Abstract
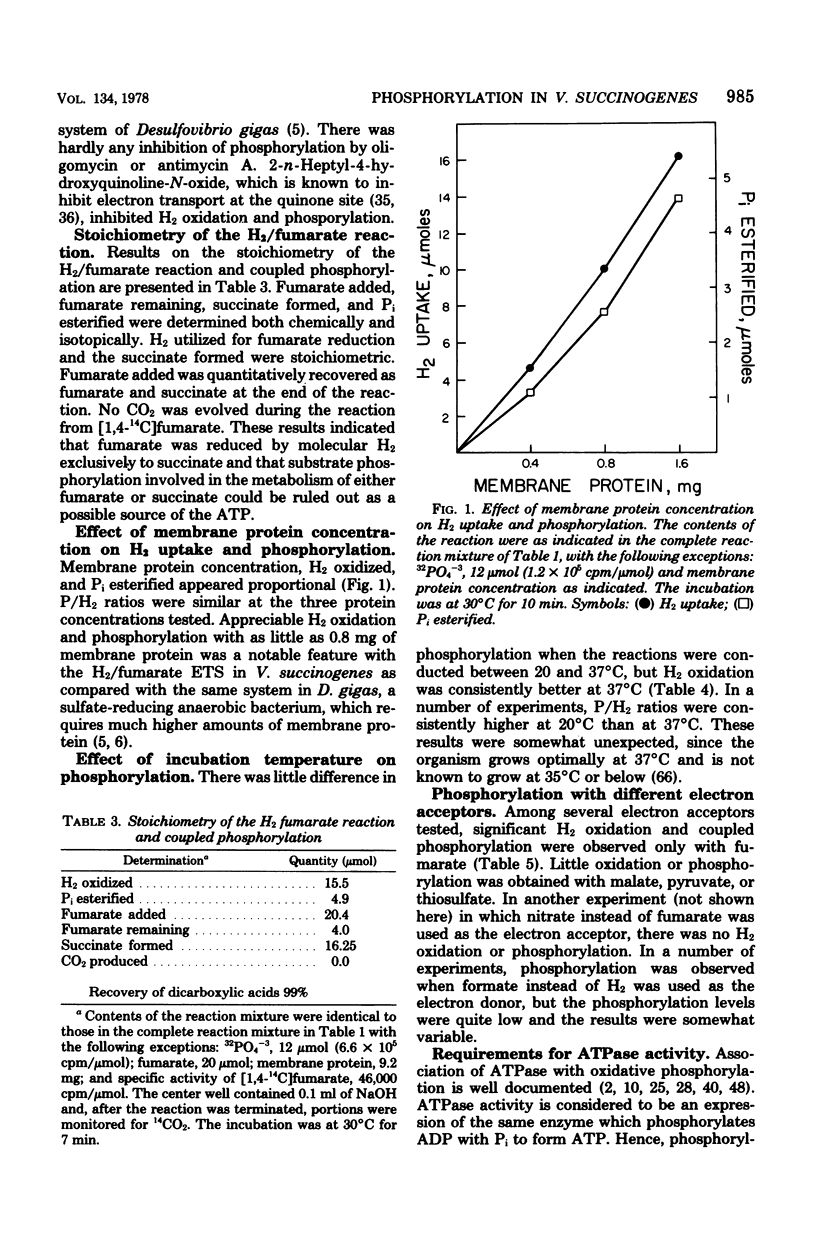
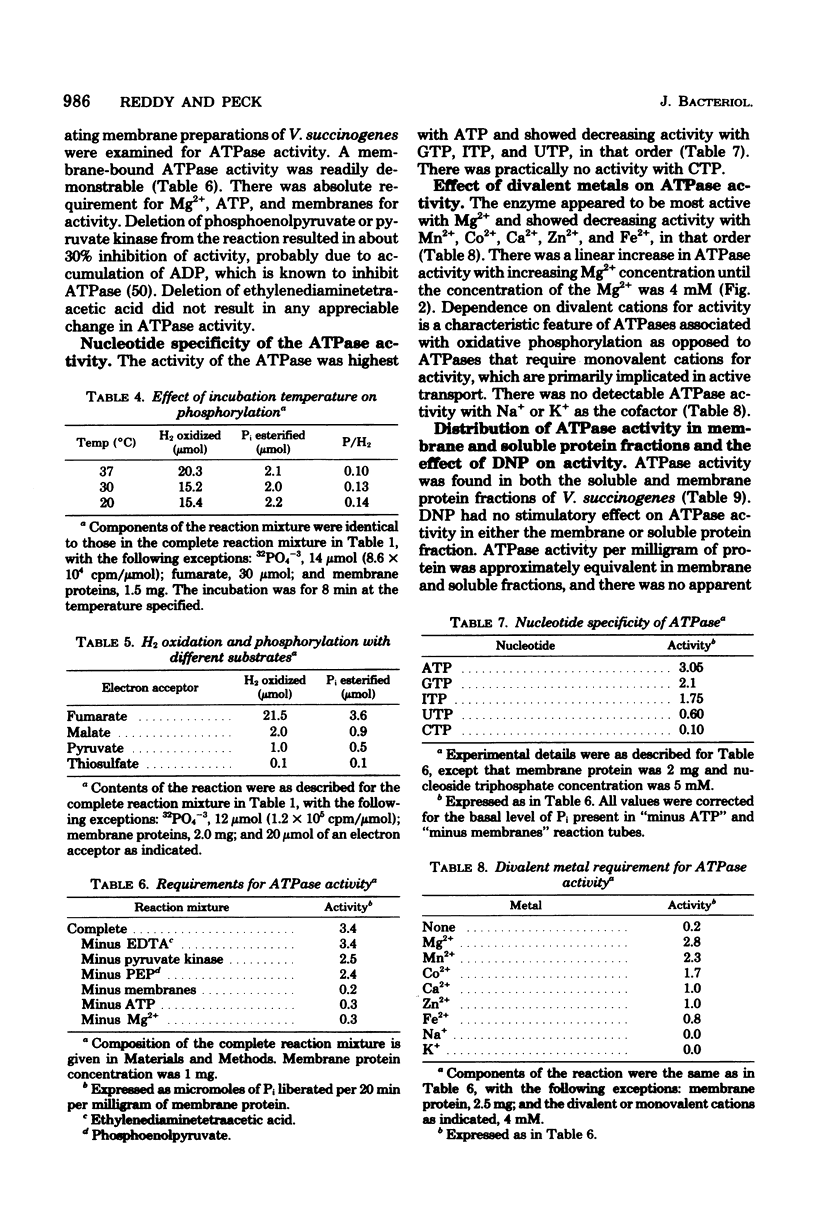
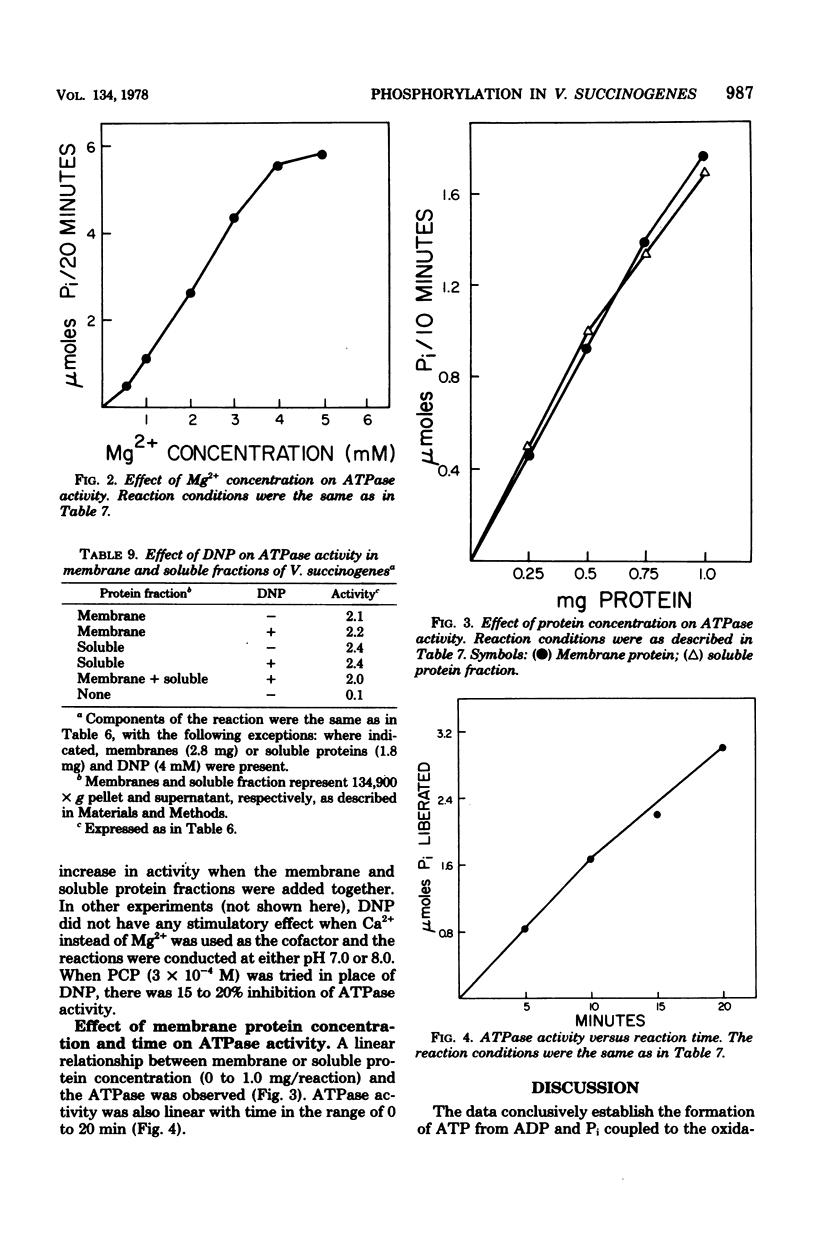
Vibrio succinogenes, an anaerobic bacterium, obtains its energy for growth from H2 or formate oxidation coupled to the reduction of fumarate to succinate. Membrane preparations have been obtained from this organism that catalyze the synthesis of ATP during H2 oxidation coupled to fumarate reduction. Esterification of orthophosphate is dependent on electron transfer, as evidenced by the requirement for both H2 and fumarate. Phosphorylation is also dependent on ADP and is destroyed by boiling the membrane preparations. H2 utilized for fumarate reduction and succinate formed are stoichiometric. The phosphorylation is markedly uncoupled by pentachlorophenol and gramicidin, but to a lesser extent by dinitrophenol and methyl viologen. 2-n-Heptyl-4-hydroxyquinoline-N-oxide causes severe inhibition of H2 oxidation as well as phosphorylation, but oligomycin or antimycin A has no demonstrable effect. Among several electron acceptors tested, significant phosphorylation is observed only with fumarate. A Mg2+-dependent adenosine triphosphatase activity is present in both the membrane and soluble protein fractions. Highest activity is obtained with ATP as the substrate, and considerably less activity is obtained with other nucleoside triphosphates. The possibility that phosphorylation during "fumarate respiration" may play an important physiological role in the growth of many anaerobic and facultatively anaerobic bacteria is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleem M. I., Nason A. PHOSPHORYLATION COUPLED TO NITRITE OXIDATION BY PARTICLES FROM THE CHEMOAUTOTROPH, NITROBACTER AGILIS. Proc Natl Acad Sci U S A. 1960 Jun;46(6):763–769. doi: 10.1073/pnas.46.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Trichardt P. F., Krizsanovich K., Coetzee J. N., Hugon Abortive transuction of motility in Proteus and Providence strains. J Gen Microbiol. 1972 Apr;70(2):361–364. doi: 10.1099/00221287-70-2-361. [DOI] [PubMed] [Google Scholar]
