Abstract
FcRγ and interleukin-10 (IL-10) are both required for chronic disease in C57BL/6 mice with Leishmania mexicana parasite infection. FcRγ is a component of several different FcRs and may be a component of some T-cell receptors. The initial antibody response to L. mexicana is an immunoglobulin G1 (IgG1) response, and IgG1 preferentially binds to FcγRIII in other systems. To begin to dissect the mechanisms by which FcγRs contribute to chronic disease, we infected FcγRIII knockout (KO) mice with L. mexicana. We show that FcγRIII KO mice are resistant to L. mexicana infection, resolving lesions in association with a stronger gamma interferon response, similar to IL-10 KO mice, with parasite control by 12 weeks. We found that the Leishmania-specific IgG response is unaltered in FcγRIII KO mice compared with that in wild-type controls. The frequencies of IL-10 production from lymph node CD25+ CD4+ T cells are the same in KO and wild-type mice, and depletion of CD25+ cells did not alter the course of infection, implying that Treg cells may not be the mechanism for susceptibility to L. mexicana infection, unlike for L. major infection. However, IL-10 mRNA was greatly diminished in the lesions of FcγRIII KO mice compared to that of B6 controls. Furthermore, macrophages from FcγRIII KO and FcRγ KO mice have the same profound defect in IL-10 production induced by IgG-opsonized amastigotes. We also found IL-10-dependent (major) and -independent (minor) inhibition of IL-12 mediated by FcγRIII, as well as parasite-mediated inhibition of IL-12 and induction of IL-10, independent of FcγR. Our data demonstrate a specific role for FcγRIII in suppressing protective immunity in L. mexicana infection, likely through macrophage IL-10 production in the lesion.
The intracellular protozoan parasite Leishmania continues to be a major cause of morbidity and mortality, with an estimated worldwide prevalence of 12 million, with increases in many areas in the world. Chronic forms of cutaneous disease occur much more commonly with Latin American strains, such as members of the Leishmania mexicana complex, than with Old World species, such as Leishmania major. Understanding why chronic lesions do not heal is important because drug therapies are toxic and resistance to current treatments has been increasing with species of Leishmania that cause cutaneous as well as visceral disease since the 1980s (12, 34, 35). In addition, understanding the pathways by which immunoglobulin G (IgG), through FcγR, can be detrimental could aid in vaccine development by pointing the way toward approaches that minimize these immunologic mechanisms.
Infection of C57BL/6 (B6) mice with L. mexicana results in chronic disease mirroring human infection, with persistently high parasite burdens and a failure to resolve the inflammatory lesions. This chronic disease is not associated with an interleukin-4 (IL-4)-driven Th2 response but rather is caused by suppression of the protective immune response by the cytokine IL-10 (6). We previously found that mice deficient in IL-10 control parasite numbers and resolve their lesions in association with enhanced gamma interferon (IFN-γ) production by T cells (6). Mice lacking the common γ chain of FcγRs also resolve L. mexicana lesions and control parasites similarly to IL-10-deficient mice (6). Furthermore, macrophages, the most abundant cell type in chronic L. mexicana lesions, secrete IL-10 in response to Leishmania amastigotes in an IgG-dependent manner (6, 19). This suggests that macrophages, which, unlike T cells, have FcγR on their surfaces, may be important in this IL-10 pathway of susceptibility. FcRγ knockout (KO) mice lack the common γ signaling chain of FcγRI, -III, and -IV as well as FcαRI and FcɛRI (11, 27). In addition, FcRγ KO mice may have immune defects due to a lack of or alteration in FcRγ-expressing T cells, which express FcRγ (but not functional FcγR) in place of the CD3ζ chain, as seen in human T cells (21). In recent studies, conventional human FcRγ− CD3ζ+ T cells had higher IFN-γ and IL-2 expression than FcRγ+ CD3ζlo T cells, which were functionally anergic and may have regulatory properties (28). Thus, a deficiency of FcRγ might alter T-cell function directly, independent of effects on FcγR-expressing antigen-presenting cells (APCs).
To start to delineate which FcRγ pathways are responsible for the nonhealing phenotype, we examined L. mexicana infection of FcγRIII KO mice. These mice resolved lesions and controlled parasites as effectively as FcRγ KO mice. This parasite control was associated with a stronger IFN-γ response than that seen in wild-type (WT) B6 mice. The IL-10 response in lesions was much lower in FcγRIII KO mice than in infected controls. We also found that bone marrow macrophages (BMMΦ) from FcγRIII KO mice had a greatly diminished IL-10 response to IgG-opsonized amastigotes indistinguishable from that of FcRγ KO macrophages. In summary, our data indicate that mice lacking FcγRIII have a defect similar to that of FcRγ KO mice, demonstrating that FcγRIII is required for chronic disease from L. mexicana. The correlation of in vitro macrophage data combined with our in vivo findings and the lack of correlation between T-cell IL-10 and parasite control support a direct role for macrophages in the suppressive IL-10 pathway. The decreased IL-10 responses in the lesions of infected FcγRIII KO mice greatly strengthen this hypothesis.
MATERIALS AND METHODS
Mice.
B6 and B6 FcγRIII KO mice were purchased from Jackson Laboratory (Bar Harbor, ME). FcRγ KO mice were purchased from Taconic Farms (Germantown, NY). Courses of infection consisted of groups of five mice per experiment. Female mice were purchased at 4 to 6 weeks and were age matched for all experiments. Animals were maintained in a specific pathogen-free environment, and the animal colony was screened regularly, and tested negative, for the presence of murine pathogens. Studies were reviewed and approved by the IACUC, Biosafety, and R&D Committees of the VA Medical Center of Philadelphia.
CD25+ T-cell depletion.
B6 mice were depleted of CD25+ T cells by using anti-CD25 (PC61) as published by others (3, 29). Mice were injected intraperitoneally (i.p.) with 0.5 mg of anti-CD25 (PC61) at −1 week, +2 weeks, and +5 weeks with respect to L. mexicana infection. PC61 was purified from ascites by using protein G agarose (Harlan, Indianapolis, IN). Depletion of CD25+ cells was confirmed by flow cytometry.
Parasites and antigens.
