Abstract
There is longstanding evidence that immunoglobulin G (IgG) has a role in protection against clinical malaria, and human antibodies of the cytophilic subclasses are thought to be particularly critical in this respect. In this cohort study, 286 Burkinabè children 6 months to 15 years old were kept under malaria surveillance in order to assess the protective role of antibody responses against four antigens which are currently being evaluated as vaccine candidates: apical membrane antigen 1 (AMA1), merozoite surface protein 1-19 (MSP1-19), MSP3, and glutamate-rich protein (GLURP). Total IgG, IgM, and IgG subclass responses were measured just before the malaria transmission season. The incidence of malaria was 2.4 episodes per child year of risk. After adjusting for the confounding effects of age, the level of total IgG to GLURP was strongly associated with reduced malaria incidence (incidence rate ratio associated with a doubling of total IgG, 0.79; 95% confidence interval, 0.66 to 0.94; P = 0.009.); there was a borderline statistically significant association between the level of total IgG to MSP3 and malaria incidence and no evidence of an association for total IgG to AMA1 and to MSP1-19. Of the IgG subclass responses studied, only IgG3 and IgG4 against GLURP and IgG1 against AMA1 were associated with reduced risk of clinical malaria. There was no evidence of an interaction between responses to AMA1 and baseline parasitemia in their effects on malaria incidence. Currently included in malaria vaccine formulations for clinical trials in humans, these blood-stage antigens, AMA1 and GLURP, offer good prospects for malaria vaccine development.
In sub-Saharan Africa, the clinical manifestations of malaria are caused by asexual blood stages of Plasmodium falciparum, and antigens on asexual parasite stages in the bloodstream are critical in the development of protective immunity to the disease. After repeated exposure, nonsterile immunity to malaria can be acquired by people living in areas of endemicity. Strong evidence exists, from the passive transfer of antibodies between immune and nonimmune individuals, that this immunity can be antibody mediated (18, 29, 40). The most efficient in vivo model for this antibody-mediated parasite control in areas where the disease is endemic requires the participation of monocytes and has been called antibody-dependent cellular inhibition (ADCI) (24, 26). This assay is assumed to mimic the in vivo cooperation between monocytes and cytophilic parasite-specific antibodies and is considered a surrogate marker of immunity against P. falciparum blood stages (21). Bouharoun-Tayoun and Druilhe observed profound differences in the distribution of immunoglobulin (Ig) subclasses between clinically protected and susceptible individuals, with cytophilic subclasses (immunoglobulin G1 [IgG1] and IgG3) being dominant in protected individuals (10). In different epidemiological settings, similar findings have been made, underscoring the importance of cytophilic antibodies against blood-stage antigens in the negative association with clinical malaria.
Merozoite surface protein 3 (MSP3) and glutamate-rich protein (GLURP) are the leading targets of cytophilic antibodies effective in ADCI. Cytophilic antibodies to these molecules were shown to be predominant in protected individuals, while noncytophilic antibodies were predominant in nonprotected individuals (35, 42). These two proteins were shown to have a complementary effect that provides a rationale for combining these two antigens in a hybrid vaccine formulation (42).
MSP1-19 and apical membrane antigen 1 (AMA1) antibodies have also been shown to be associated with a reduced risk of clinical malaria (5, 12). The antibodies to AMA1 have been reported to have high levels of parasite growth inhibitory activity in a growth inhibition assay (37). Bivalent monoclonal and polyclonal antibodies, as well as their respective monovalent Fab segments, inhibit the invasion of Plasmodium merozoites into erythrocytes. ADCI was not reported as an important effector mechanism for AMA1 and MSP1-19 antibodies, but their biological activity is linked to the specificity/avidity of the Fab portion and, most likely, not to the Fc portion.