- Aspen A. J., Wolin M. J. Solubilization and reconstitution of a particulate hydrogenase from Vibrio succinogenes. J Biol Chem. 1966 Sep 25;241(18):4152–4156. [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BENNUN A., DEPAHN E. M., STOPPANI A. O. SOME PROPERTIES OF PARTICLE-BOUND INTRACELLULAR ATPASE FROM BAKER'S YEAST. Biochim Biophys Acta. 1964 Sep 18;89:532–539. doi: 10.1016/0926-6569(64)90079-3. [DOI] [PubMed] [Google Scholar]
- BOSE S. K., GEST H. PROPERTIES OF ADENOSINE TRIPHOSPHATASE IN A PHOTOSYNTHETIC BACTERIUM. Biochim Biophys Acta. 1965 Jan;96:159–162. doi: 10.1016/0005-2787(65)90621-0. [DOI] [PubMed] [Google Scholar]
- BRODIE A. F. Oxidative phosphorylation in fractionated bacterial systems. I. Role of soluble factors. J Biol Chem. 1959 Feb;234(2):398–404. [PubMed] [Google Scholar]
- Baccarini-Melandri A., Gest H., Pietro A. S. A coupling factor in bacterial photophosphorylation. J Biol Chem. 1970 Mar 10;245(5):1224–1226. [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Oxidative phosphorylation in Escherichia coli. Can J Biochem. 1968 Jul;46(7):631–641. doi: 10.1139/o68-099. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Faust P. J., Vandemark P. J. Phosphorylation coupled to NADH oxidation with fumarate in Streptococcus faecalis 10Cl. Arch Biochem Biophys. 1970 Apr;137(2):392–398. doi: 10.1016/0003-9861(70)90454-6. [DOI] [PubMed] [Google Scholar]
- Guarraia L. J., Peck H. D., Jr Dinitrophenol-stimulated adenosine triphosphatase activity in extracts of Desulfovibrio gigas. J Bacteriol. 1971 Jun;106(3):890–895. doi: 10.1128/jb.106.3.890-895.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D. L., Kanner B. I., Postma P. W. Oxidative phosphorylation in mutants of Escherichia coli defective in energy transduction. Biochim Biophys Acta. 1972 Nov 17;283(2):217–222. doi: 10.1016/0005-2728(72)90237-x. [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Kennedy E. P. Energy-transducing adenosine triphosphatase from Escherichia coli: purification, properties, and inhibition by antibody. J Bacteriol. 1973 May;114(2):772–781. doi: 10.1128/jb.114.2.772-781.