiol. 1567-16. [DOI] [PubMed] [Google Scholar]
- 3.Burrowes, E., C. Baysse, C. Adams, and F. O'Gara. 2006. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152405-418. [DOI] [PubMed] [Google Scholar]
- 4.Choi, K. H., J. B. Gaynor, K. G. White, C. Lopez, C. M. Bosio, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2443-448. [DOI] [PubMed] [Google Scholar]
- 5.Chopra, I., A. J. O'Neill, and K. Miller. 2003. The role of mutators in the emergence of antibiotic-resistant bacteria. Drug Resist. Updates 6137-145. [DOI] [PubMed] [Google Scholar]
- 6.Comolli, J. C., A. R. Hauser, L. Waite, C. B. Whitchurch, J. S. Mattick, and J. N. Engel. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 673625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deretic, V., M. J. Schurr, and H. Yu. 1995. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol. 3351-356. [DOI] [PubMed] [Google Scholar]
- 9.Ernst, R. K., D. A. D'Argenio, J. K. Ichikawa, M. G. Bangera, S. Selgrade, J. L. Burns, P. Hiatt, K. McCoy, M. Brittnacher, A. Kas, D. H. Spencer, M. V. Olson, B. W. Ramsey, S. Lory, and S. I. Miller. 2003. Genome mosaicism is conserved but not unique in Pseudomonas aeruginosa isolates from the airways of young children with cystic fibrosis. Environ. Microbiol. 51341-1349. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, R., H. Geier, K. M. Weigel, J. Do, T. E. Ford, and G. A. Cangelosi. 2006. Roles for cell wall glycopeptidolipid in surface adherence and planktonic dispersal of Mycobacterium avium. Appl. Environ. Microbiol. 727554-7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman, L., and R. Kolter. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51675-690. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa, S., S. L. Kuchma, and G. A. O'Toole. 2006. Keeping their options open: acute versus persistent infections. J. Bacteriol. 1881211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg, J. B., and G. B. Pler. 1996. Pseudomonas aeruginosa lipopolysaccharides and pathogenesis. Trends Microbiol. 4490-494. [DOI] [PubMed] [Google Scholar]
- 14.Goodman, A. L., B. Kulasekara, A. Rietsch, D. Boyd, R. S. Smith, and S. Lory. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7745-754. [DOI] [PubMed] [Google Scholar]
- 15.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 1835395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heurlier, K., F. Williams, S. Heeb, C. Dormond, G. Pessi, D. Singer, M. Camara, P. Williams, and D. Haas. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 1862936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain, M., D. Ramirez, R. Seshadri, J. F. Cullina, C. A. Powers, G. S. Schulert, M. Bar-Meir, C. L. Sullivan, S. A. McColley, and A. R. Hauser. 2004. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J. Clin. Microbiol. 425229-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen, H. K., F. Espersen, S. J. Cryz, Jr., H. P. Hougen, A. Fomsgaard, J. Rygaard, and N. Hoiby. 1994. Immunization with Pseudomonas aeruginosa vaccines and adjuvant can modulate the type of inflammatory response subsequent to infection. Infect. Immun. 623146-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay, E., B. Humair, V. Denervaud, K. Riedel, S. Spahr, L. Eberl, C. Valverde, and D. Haas. 2006. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J. Bacteriol. 1886026-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labat, F., O. Pradillon, L. Garry, M. Peuchmaur, B. Fantin, and E. Denamur. 2005. Mutator phenotype confers advantage in Escherichia coli chronic urinary tract infection pathogenesis. FEMS Immunol. Med. Microbiol. 44317-321. [DOI] [PubMed] [Google Scholar]
- 21.Laskowski, M. A., E. Osborn, and B. I. Kazmierczak. 2004. A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa. Mol. Microbiol. 541090-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lujan, A. M., A. J. Moyano, I. Segura, C. E. Argarana, and A. M. Smania. 2007. Quorum-sensing-deficient (lasR) mutants emerge at high frequency from a Pseudomonas aeruginosa mutS strain. Microbiology 153225-237. [DOI] [PubMed] [Google Scholar]