Each of these antigens (MSP3, GLURP, MSP1-19, and AMA1) has been included in malaria vaccine candidates which have already undergone phase 1 trials in Europe, the United States, Africa, and Australia, and the protective efficacies of these malaria vaccine antigens will ultimately be tested in phase II or III vaccine trials in Africa. In preparation for evaluating the efficacy of the vaccine in field trials, it is important to investigate the natural immune response to the vaccine antigens and to determine the association between immune responses and protection against clinical malaria.
The present study was designed to (i) characterize the profiles of IgG, IgG subclass, and IgM responses to MSP3, GLURP, MSP1-19, and AMA1 antigens and (ii) examine the relationship between natural antibody isotype responses to these antigens and protection against clinical malaria. This study is part of the work of the Afro-immunoassay network (AIA) which aims to develop standardized immunological assays to contribute to the validation of putative malaria vaccine candidate antigens for development and inclusion in a future malaria vaccine.
MATERIALS AND METHODS
Study area.
The study was conducted in the village of Balonghin, located in the province of Bazega 50 km southwest of Ouagadougou, the capital city of Burkina Faso. The climate in this area is characteristic of the Sudanese savannah, with a dry season from November to May and a rainy season from June to October. Malaria transmission is markedly seasonal; most transmission occurs during the rainy season. The entomological inoculation rate in Balonghin was estimated at 0.3 and 44.4 infective bites/person/month during the dry and rainy seasons, respectively, of 2001. The main vectors are Anopheles gambiae, Anopheles arabiensis and Anopheles funestus. P. falciparum is the predominant malaria parasite, accounting for more than 95% of infections in children under 5 years of age (unpublished data). The use of insecticide-treated nets was uncommon in this area at the time of the study (about 1%); the use of indoor residual spraying is nonexistent in the area, and malaria control relies mainly on the treatment of clinical cases. In 2003, rainfall started on 16 June 2003.
Study population.
The population of Balonghin (approximately 1,600) belongs almost exclusively to the Mossi ethnic group and lives by subsistence farming. All children aged 0.5 to 15 years in June 2003 and resident in the village were listed from the Demographic Surveillance database and invited to participate.
Study design and sample collection.
The study was approved by the Burkina Faso Ministry of Health. Consent was obtained from a parent or guardian of each participating child. A cross-sectional survey was carried out in June, before the beginning of the high malaria transmission season, to obtain a venous blood sample from each child for immunological assays. Samples of 5 ml of blood were taken in a tube containing EDTA as an anticoagulant. Thick and thin blood films were prepared for microscopic examination, and plasma samples were stored at −20°C. The axillary temperature was taken twice, once under each arm, with appropriate quality control procedures. Children with fever, defined as a mean axillary temperature of ≥37.5°C or a history of fever in the last 48 h were given presumptive malaria treatment (with chloroquine) and an antipyretic (paracetamol).
Active case detection for malaria episodes was conducted during the high malaria transmission season from July to October by a team of study nurses who were resident in the village. Each child was visited daily to take his or her axillary temperature, and if the child had fever, presumptive treatment with chloroquine and paracetamol was given according to the national guidelines that applied at the time of the study. A blood sample was taken, and thick and thin blood films were prepared for measurement of parasitemia by microscopy.
Parasitological diagnosis.
Thick and thin blood films were air dried, thin blood films were fixed with methanol, and both were stained with Giemsa 3% solution. One hundred high-power fields (HPF) were examined, and the number of malaria parasites of each species and stage recorded. The number of parasites per microliter of blood was calculated by assuming 20 white blood cells per high-power field and a fixed white cell count of 8,000/μl.
Malaria antigens used for antibody measurement.
The following antigens were used to measure the antibodies: AMA1 (25), GLURP (44), MSP1-19 (8), and MSP3 (7). The antigen description is summarized in Table 1.
TABLE 1.