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchikian E. C., Le Gall J. Evidence for the presence of a b-type cytochrome in the sulfate-reducing bacterium Desulfovibrio gigas, and its role in the reduction of fumarate by molecular hydrogen. Biochim Biophys Acta. 1972 Jun 23;267(3):479–484. doi: 10.1016/0005-2728(72)90175-2. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Mustafa T. Invertebrate facultative anaerobiosis. Science. 1972 Dec 8;178(4065):1056–1060. doi: 10.1126/science.178.4065.1056. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. I. Preparation and properties of phosphorylating membrane fragments. Biochim Biophys Acta. 1967;143(3):462–476. doi: 10.1016/0005-2728(67)90052-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Properties of an oxidative phosphorylation system reconstituted from coupling factors in Micrococcus lysodeikticus. J Biochem. 1970 Feb;67(2):297–312. doi: 10.1093/oxfordjournals.jbchem.a129253. [DOI] [PubMed] [Google Scholar]
- JACOBS N. J., WOLIN M. J. Electron-transport system of Vibrio succinogenes. I. Enzymes and cytochromes of electron-transport system. Biochim Biophys Acta. 1963 Jan 1;69:18–28. doi: 10.1016/0006-3002(63)91221-6. [DOI] [PubMed] [Google Scholar]
- JACOBS N. J., WOLIN M. J. Electron-transport system of Vibrio succinogenes. II. Inhibition of electron transport by 2-heptyl-4-hydroxyquinoline N-oxide. Biochim Biophys Acta. 1963 Jan 1;69:29–39. doi: 10.1016/0006-3002(63)91222-8. [DOI] [PubMed] [Google Scholar]
- Joyner A. E., Jr, Baldwin R. L. Enzymatic studies of pure cultures of rumen microorganisms. J Bacteriol. 1966 Nov;92(5):1321–1330. doi: 10.1128/jb.92.5.1321-1330.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. W., Hanson J. B. Activation of 2,4-dinitrophenol-stimulated ATPase activity in cauliflower and corn mitochondria. Arch Biochem Biophys. 1975 Jun;168(2):358–368. doi: 10.1016/0003-9861(75)90264-7. [DOI] [PubMed] [Google Scholar]