L. mexicana (MNYC/BZ/62/M379) promastigotes were grown at 27°C in Grace's medium (pH 6.3; Life Technologies, Grand Island, NY) supplemented with 20% heat-inactivated fetal bovine serum (FBS; HyClone Labs, Logan, UT), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Stationary-phase promastigotes (day 7 of culture) were washed three times in phosphate-buffered saline (PBS), and 5 × 106 parasites (in 50 μl PBS) were injected into the hind footpads of mice. Lesions were monitored using a metric dial caliper, and lesion size was defined as footpad thickness in the infected foot minus the thickness of the contralateral uninfected foot. Lesion-derived amastigotes were obtained by grinding footpad lesions of mice chronically infected with L. mexicana. Axenic amastigotes, grown free of mammalian cells, were prepared by placing L. mexicana stationary-phase promastigote cultures (day 7) at 33°C for 3 days, with passage every 7 to 10 days at a 1/100 dilution into acidic Grace's medium (pH 5.5) supplemented as described above. Freeze-thaw antigen (FTAg) was prepared from L. mexicana stationary-phase promastigotes that were washed four times in PBS, resuspended at 109/ml, and frozen (−80°C) and thawed rapidly (37°C) for five cycles. FTAg was assayed for protein content by the bicinchoninic acid method (Pierce, Rockford, IL), brought to 1 mg/ml protein, and aliquoted at −80°C. “Washed membranes” were prepared from axenic amastigotes by hypotonic lysis as described for African trypanosomes (24). Briefly, axenic amastigotes were washed in PBS and then hypotonically lysed at 109/ml in endotoxin-free water containing 0.1 mM N-tosyl-l-lysine-chloromethyl ketone (TLCK; Sigma-Aldrich, St. Louis, MO) and 1 μg/ml leupeptin (Sigma-Aldrich) for 5 min on ice. Then, an equal volume of 0.1 mM TLCK, 1 μg/ml leupeptin, 20% glycerol was added and parasites were frozen at −80°C. Thawed lysate was washed in PBS (6,100 × g, 10 min, 4°C) to remove soluble proteins and protease inhibitors and resuspended at 109/ml in PBS. This lysate was assayed for protein content by bicinchoninic acid, brought to 1 mg/ml protein, aliquoted, and stored at −80°C.
Cytokine assays.
Single-cell suspensions were prepared from draining lymph nodes (LNs), and 200-μl samples (8 × 105 cells) were cultured in duplicate in 96-well tissue culture plates in Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA) supplemented with 10% heat-inactivated FBS, 25 mM HEPES (pH 7.4), 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were stimulated with 10 μg/ml (∼107 cell equivalents/ml) L. mexicana FTAg for 3 days at 37°C in a 5% CO2 incubator, and supernatants were assayed by an enzyme-linked immunosorbent assay (ELISA) for IFN-γ and IL-4 as previously described (31) and for IL-10 by using commercial antibodies as recommended by the manufacturer (BD Bioscience, San Diego, CA). Cells from uninfected mice had no detectable IL-10, IL-4, or IFN-γ production with antigen stimulation in these experiments. Macrophage supernatants were assayed by ELISA using antibody pairs for IL-12p40 and IL-10 per the manufacturer's recommendations (BD Bioscience).
In vitro infection of BMMΦ.
For BMMΦ preparation, bone marrow from the femurs and tibias was lysed (5 min at room temperature in 5 ml of 144 mM NH4Cl, 17 mM Tris, pH 7.65) and washed, and cells (5 × 106 cells per petri dish) were grown in 10 ml of complete macrophage medium (Dulbecco's modified Eagle's medium containing 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 20% L929 cell-conditioned medium) for 6 days, with an additional 10 ml of complete macrophage medium added on day 3 (19, 38, 39). Macrophages were harvested by gentle scraping in cold PBS (4°C), washed, and replated at 5 × 105/ml in 24-well plates in 0.5 ml complete macrophage medium lacking L929 cell-conditioned medium. After resting overnight and being washed with fresh medium, cells were incubated with 0.5 ml complete medium, and when included, lipopolysaccharide (LPS) from Escherichia coli 0111:LB4 (Sigma-Aldrich, St. Louis, MO) was added at 100 ng/ml. Macrophages were infected at a 10:1 multiplicity of infection with axenic amastigotes or axenic amastigotes opsonized for 30 min at 4°C with 50 μl of a dilution of pooled B6 mouse serum from early infections with L. mexicana. Amastigotes incubated with uninfected mouse serum yielded results identical to those for unopsonized parasites in several experiments. In addition, purified IgG led to the same effects as those seen with IgG-containing serum, demonstrating that components of serum other than IgG were not required for the cytokine induction seen. After 20 h, supernatants were collected, frozen at −20°C, and assayed later for IL-10 and IL-12p40 by ELISA as described above. In addition, anti-IL-10R (1B1.3a; a generous gift from DNAX) was added to some BMMΦ cultures at 6.3 μg/ml to block IL-10 uptake and degradation and block effects of IL-10; the effect of anti-IL-10R saturated around 3.2 μg/ml, with identical IL-10 production seen with a broad range of concentrations (3.2 to 30 μg/ml).
Flow cytometry.
Mouse IgG on the surfaces of amastigotes was measured using phycoerythrin (PE)-conjugated anti-mouse IgG1 (A85-1) and fluorescein isothiocyanate-conjugated anti-mouse IgG2a/c (R19-15). Lesion-derived amastigotes, but not untreated axenic amastigotes, had cell surface-associated IgG. When axenic amastigotes were opsonized (4°C, 30 min in a 50-μl volume of IgG-containing serum dilution), surface IgG was similar to that of lesion-derived amastigotes. LN cells from infected mice were incubated with or without L. mexicana FTAg for 3 days and then were stimulated with phorbol myristate acetate (50 ng/ml), ionomycin (0.5 μg/ml), and brefeldin A (10 μg/ml) for 4 h, followed by staining for CD3ɛ (fluorescein isothiocyanate-145-2C11), CD8α (peridinin chlorophyll protein-53-6.7), and CD25 (PE-PC61 5.3), fixing with 1% formaldehyde, and staining for intracellular IL-10 (APC-JES5-16E3) with saponin (1%). We used CD3+ CD8− staining to determine CD4 cells because of the downregulation of CD4 with antigen stimulation. Antibodies were from BD Biosciences or Caltag (CD25), and flow cytometry was acquired and analyzed on a FACSCalibur flow cytometer with CellQuest Pro software (BD Biosciences). Representative fluorescence-activated cell sorting plots from groups of three to five mice are shown.
Measurement of Leishmania-specific serum IgG.
Sera from infected mice were assayed for Leishmania-specific IgG1 and IgG2a/c by ELISA using FTAg or axenic amastigote “washed membranes” for capture and biotin-conjugated anti-mouse IgG1 and IgG2a/c (BD Biosciences) with peroxidase-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA). IgG quantitation shows means and standard errors of the means for five mice per group. Note that B6 mice actually have IgG2c (also referred to as the Igh1-b allele of IgG2a) rather than IgG2a, found in many other mouse strains (18). However, IgG2a and IgG2c appear to function interchangeably, and the ELISA reagents do not distinguish these two isotypes, so the term IgG2a/c is used throughout the paper.