Description of the antigens used for the measurement of antibodies
| Antigen | Length in amino acids | P. falciparum strain | Type of synthesis | Expression system |
|---|---|---|---|---|
| AMA1 | 25-545 | FVO | Recombinant | Pichia pastoris |
| GLURP | 25-514 | F32 | Recombinant | Escherichia coli |
| MSP1-19 | 20-43, 1615-1723 | Uganda Palo-Alto | Recombinant | Baculovirus |
| MSP3 | 181-276 | FC27 | Synthetic |
All the antigens were provided through the AIA network, six African institutions that are developing standard methods for evaluating malaria vaccines, sponsored by the African Malaria Network Trust (AMANET [www.amanet-trust.org]).
Measurement of antibody responses.
At the baseline survey, children were screened for the presence of hemoglobinopathy, and antibody responses were measured only in children whose blood was of the normal hemoglobin type, AA. Malaria is less common in those with abnormal hemoglobin (3, 4, 15), and the protective effect of abnormal hemoglobin types against malaria morbidity is reported to be due to the impairment of parasite growth inside red blood cells which, in addition, may involve a modulation of immune response leading to a faster acquisition of immunity (2, 28, 34). Since it might be difficult to adjust for these effects in the analysis, children with hemoglobinopathies were excluded.
Specific IgG, IgM, and IgG subclass levels were measured by enzyme-linked immunosorbent assay for the long synthetic peptide MSP3, recombinant GLURP, recombinant MSP1-19, and recombinant AMA1. The enzyme-linked immunosorbent assay was done by following a standardized methodology described in the AIA standard operating procedures (procedure numbers AIA-007-03, AIA-001-03, and AIA-013-03), as described elsewhere (27, 41). In brief, microtiter plates (Maxisorp F 96 439454; NUNC) were coated with long synthetic peptides LR55 MSP3 (1 μg/ml in phosphate-buffered saline [PBS]), recombinant GLURP (0.5 μg/ml in PBS), and recombinant MSP1-19 (1 μg/ml in PBS), incubated overnight at 4°C, and blocked with 3% dry, nonfat skim milk powder in PBS-Tween 20 for 1 h. Plasma samples diluted 1:200 (IgG and IgM) or 1:25 (IgG subclasses) were added in duplicate and incubated at room temperature for 2 h. Plates were washed four times between steps. Plates were developed with either peroxidase-conjugated goat anti-human IgG or IgM (secondary antibody) (H10007 and H15007; Caltag).
For IgG subclasses, the secondary antibody was a mouse anti-human monoclonal IgG subclass (I-9513, clone HP-6002 for IgG1 and IgG2 [Sigma]; M08011, clone ZG4 for IgG3 [Sky Bio], and M11014, clone RJ4 for IgG4) and the revealing was done with peroxidase-conjugated goat anti-mouse IgG (M3007; Caltag).
Bound secondary antibodies for IgG and IgM and the third antibodies for IgG subclasses were quantified by staining with ready-to-use TMB (3,3′,5,5′-tetramethylbenzidine) substrate. The optical density was read at 450 nm, with a reference at 620 nm, in a plate reader (Multiskan Ascent, Finland), and the optical density value of the test sample was converted into arbitrary units by means of a standard curve for each plate.
The positive-control plasma samples were from positive Liberian plasma samples, and the negative controls were Danish plasma samples from Statens Serum Institute (Copenhagen, Denmark).
Statistical analysis.