- 23.Luzar, M. A., and T. C. Montie. 1985. Avirulence and altered physiological properties of cystic fibrosis strains of Pseudomonas aeruginosa. Infect. Immun. 50572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macia, M. D., N. Borrell, M. Segura, C. Gomez, J. L. Perez, and A. Oliver. 2006. Efficacy and potential for resistance selection of antipseudomonal treatments in a mouse model of lung infection by hypermutable Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mena, A., M. D. Macia, N. Borrell, B. Moya, T. de Francisco, J. L. Perez, and A. Oliver. 2007. Inactivation of the mismatch repair system in Pseudomonas aeruginosa attenuates virulence but favors persistence of oropharyngeal colonization in cystic fibrosis mice. J. Bacteriol. 1893665-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee, A., Y. Cui, W. Ma, Y. Liu, and A. K. Chatterjee. 2000. hexA of Erwinia carotovora ssp. carotovora strain Ecc71 negatively regulates production of RpoS and rsmB RNA, a global regulator of extracellular proteins, plant virulence and the quorum-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Environ. Microbiol. 2203-215. [DOI] [PubMed] [Google Scholar]
- 28.Mulcahy, H., J. O'Callaghan, E. P. O'Grady, C. Adams, and F. O'Gara. 2006. The posttranscriptional regulator RsmA plays a role in the interaction between Pseudomonas aeruginosa and human airway epithelial cells by positively regulating the type III secretion system. Infect. Immun. 743012-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Grady, E. P., H. Mulcahy, J. O'Callaghan, C. Adams, and F. O'Gara. 2006. Pseudomonas aeruginosa infection of airway epithelial cells modulates expression of Kruppel-like factors 2 and 6 via RsmA-mediated regulation of type III exoenzymes S and Y. Infect. Immun. 745893-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver, A., F. Baquero, and J. Blazquez. 2002. The mismatch repair system (mutS, mutL and uvrD genes) in Pseudomonas aeruginosa: molecular characterization of naturally occurring mutants. Mol. Microbiol. 431641-1650. [DOI] [PubMed] [Google Scholar]
- 31.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 2881251-1254. [DOI] [PubMed] [Google Scholar]
- 32.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 1836676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsey, B. W., M. S. Pepe, J. M. Quan, K. L. Otto, A. B. Montgomery, J. Williams-Warren, K. M. Vasiljev, D. Borowitz, C. M. Bowman, B. C. Marshall, S. Marshall, and A. L. Smith. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N. Engl. J. Med. 34023-30. [DOI] [PubMed] [Google Scholar]
- 34.Riedel, C. U., P. G. Casey, H. Mulcahy, F. O'Gara, C. G. M. Gahan, and C. Hill. 2007. Construction of p16Slux, a novel vector for improved bioluminescent-labeling of gram-negative bacteria. Appl. Environ. Microbiol. 737092-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Heeckeren, A. M., and M. D. Schluchter. 2002. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 36291-312. [DOI] [PubMed] [Google Scholar]
- 36.Ventre, I., A. L. Goodman, I. Vallet-Gely, P. Vasseur, C. Soscia, S. Molin, S. Bleves, A. Lazdunski, S. Lory, and A. Filloux. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. USA 103171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yahr, T. L., and E. P. Greenberg. 2004. The genetic basis for the commitment to chronic versus acute infection in Pseudomonas aeruginosa. Mol. Cell 16497-498. [DOI] [PubMed] [Google Scholar]
- 38.Yahr, T. L., and M. C. Wolfgang. 2006. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62631-640. [DOI] [PubMed] [Google Scholar]
- 39.Zolfaghar, I., D. J. Evans, R. Ronaghi, and S. M. Fleiszig. 2006. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect. Immun. 743880-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]