Parasite quantitation.
Parasite quantitation was performed by limiting dilution as described previously for three to five mice per group (5). The limit of detection was 25 parasites/lesion.
RNA isolation/qRT-PCR.
At 12 weeks postinfection, mice were sacrificed and the infected feet were harvested. The feet were immediately skinned, had the toes removed, and were cut into small pieces, which were stored in 0.5 ml RNAlater solution (Qiagen, CA) at 4°C. RNA was extracted from the lesions by using a Tissuelyser (Qiagen) and RNA STAT-60 (Tel-Test Inc., TX), according to the manufacturers' instructions. A Superscript II reverse transcription-PCR kit (Invitrogen, NY) was used to generate cDNA from RNA, using random hexamer primers. Quantitative real-time PCR (qRT-PCR) was performed on these cDNA preparations by using QuantiTect mouse IL-10, β-actin real-time PCR primers (Qiagen), and an ABI Prism 7000 sequence detection system with SYBR green PCR reagents (Bio-Rad, CA). The relative differences for IL-10 mRNA levels among samples were determined using the ΔΔCT method (22). Threshold cycle (CT) values obtained from the qRT-PCR were converted to ΔCT values by subtracting β-actin internal control values from the same samples, and the mean of B6 control ΔCT values was subtracted from the individual FcγRIII KO and B6 ΔCT values to obtain ΔΔCT. The difference from the B6 control level was calculated by using the formula 2−(ΔΔCT).
Statistical analysis.
Except where indicated, experiments were performed two to four times and representative data are shown. A two-tailed, unequal-variance Student t test was used to compare means of lesion sizes, log parasite burdens, and cytokine production from different groups of mice. Cytokine analysis by ELISA or flow cytometry and serum IgG assays consisted of four or five mice per group. In vitro BMMΦ experiments were performed in quadruplicate. For relative mRNA expression, ΔΔCT values for B6 and FcγRIII KO samples from individual mice were compared by a Student t test. Data are presented as means ± standard errors of the means, and differences were considered significant at P values of <0.05.
RESULTS
FcγRIII KO mice control parasite numbers and resolve L. mexicana lesions.
To determine the role of FcγRIII in L. mexicana infection, we infected B6 FcγRIII KO and WT B6 mice and followed the course of disease. Lesions developed similarly in the two mouse strains for the first 9 weeks, but by 10 weeks of infection FcγRIII KO mice began to heal their lesions (Fig. 1A). By 23 weeks postinfection, FcγRIII KO mice had nearly resolved all lesions. Parasite loads were the same in KO and WT mice for the first 8 weeks of infection, but by 12 weeks postinfection, FcγRIII KO mice had controlled parasite numbers, with 4.3 logs fewer parasites than in control mice, with the difference growing to 5.6 logs by 23 weeks (Fig. 1B). It is important to note that parasite uptake is well preserved in FcγRIII KO (Fig. 1B), FcRγ KO (6), and β2-microglobulin KO (6) mice (that have undetectable IgG), as all of these mice have parasite burdens comparable to or higher than those of B6 mice early in infection and have low parasite burdens only once the Th1 response is initiated. Thus, resolution of disease is not simply due to loss of FcγR/immune complex-mediated parasite uptake as originally proposed (20).
FIG. 1.
FcγRIII KO mice control parasite numbers with an enhanced IFN-γ response and resolve L. mexicana lesions. (A) FcγRIII KO (KO) and C57BL/6 (B6) mice were infected in the right hind footpad with 5 × 106 stationary-phase L. mexicana promastigotes, and lesion size was monitored. Lesion sizes were different at 12 weeks and thereafter (P < 0.05). (B) At the times indicated, lesion parasite burdens from FcγRIII KO and B6 mice were determined by limiting dilution. *, P < 0.01. At the indicated times postinfection, draining LN cells were stimulated with FTAg for 3 days and supernatants were assayed for IFN-γ (C), IL-4 (D), and IL-10 (E). The IL-10 data represent pooled data from two experiments at 8 weeks and three at 17 to 23 weeks. No measurable cytokine was detectable with unstimulated media controls. *, P = 0.02; #, P = 0.004.
FcγRIII KO mice have a stronger IFN-γ response.
We next determined if the control of parasites was associated with a stronger IFN-γ response, as seen with FcRγ KO and IL-10 KO mice (6). We found that the draining LN cells from L. mexicana-infected FcγRIII KO mice produced 2.6-fold and 4-fold more IFN-γ than those from infected B6 mice when restimulated in vitro with FTAg from L. mexicana at 8 and 17 weeks postinfection, respectively (Fig. 1C). IL-4 responses at 8 and 23 weeks were very low (<1 U/ml) and not different for KO and WT mice (Fig. 1D).
T-cell IL-10 does not correlate with lesion resolution.
We next examined IL-10 production from draining LN cells from L. mexicana-infected FcγRIII KO and B6 mice. We found that IL-10 production from B6 LN cells was quite low upon restimulation with antigen and that FcγRIII KO cells had no clear defect (Fig. 1E). Because of the low levels seen, we also examined IL-10 by flow cytometry. Reports of the role of CD25+ CD4+ T cells in L. major persistence (3) led us to examine whether IL-10 from these cells could explain chronic disease with L. mexicana. We found that nearly all (92%) of the IL-10 from restimulated LN cells from both WT and FcγRIII KO mice came from T cells, and the vast majority (82%) of IL-10 from CD4+ T cells came from CD25+ CD4+ cells (a population that includes Treg cells). At 8 weeks of infection, the percentages of CD25+ CD4+ T cells producing IL-10 were not different in FcγRIII KO and B6 mice, with 7.9% ± 0.9% and 8.3% ± 1.5%, respectively (P = 0.82) (Fig. 2A). Data from 12 and 23 weeks were comparable (data not shown). This demonstrates that IL-10 production from LN T cells, and in particular IL-10 from CD25+ CD4+ T cells, is not found to correlate with susceptibility, parasite burdens, and the strength of the IFN-γ response when FcγRIII KO and B6 mice are compared.
FIG. 2.