- Kröger A., Dadák V., Klingenberg M., Diemer F. On the role of quinones in bacterial electron transport. Differential roles of ubiquinone and menaquinone in Proteus rettgeri. Eur J Biochem. 1971 Aug 16;21(3):322–333. doi: 10.1111/j.1432-1033.1971.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Kröger A. Electron-transport phosphorylation coupled to fumarate reduction in anaerobically grown Proteus rettgeri. Biochim Biophys Acta. 1974 May 22;347(2):273–289. doi: 10.1016/0005-2728(74)90051-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macy J., Probst I., Gottschalk G. Evidence for cytochrome involvement in fumarate reduction and adenosine 5'-triphosphate synthesis by Bacteroides fragilis grown in the presence of hemin. J Bacteriol. 1975 Aug;123(2):436–442. doi: 10.1128/jb.123.2.436-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Enzyme complex which couples glycerol-3-phosphate dehydrogenation to fumarate reduction in Escherichia coli. J Bacteriol. 1973 May;114(2):767–771. doi: 10.1128/jb.114.2.767-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Molecular weight, amino acid composition and other properties of membrane-bound ATPase from Bacillus megaterium KM. Biochim Biophys Acta. 1973 Jan 26;291(2):480–488. doi: 10.1016/0005-2736(73)90499-9. [DOI] [PubMed] [Google Scholar]
- NIELSEN S. O., LEHNINGER A. L. Phosphorylation coupled to the oxidation of ferrocytochrome c. J Biol Chem. 1955 Aug;215(2):555–570. [PubMed] [Google Scholar]
- Niederman R. A., Wolin M. J. Requirement of succinate for the growth of Vibrio succinogenes. J Bacteriol. 1972 Feb;109(2):546–549. doi: 10.1128/jb.109.2.546-549.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, SMITH O. H., GEST H. Comparative biochemistry of the biological reduction of fumaric acid. Biochim Biophys Acta. 1957 Jul;25(1):142–147. doi: 10.1016/0006-3002(57)90431-6. [DOI] [PubMed] [Google Scholar]
- PINCHOT G. B. Phosphorylation coupled to electron transport in cell-free extracts of Alcaligenes faecalis. J Biol Chem. 1953 Nov;205(1):65–74. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Rizza V., Sinclair P. R., White D. C., Cuorant P. R. Electron transport system of the protoheme-requiring anaerobe Bacteroides melaninogenicus. J Bacteriol. 1968 Sep;96(3):665–671. doi: 10.1128/jb.96.3.665-671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smibert R. M., Holdeman L. V. Clinical isolates of anaerobic gram-negative rods with a formate-fumarate energy metabolism: Bacteroides corrodens, Vibrio succionogenes, and unidentified strains. J Clin Microbiol. 