T-cell IL-10 does not correlate with lesion resolution in FcγRIII KO mice and depletion of CD25+ T cells does not alter chronic disease. (A) LN cells from FcγRIII KO and B6 mice infected for 8 weeks were stimulated with FTAg for 3 days and phorbol myristate acetate-ionomycin-brefeldin A for 4 h before surface staining and intracellular IL-10 staining. Cells were gated as live CD3+ CD8− and stained for CD25 and IL-10. The numbers shown in the upper right are percentages of CD25+ CD4+ cells that are IL-10-positive. CD25+ T cells were depleted using anti-CD25 antibody (PC61) administered i.p. at 1 week before and at 2 weeks and 5 weeks after infection with L. mexicana. Control mice received rat IgG on the same schedule. (B) Lesion size was monitored as in Fig. 1. (C) Lesion parasite burdens were determined at 18 weeks of infection by limiting dilution. At the indicated times postinfection, PC61 treated and control mice had draining LN cells stimulated with FTAg for 3 days and supernatants were assayed for IFN-γ (D) and IL-4 (E). At the time of infection, 1 week after PC61 injection, white blood cells from peripheral blood were isolated and stained for CD25 and analyzed by flow cytometry (F). Isotype control staining and samples from PC61-treated and rat IgG-treated mice are shown, with the mean percentages of CD25+ cells of gated lymphocytes in the upper right corners; plots were gated for lymphocytes and plotted as forward scatter versus CD25 PE. The percentage of CD25+ cells was significantly greater (P < 0.002) in rat IgG controls than in PC61-treated mice.
Depletion of CD25+ T cells does not alter the course of L. mexicana infection.
Because depletion of CD25+ CD4+ T cells can lead to healing of L. major infection in BALB/c mice (3), we tested whether depletion of CD25+ Treg cells would induce resistance to L. mexicana infection of B6 mice. Mice were injected i.p. with anti-CD25 at −1 week, +2 weeks, and +5 weeks with respect to infection with L. mexicana. We did not see any alteration in the course of infection (Fig. 2B), parasite burdens (Fig. 2C), or immune responses (Fig. 2D and E), despite depletion of CD25+ cells, which include Treg cells and some effector T cells. Depletion was confirmed by flow cytometry, with 96 to 100% depletion occurring when assessed at 1 week after antibody administration (Fig. 2F). Thus, consistent with our findings that resistant FcγRIII KO mice do not have a lower frequency of IL-10 from T cells, CD25+ Treg-cell depletion did not lead to healing in B6 mice.
We did not find, as did Ji et al. (16) with Leishmania amazonensis infection, that depletion of CD25+ cells had a beneficial effect on host resistance; however, the role of IL-10 in L. amazonensis infection is clearly different from that in L. mexicana infection, as L. amazonensis-infected IL-10 KO mice do not heal (17).
Anti-Leishmania IgG responses of FcγRIII KO and B6 mice do not differ.
IL-4 can drive isotype class switching to IgG1, and IFN-γ can drive switching to IgG2a (4, 33). It would therefore be expected that the stronger IFN-γ response seen in FcγRIII KO mice would lead to a stronger IgG2a/c response and perhaps a weaker IgG1 response as well. Contrary to this expectation, we found that the IgG1 and IgG2a/c responses of B6 and FcγRIII KO mice against L. mexicana were essentially identical, both early (8 weeks), when IgG1 predominates, and late (23 weeks) in infection, when both IgG2a/c and IgG1 are present (Fig. 3). Parasite-specific IgG2a/c and IgG1 were undetectable at 4 weeks in both B6 and FcγRIII KO mice (data not shown). These data suggest that to a great extent, the IgG2a/c response is IFN-γ independent in this infection and that the IgG response may in fact help to determine the IFN-γ cytokine response. In this model, IgG, which takes >4 weeks to develop, induces IL-10, which in turn decreases the IFN-γ response and leads to chronic disease; when this pathway is blocked either by a lack of FcγR or by IL-10 itself, Th1-mediated healing occurs instead (see Fig. 4).
FIG. 3.
IgG responses of FcγRIII KO and B6 mice do not differ. Serum from FcγRIII KO (KO) and B6 mice infected with L. mexicana for 8 weeks (A) and 23 weeks (B) were assayed for L. mexicana-specific IgG1 and IgG2a/c by ELISA, using FTAg as a capture reagent. OD405, optical density at 405 nm.
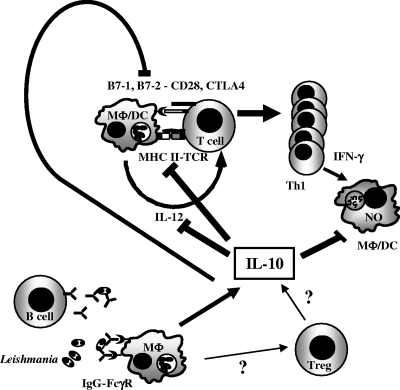
FIG. 4.
Model for IgG-FcγR induction of chronic Leishmania disease through IL-10. B cells secrete anti-Leishmania IgG, which binds to amastigotes forming immune complexes. IgG-Leishmania complexes bind to FcγR on macrophages (MΦ) inducing IL-10. IL-10 has many effects, including direct downregulation of iNOS, with decreased NO-mediated killing of Leishmania and decreased IL-12 secretion by APCs, as well as decreased antigen presentation through major histocompatibility class II (MHC II) and B7 expression, which combine to decrease Th1 development and IFN-γ. The decrease in IFN-γ also leads to lower iNOS and lower NO-mediated parasite killing. Treg cells may also be stimulated by macrophage IL-10 or other factors to contribute their own IL-10, although our data do not directly support this. DC, dendritic cells; TCR, T-cell receptor.
Macrophages from FcγRIII KO and FcRγ KO mice have a defect in IL-10 production in response to opsonized parasites.
We and others have shown previously that LPS-stimulated macrophages secrete IL-10 in response to IgG-opsonized Leishmania amastigotes (6, 19). In addition, macrophages from FcRγ KO mice have a defect in immune complex-induced IL-10 production (19). However, with sheep erythrocytes and rabbit antisera, it was proposed that FcγRI was the main FcγR responsible for macrophage IL-10 production rather than FcγRIII (36). This is in conflict with our in vivo findings, if indeed macrophage FcγR-induced IL-10 is the mechanism for the development of chronic disease in L. mexicana infection. Having demonstrated that FcγRIII KO mice resolve lesions, control parasite numbers, and produce an IFN-γ response very similarly to FcRγ KO mice, we next wished to determine if macrophages from these mice behave in similar manners in terms of opsonized parasite-induced IL-10 production. BMMΦ from FcγRIII KO, FcRγ KO, and B6 control mice were stimulated with LPS and infected with unopsonized amastigotes or IgG-opsonized amastigotes (opsonized with serum from L. mexicana-infected mice). Whereas BMMΦ from B6 mice stimulated with LPS and opsonized parasites produced large amounts of IL-10, FcγRIII KO and FcRγ KO macrophages had a defect in IL-10 production under these conditions (fourfold less IL-10) (Fig. 5A). The levels of IL-10 from FcγRIII KO and FcRγ KO macrophages stimulated with IgG-opsonized parasites and LPS were the same as those seen with unopsonized parasites plus LPS, indicating that the two types of FcγR-deficient macrophages do not show any increase in IL-10 due to the presence of IgG on the parasites, at least under these experimental conditions. We also saw a modest but reproducible induction of IL-10 from macrophages stimulated with LPS and infected with unopsonized L. mexicana amastigotes. This occurred with B6, FcγRIII KO, and FcRγ KO macrophages and without opsonization, so this pathway of IL-10 induction is not FcγR mediated. Note that opsonization with sera from uninfected mice did not induce IL-10, and purified IgG worked as well as IgG-containing serum, demonstrating that this is an IgG effect (data not shown).
FIG. 5.
FcγRIII KO and FcRγ KO macrophages have a defect in IL-10 production in response to opsonized parasites. BMMΦ were prepared from B6, FcγRIII KO, and FcRγ KO mice and incubated with media alone, LPS, unopsonized axenic amastigotes (AA), AA with LPS (AA/LPS), or AA opsonized with serum from L. mexicana-infected mice with LPS (opsAA/LPS) for 20 h, and IL-10 was measured in the supernatants by ELISA. BMMΦ were incubated without (A) or with (B) anti-IL-10R to block IL-10 uptake and signaling. P was <0.01 for B6 BMMΦ incubated with opsAA/LPS and all other groups and for all macrophage types incubated with AA/LPS compared to the same macrophage type incubated with media, LPS, or AA alone. Opsonized parasites generate amounts of IL-10 similar to those from unopsonized parasites when LPS is not present.
Because IL-10 can be consumed in cultures, leading to less reliable results from ELISA, we also incubated BMMΦ in the presence of anti-IL-10R and found essentially the same results except that IL-10 levels were 2.6-fold higher (Fig. 5B). In these experiments (Fig. 5A and B), serum from early infection that contained IgG1 but no detectable IgG2a/c was used and the presence of parasite surface-associated antibodies was confirmed by flow cytometry (data not shown). These data indicate that FcγRIII is responsible for essentially all of the immune complex-induced IL-10 from our BMMΦ, implying that the defect seen in FcRγ KO BMMΦ may be explained fully by a defect in FcγRIII rather than other FcRs found on macrophages. This is consistent with the in vivo data shown above.
IL-12 production is inhibited by IL-10, by parasite infection, and by parasite immune complexes.
IgG-amastigote immune complexes can induce IL-10 from macrophages. This IL-10 is known to have many immunosuppressive effects, including suppression of IL-12 secretion. In vivo, we have shown that IL-12 KO mice have the same immune response and chronic disease as that seen in B6 mice but that anti-IL-12 abrogates the disease resolution in IL-10 KO mice (6). This implies that IL-12 drives healing in IL-10 KO mice but is unable to do this in B6 mice because of the presence of IL-10. We therefore examined the role that paracrine/autocrine IL-10 might have on macrophage production of IL-12. It has been shown that supernatants of L. major amastigote-infected BMMΦ can inhibit the production of IL-12 induced by IFN-γ plus LPS and that this is completely abolished by blockade of IL-10 action by anti-IL-10R (19). We found that LPS induced substantial amounts of IL-12 from B6, FcγRIII KO, and FcRγ KO macrophages (Fig. 6A), as expected. Infection with unopsonized amastigotes diminished the LPS-induced IL-12 significantly in WT and both types of KO cells, showing once again that Leishmania infection diminishes macrophage IL-12 production. Infection with IgG-opsonized amastigotes further diminished IL-12 production in B6 but not FcγRIII KO macrophages, showing that the majority of FcγR effects are mediated through FcγRIII under these conditions. The negative correlation of IL-10 and IL-12 is shown in Fig. 7A. We next examined IL-12 production in the presence of blockade of IL-10R to determine if the drop in IL-12 is solely mediated by IL-10. When IL-10R was blocked, there was a >10-fold increase in LPS-induced IL-12, demonstrating that even the small amount of IL-10 induced by LPS stimulation (without parasites) decreases IL-12 production significantly (P < 0.006; note scale difference between Fig. 6A and B). This was seen in B6, FcγRIII KO, and FcRγ KO macrophages. Even in the absence of IL-10 signaling, amastigote infection decreased LPS-induced IL-12 production in B6 and KO macrophages (two- to fourfold decreases) (Fig. 6B). Infection with opsonized amastigotes further decreased IL-12 production in B6 but not FcγRIII KO or FcRγ macrophages. These data suggest that IL-10 has a strong inhibitory affect on IL-12 production from macrophages but that there are IL-10-independent IL-12 suppression pathways mediated by parasite infection and by FcγR engagement. To rule out the possibility that the IL-10-independent effects were due to insufficient blockade of IL-10R, we added twofold less and up to fivefold more anti-IL-10R and obtained essentially identical results (data not shown). There is a strong inverse correlation between IL-10 and IL-12 levels even in the absence of IL-10 signaling (Fig. 7B) that is similar to the data in the presence of IL-10 signaling (Fig. 7A). Thus, opsonized parasites binding to FcγRIII suppress IL-12 production from macrophages through IL-10 and by an IL-10-independent pathway.
FIG. 6.
IL-12 production is inhibited by IL-10, parasite infection, and immune complexes. The supernatants shown in Fig. 5 without (A) or with (B) anti-IL-10R were analyzed for the presence of IL-12 by ELISA. P was <0.02 for LPS versus other conditions for B6, FcRγ KO, and FcγRIII KO cells and axenic amastigotes with LPS (AA/LPS) versus opsonized AA with LPS (opsAA/LPS) for B6 cells.
FIG. 7.
Amastigotes and opsonized amastigotes inhibit LPS-induced IL-12 and potentiate production of IL-10 in a reciprocal manner even with an IL-10R blockade. IL-10 and IL-12 were correlated from supernatants of B6 BMMΦ stimulated with LPS alone, axenic amastigotes and LPS (AA/LPS), opsonized axenic amastigotes and LPS (opsAA/LPS), and media alone in the absence (A) or presence (B) of anti-IL-10R monoclonal antibodies (data from Fig. 5 and 6).
IL-10 expression is diminished in lesions of L. mexicana-infected FcγRIII KO mice compared with control levels.
As shown above, T-cell and CD25+ CD4+ T-cell IL-10 from the draining LNs do not correlate with resistance to L. mexicana infection in FcγRIII KO mice. Furthermore, macrophages from FcγRIII KO mice secrete less IL-10 in response to IgG-Leishmania immune complexes. We therefore decided to examine IL-10 expression from lesions, as the site of infection contains an abundance of macrophages that are likely to have an effect on the disease progression. When IL-10 mRNAs from L. mexicana-infected lesions of FcγRIII KO and B6 mice were compared, we found that the KO lesions had 7.5-fold less expression at 12 weeks of infection (Fig. 8). As macrophages are very abundant in L. mexicana lesions, whereas T cells are not (data not shown), this supports a role for IgG-FcγR induction of IL-10 from macrophages as a crucial factor in susceptibility to L. mexicana infection.