1976 Apr;3(4):432–437. doi: 10.1128/jcm.3.4.432-437.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N. The redox reactions in propionic acid fermantation. I. Occurrence and nature of an electron transfer system in Propionibacterium arabinosum. J Biochem. 1972 Jun;71(6):931–940. doi: 10.1093/oxfordjournals.jbchem.a129864. [DOI] [PubMed] [Google Scholar]
- TISSIERES A., SLATER E. C. Respiratory chain phosphorylation in extracts of Azotobacter vinelandii. Nature. 1955 Oct 15;176(4485):736–737. doi: 10.1038/176736a0. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. A., Cascarano J. The energy-yielding oxidation of NADH by fumarate in submitochondrial particles of rat tissues. Biochim Biophys Acta. 1970 Aug 4;216(1):54–62. doi: 10.1016/0005-2728(70)90158-1. [DOI] [PubMed] [Google Scholar]
- Wolin M. J. Lysis of Vibrio succinogenes by ethylenediamine-tetraacetic acid or lysozyme. J Bacteriol. 1966 May;91(5):1781–1786. doi: 10.1128/jb.91.5.1781-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sastre M. B., Stoppani A. O. Demonstration of a Mg 2+ -activated adenosine triphosphatase in Trypanosoma cruzi. FEBS Lett. 1973 Apr 1;31(1):137–142. doi: 10.1016/0014-5793(73)80091-2. [DOI] [PubMed] [Google Scholar]
- de Vries W., Wijck-Kapteijn W. M., Stouthamer A. H. Influence of oxygen on growth, cytochrome synthesis and fermentation pattern in propionic acid bacteria. J Gen Microbiol. 1972 Aug;71(3):515–524. doi: 10.1099/00221287-71-3-515. [DOI] [PubMed] [Google Scholar]
- de Vries W., van Wijck-Kapteyn W. M., Oosterhuis S. K. The presence and function of cytochromes in Selenomonas ruminantium, Anaerovibrio lipolytica and Veillonella alcalescens. J Gen Microbiol. 1974 Mar;81(1):69–78. doi: 10.1099/00221287-81-1-69. [DOI] [PubMed] [Google Scholar]
- de Vries W., van Wyck-Kapteyn W. M., Stouthamer A. H. Generation of ATP during cytochrome-linked anaerobic electron transport in propionic acid bacteria. J Gen Microbiol. 1973 May;76(1):31–41. doi: 10.1099/00221287-76-1-31. [DOI] [PubMed] [Google Scholar]