FIG. 8.
IL-10 expression is diminished in FcγRIII KO lesions compared with control levels. Lesions from FcγRIII KO (KO) and B6 (WT) mice were analyzed for IL-10 expression by qRT-PCR at 12 weeks of infection as described in Materials and Methods. Relative expression of IL-10 in KO animals was normalized to WT levels. *, P < 0.05.
DISCUSSION
We previously demonstrated that FcRγ was required for chronic disease in B6 mice caused by the intracellular protozoan parasite L. mexicana. The common γ signaling chain is shared by and required for proper expression and function of the activating FcγRs that contain immunoreceptor tyrosine-based activation motifs, namely, FcγRI, -III, and -IV, as well as FcαRI and FcɛRI. It is therefore possible that defects in any of these FcRs might abrogate the suppressive IL-10 pathway seen in L. mexicana infection of B6 mice. Initial analysis pointed to a role for FcγRI in macrophage IL-10 production. However, this work was performed with sheep erythrocytes and rabbit antisera and isotype differences in binding to various mouse FcγRs may have skewed these data (36). FcγRI is a high-affinity receptor that binds monomeric (uncomplexed) IgG2a/c as well as immune complexes. It might therefore be less likely that FcγRI would be the main FcγR in an infection in which IgG1 predominates at the time when a transition to chronic disease occurs. IgG1, but not IgG2a/c, is present at 8 to 10 weeks, at this transition to chronic disease (see the present studies and reference 6). The specificities of FcγR for different IgG isotypes vary, and particular isotypes have been shown to be important in various disease processes. For instance, macrophages from FcγRIII KO mice have an in vitro defect in phagocytosis of IgG1-coated erythrocytes but not IgG2a-coated erythrocytes (13); FcγRIII KO mice are also less susceptible to IgG1-mediated autoimmune hemolytic anemia and other IgG1-mediated phenomena in vivo (14, 25). By using a panel of IgG molecules that have the same antigen combining site but different isotypes, it has been shown that hemolytic anemia is specifically mediated through FcγRIII and IgG1 (9). In addition, neutrophil infiltration, as well as secretion of some macrophage cytokines, is mediated by IgG1-antigen complexes and FcγRIII (37). Thus, it is reasonable to suspect that IgG1-containing immune complexes can be mediated by FcγRIII-dependent pathways.
We have now shown in vivo that FcγRIII KO mice have a resistant phenotype very similar to that of FcRγ KO mice. Furthermore, in vitro, macrophages from FcγRIII KO and FcRγ KO mice have similar defects in IL-10 production induced by IgG-amastigote immune complexes. In fact, all of the IL-10 attributable to immune complexes is abrogated in FcγRIII KO BMMΦ (Fig. 5). It may be that the lack of FcγRIII completely explains the defect in IL-10 production in FcRγ KO macrophages and the resistant phenotype to L. mexicana infection seen in these mice, although we cannot yet rule out the possibility that FcγRI, FcγRIII, and FcγRIV are all required for chronic disease in vivo.
We found that IL-10 had a very strong inhibitory effect on LPS-induced IL-12 production by macrophages in vitro, even at the low levels found in the absence of immune complex stimulation (90% decrease). However, immune complexes also exert an inhibition on IL-12 production in the absence of IL-10 signaling (Fig. 7B). We titrated anti-IL-10R to be sure that we did not have incomplete blockade of this receptor. In addition, similar IL-12 results were obtained with IL-10 KO BMMΦ and with B6 cells treated with anti-IL-10R (data not shown). Thus, FcγRs exert their effects through IL-10-dependent and -independent pathways. This may explain why the phenotypes of FcRγ and FcγRIII KO mice are somewhat stronger than that seen in IL-10 KO mice, with somewhat lower parasite burdens late in infection (see current studies and reference 6). Leishmania infection of macrophages induces phosphatidylinositol 3-kinase (PI3-kinase) activity (30), as does FcγR ligation (8, 15). PI3-kinase has been shown to downregulate IL-12 production, and BALB/c PI3-kinase p85 KO mice are resistant to L. major (10). Thus, PI3-kinase may be a potential mechanism by which FcγR ligation downregulates IL-12 independent of IL-10. There also appears to be a parasite-induced FcγR-independent increase in IL-10 (in the absence of IgG) as well as a parasite-induced IL-10-independent and FcγR-independent inhibition of IL-12, both of which will require further study.
FcγRIII is critical for chronic disease, likely through IL-10 induction but perhaps through other pathways that can suppress IL-12. We previously showed that, whereas IL-12p40 KO mice still had chronic disease with L. mexicana infection, blockade of IL-12p40 in IL-10 KO mice abrogated lesion resolution, indicating that IL-10 is responsible for blocking an IL-12-dependent healing immune response (6). Here, we found that FcγRIII ligation induced IL-10-dependent and -independent suppression of IL-12.
IL-10 KO mice are resistant to L. mexicana infection (6). The source of this IL-10 is not yet determined. IL-10 from CD25+ CD4+ Treg cells is crucial in susceptibility of BALB/c mice to L. major and in persistence of low levels of parasites in B6 mice as well (3). Whereas T cells do not have functional FcγR, some FcRγ-expressing T cells in mice may express FcRγ in place of the CD3ζ chain, as seen in human T cells (21). We therefore examined whether T-cell IL-10 is responsible for chronic disease in our system. The levels of IL-10 production from LN cells were low and did not show a defect in FcγRIII KO mice. We found that the frequencies of IL-10 production from CD25+ CD4+ cells (which includes most Treg cells) are the same from WT and FcγRIII KO mice (Fig. 2A), despite the fact that KO mice resolve lesions rather than having chronic disease. Thus, there is not a clear negative correlation between CD25+ CD4+ T-cell IL-10 or even T-cell IL-10 and healing, suggesting that the relevant source of IL-10 lacking in IL-10 KO mice is not LN T cells. It is always possible that recall responses do not represent in vivo IL-10 responses, although they have been used in many systems, including ours, as a good surrogate marker. Furthermore, we did not see any effect on the course of infection, parasite loads, or immune responses when CD25+ T cells were depleted in vivo. Importantly, we verified that the CD25 depletion was successful despite a lack of any alteration in the course of infection. The facts that the vast majority of IL-10 from LN CD4+ T cells came from CD25+ cells and that T-cell IL-10 in general did not correlate with resistance in FcγRIII KO mice argue against a role for IL-10 from the recently described CD4+ CD25− Foxp3− Treg cells, which are important in chronic lesions caused by a particular strain of L. major in B6 mice (1). In addition, we cannot attribute disease resolution in FcRγ KO mice (6) exclusively to a lack of or dysfunction of FcRγ-expressing T cells, because FcγRIII KO mice, which also heal, do have FcRγ. The more straightforward explanation is that IL-10 production in lesions, where macrophages are abundant and lymphocytes are rare, may more closely match parasite control and lesion resolution. In fact, we found that lesion expression of IL-10 mRNA was 7.5-fold lower in FcγRIII KO mice than in B6 controls. Our work supports the growing evidence for a role for macrophages in the suppressive IL-10 pathway rather than Treg cells, as demonstrated for L. major infection (1-3). Miles et al. have similarly shown the importance of IgG and IL-10 in infection of BALB/c mice with L. major (26), calling into question whether Treg cells are the only determining factor in healing of cutaneous leishmaniasis even in L. major infection, although an important interaction between macrophage IL-10 and Treg cells (perhaps through effector molecules other than IL-10) cannot yet be ruled out.
In early studies of Leishmania infection, IgG2a and IgG1 responses were examined as a measure of Th1/Th2 immune responses. It is known that cytokines can influence the IgG isotype distribution in many immunologic systems. For instance, in L. major infection, healing B6 mice generate strong IgG2a/c responses, whereas nonhealing BALB/c mice have strong IgG1 responses (32). It has been shown that IFN-γ can drive class switching to IgG2a/c and IL-4 can drive class switching to IgG1 and IgE (4, 33); thus, IFN-γ drives Th1-type IgG responses and IL-4 drives Th2-type responses. However, IgG2a/c responses to Trypanosoma cruzi, Toxoplasma gondii, and lactate dehydrogenase-elevating virus infections can occur in the absence of IFN-γ (23). In one instance, it was found that CD40 can drive IgG2a responses in the absence of IFN-γ (7). Furthermore, in L. mexicana infection of B6 mice, parasite-specific IgG1 occurs early despite a very low IL-4 response. In the present study, we found that despite an enhanced IFN-γ response in FcγRIII KO mice, the IgG1 and IgG2a/c responses were completely unaltered from those in infected B6 mice (Fig. 3). This argues that in L. mexicana infection, the IgG isotype profile is not merely a consequence of cytokines such as IFN-γ and IL-4, but in fact, determination of the IgG response may occur earlier than the cytokine response. This is consistent with our hypothesis that IgG-amastigote immune complexes drive an IL-10 response, which in turn blocks the development of a protective Th1 response and inducible nitric oxide synthase (iNOS) expression needed to kill parasites (model in Fig. 4). The data shown here indicate that the increased IFN-γ response in FcγRIII KO mice (seen at 8 weeks) precedes the drop in parasite load (seen at 12 weeks but not 8 weeks). In any case, the differences in IFN-γ between resistant FcγRIII KO mice and susceptible B6 mice were not reflected in the IgG2a/c responses, indicating that IgG2a/c levels were not driven by IFN-γ levels.
In summary, we have shown that FcγRIII deficiency mirrors deficiency in FcRγ, both in vivo and in macrophage cultures, at least with IgG1-rich serum opsonization (from early L. mexicana infection), suggesting that FcγRIII plays a critical role in chronic disease and may fully explain the resistance seen in FcRγ KO mice. These data also argue against an important role for FcRγ directly in T cells in L. mexicana infection, as FcγRIII KO mice are not deficient in FcRγ but are also resistant to this parasite infection. Furthermore, the kinetics of lesion resolution in FcγR KO and IL-10 KO mice correlate with the appearance of the IgG1 response against parasite antigens (present at 8 to 10 weeks of infection and absent at 3 to 4 weeks) (6), making plausible the argument that chronic disease is initiated by IgG responses through FcγR-induced IL-10. Furthermore, the fact that the IgG responses in FcγRIII KO and B6 mice appear identical, despite the difference in IFN-γ responses and parasite control, supports a model by which the developing IgG response may in turn shape the cytokine response, in addition to the opposite, perhaps because L. mexicana infection is such a slowly developing process. We have also shown that the frequency of IL-10-producing T cells is not more pronounced in WT mice than in FcγRIII KO mice and that depletion of CD25+ T cells does not lead to resistance to L. mexicana, also suggesting a pivotal role for cells such as macrophages rather than or in addition to Treg cells in determining the chronic disease picture seen with L. mexicana infection. The decrease in IL-10 in lesions in mice lacking FcγRIII (Fig. 8) further supports this model, as macrophages, but not T cells, are abundant in L. mexicana lesions.
Acknowledgments
This work was supported by a Veterans Affairs Merit Review grant and by the University of Pennsylvania.
We thank Andrea Rosso and Niansheng Chu for their technical support and David Artis, Jay Farrell, Christopher Hunter, and Victoria Werth for critical reading of the manuscript.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Anderson, C. F., M. Oukka, V. J. Kuchroo, and D. Sacks. 2007. CD4+CD25-Foxp3- Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204285-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aseffa, A., A. Gumy, P. Launois, H. R. MacDonald, J. A. Louis, and F. Tacchini-Cottier. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 1693232-3241. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420502-507. [DOI] [PubMed] [Google Scholar]
- 4.Berton, M. T., J. W. Uhr, and E. S. Vitetta. 1989. Synthesis of germ-line gamma 1 immunoglobulin heavy-chain transcripts in resting B cells: induction by interleukin 4 and inhibition by interferon gamma. Proc. Natl. Acad. Sci. USA 862829-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxbaum, L. U., H. Denise, G. H. Coombs, J. Alexander, J. C. Mottram, and P. Scott. 2003. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J. Immunol. 1713711-3717. [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum, L. U., and P. Scott. 2005. Interleukin 10- and Fcγ receptor-deficient mice resolve Leishmania mexicana lesions. Infect. Immun. 732101-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, J. T., J. Shi, B. E. Burrell, D. K. Bishop, and W. A. Dunnick. 2006. Induced expression of murine gamma2a by CD40 ligation independently of IFN-gamma. J. Immunol. 1775414-5419. [DOI] [PubMed] [Google Scholar]
- 8.Deane, J. A., and D. A. Fruman. 2004. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 22563-598. [DOI] [PubMed] [Google Scholar]
- 9.Fossati-Jimack, L., A. Ioan-Facsinay, L. Reininger, Y. Chicheportiche, N. Watanabe, T. Saito, F. M. Hofhuis, J. E. Gessner, C. Schiller, R. E. Schmidt, T. Honjo, J. S. Verbeek, and S. Izui. 2000. Markedly different pathogenicity of four immunoglobulin G isotype-switch variants of an antierythrocyte autoantibody is based on their capacity to interact in vivo with the low-affinity Fcgamma receptor III. J. Exp. Med. 1911293-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukao, T., M. Tanabe, Y. Terauchi, T. Ota, S. Matsuda, T. Asano, T. Kadowaki, T. Takeuchi, and S. Koyasu. 2002. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3875-881. [DOI] [PubMed] [Google Scholar]
- 11.Gerber, J. S., and D. M. Mosser. 2001. Stimulatory and inhibitory signals originating from the macrophage Fcgamma receptors. Microbes Infect. 3131-139. [DOI] [PubMed] [Google Scholar]
- 12.Hadighi, R., M. Mohebali, P. Boucher, H. Hajjaran, A. Khamesipour, and M. Ouellette. 2006. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 3e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazenbos, W. L., J. E. Gessner, F. M. Hofhuis, H. Kuipers, D. Meyer, I. A. Heijnen, R. E. Schmidt, M. Sandor, P. J. Capel, M. Daeron, J. G. van de Winkel, and J. S. Verbeek. 1996. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity 5181-188. [DOI] [PubMed] [Google Scholar]
- 14.Hazenbos, W. L., I. A. Heijnen, D. Meyer, F. M. Hofhuis, C. R. Renardel de Lavalette, R. E. Schmidt, P. J. Capel, J. G. van de Winkel, J. E. Gessner, T. K. van den Berg, and J. S. Verbeek. 1998. Murine IgG1 complexes trigger immune effector functions predominantly via Fc gamma RIII (CD16). J. Immunol. 1613026-3032. [PubMed] [Google Scholar]
- 15.Huang, Z. Y., D. R. Barreda, R. G. Worth, Z. K. Indik, M. K. Kim, P. Chien, and A. D. Schreiber. 2006. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J. Leukoc. Biol. 801553-1562. [DOI] [PubMed] [Google Scholar]
- 16.Ji, J., J. Masterson, J. Sun, and L. Soong. 2005. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J. Immunol. 1747147-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, D. E., M. R. Ackermann, U. Wille, C. A. Hunter, and P. Scott. 2002. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect. Immun. 702151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jouvin-Marche, E., M. G. Morgado, C. Leguern, D. Voegtle, F. Bonhomme, and P. A. Cazenave. 1989. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics 29 92-97. [DOI] [PubMed] [Google Scholar]
- 19.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 1661141-1147. [DOI] [PubMed] [Google Scholar]
- 20.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, M. J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 1911063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan, S., V. G. Warke, M. P. Nambiar, G. C. Tsokos, and D. L. Farber. 2003. The FcR gamma subunit and Syk kinase replace the CD3 zeta-chain and ZAP-70 kinase in the TCR signaling complex of human effector CD4 T cells. J. Immunol. 1704189-4195. [DOI] [PubMed] [Google Scholar]
- 22.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 23.Markine-Goriaynoff, D., J. T. van der Logt, C. Truyens, T. D. Nguyen, F. W. Heessen, G. Bigaignon, Y. Carlier, and J. P. Coutelier. 2000. IFN-gamma-independent IgG2a production in mice infected with viruses and parasites. Int. Immunol. 12223-230. [DOI] [PubMed] [Google Scholar]
- 24.Masterson, W. J., T. L. Doering, G. W. Hart, and P. T. Englund. 1989. A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell 56793-800. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, D., C. Schiller, J. Westermann, S. Izui, W. L. Hazenbos, J. S. Verbeek, R. E. Schmidt, and J. E. Gessner. 1998. FcgammaRIII (CD16)-deficient mice show IgG isotype-dependent protection to experimental autoimmune hemolytic anemia. Blood 923997-4002. [PubMed] [Google Scholar]
- 26.Miles, S. A., S. M. Conrad, R. G. Alves, S. M. Jeronimo, and D. M. Mosser. 2005. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 201747-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nimmerjahn, F., P. Bruhns, K. Horiuchi, and J. V. Ravetch. 2005. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23 41-51. [DOI] [PubMed] [Google Scholar]
- 28.Okoye, F. I., G. C. Tsokos, and D. L. Farber. 2006. Expression of the signaling subunit FcR-γ defines a class of regulatory T cells distinct from endogenous Tregs. J. Immunol. 176(Suppl.)S197. [Google Scholar]
- 29.Oldenhove, G., M. de Heusch, G. Urbain-Vansanten, J. Urbain, C. Maliszewski, O. Leo, and M. Moser. 2003. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J. Exp. Med. 198259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruhland, A., N. Leal, and P. E. Kima. 2007. Leishmania promastigotes activate PI3K/Akt signalling to confer host cell resistance to apoptosis. Cell. Microbiol. 984-96. [DOI] [PubMed] [Google Scholar]
- 31.Scharton-Kersten, T., L. C. Afonso, M. Wysocka, G. Trinchieri, and P. Scott. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 1545320-5330. [PubMed] [Google Scholar]
- 32.Scott, P. A., and J. P. Farrell. 1982. Experimental cutaneous leishmaniasis: disseminated leishmaniasis in genetically susceptible and resistant mice. Am. J. Trop. Med. Hyg. 31230-238. [DOI] [PubMed] [Google Scholar]
- 33.Snapper, C. M., and W. E. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236944-947. [DOI] [PubMed] [Google Scholar]
- 34.Soto, J., J. Toledo, J. Vega, and J. Berman. 2005. Short report: efficacy of pentavalent antimony for treatment of colombian cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 72421-422. [PubMed] [Google Scholar]
- 35.Sundar, S., D. K. More, M. K. Singh, V. P. Singh, S. Sharma, A. Makharia, P. C. Kumar, and H. W. Murray. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 311104-1107. [DOI] [PubMed] [Google Scholar]
- 36.Sutterwala, F. S., G. J. Noel, P. Salgame, and D. M. Mosser. 1998. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J. Exp. Med. 188217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taube, C., A. Dakhama, Y. H. Rha, K. Takeda, A. Joetham, J. W. Park, A. Balhorn, T. Takai, K. R. Poch, J. A. Nick, and E. W. Gelfand. 2003. Transient neutrophil infiltration after allergen challenge is dependent on specific antibodies and Fc gamma III receptors. J. Immunol. 1704301-4309. [DOI] [PubMed] [Google Scholar]
- 38.Tushinski, R. J., I. T. Oliver, L. J. Guilbert, P. W. Tynan, J. R. Warner, and E. R. Stanley. 1982. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 2871-81. [DOI] [PubMed] [Google Scholar]
- 39.Warren, M. K., and S. N. Vogel. 1985. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J. Immunol. 134982-989. [PubMed] [Google Scholar]