Data were double entered using Epi Info version 6.0 and analyzed using Stata version 9.2 (Statcorp, TX). Children were considered to have a clinical malaria episode if they had an axillary temperature of ≥37.5°C and parasitemia of ≥5,000 parasites/μl (30). The clinical malaria incidence rates were calculated as the number of episodes divided by the time at risk. For each antigen, negative binomial regression was used to investigate the association between the levels of antibody measured at baseline and the incidence rate of clinical malaria. The levels of total IgG and IgM and each IgG subclass were analyzed for each antigen in turn. The antibody values were transformed to log base 2 so that the rate ratio (RR) would correspond to a doubling of antibody level. To investigate whether the relationship between malaria incidence and antibody level was nonlinear, a likelihood ratio test was used to compare the fit of the model when antibody level was included as a categorical or a continuous variable. When antibody values were 0, indicating levels below the detection limit, two approaches were used. If fewer than 10% of observations were below the detection limit, the 0 values were assigned a nominal value equal to half the smallest measured value for that variable. If 10% or more of the observations were 0 values, then the variable was treated as categorical, with the first category containing the 0 values and the measured values divided into two groups, above and below the median; a likelihood ratio test was used to determine the P value for the association with that antibody. Age at enrolment was considered to be an important potential confounder and was included in the regressions as a factor with categories defined by quintiles. To model seasonality in malaria incidence, the month of surveillance was included in the models as a factor. To avoid double counting of malaria episodes, any episode occurring within 28 days of a primary episode was ignored, and the 28 days after each episode did not contribute to the total time at risk. To explore the impact of presumptive treatments on the results, a time-dependent variable defined to represent the effect of treatment on the risk of malaria was included in the model. To construct a parsimonious model using all the immunological variables, first, a model was produced for each antigen; in this model, each IgG subclass and total IgG and IgM were candidates for inclusion, provided that, when considered singly, the P value for association with malaria incidence was 0.1 or less. Variables were then removed if the P value for the likelihood ratio test was more than 0.1 and provided that removal did not change the coefficients of variables in the model by more than 10%. Second, the variables in these models were combined in a final model in a similar way. In the secondary analyses, the interactions between the immunological variables and the presence of parasitemia at baseline were examined for their effects on malaria incidence; baseline parasitemia was not considered as a potential confounder in the primary analyses, but its effects were considered in secondary analyses. LOESS smoothing was used to produce plots of antibody level against age using R software. The antibody response associations with age were analyzed by Spearman rank correlation.
RESULTS
Characteristics of the study cohort.
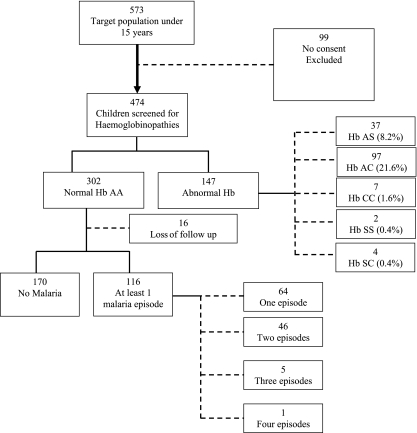
A cohort of 573 children aged 0.5 to 15 years was identified from the demographic surveillance database; 474 agreed to participate and provided a venous blood sample, of which 67.3% were hemoglobin sickle cell-negative genotype AA. Immunological assays were performed on samples from 286 children with the AA hemoglobin genotype who were present at the first surveillance visit and completed the longitudinal surveillance (Fig. 1). Characteristics of the children at baseline are shown in Table 2. The cumulative incidence of malaria was 40% in the 4-month period.
FIG. 1.
Study participant flow chart. Hb, hemoglobin.
TABLE 2.
Characteristics of the study population
| Characteristic | No. (%) of children | Cumulative incidence of clinical malaria | No. of malaria episodes (child yr of risk) | Incidence rate of clinical malaria per child per yr (95% CI) |
|---|---|---|---|---|
| Age (yr) | ||||
| 0-3.5 | 56 (20) | 88% (49/56) | 82 (11.18) | 7.3 (5.9-9.1) |
| 3.6-5.9 | 49 (17) | 53% (26/49) | 40 (11.88) | 3.4 (2.5-4.6) |
| 6.0-7.9 | 51 (18) | 47% (24/51) | 35 (12.92) | 2.7 (1.9-3.8) |
| 8.0-10.9 | 65 (23) | 17% (11/65) | 11 (18.73) | 0.59 (0.33-1.06) |
| 11.0-15 | 65 (23) | 9.2% (6/65) | 7 (18.92) | 0.37 (0.18-0.78) |
| Baseline P. falciparum parasitemia level | ||||
| Negative | 114 (40) | 50% (57/114) | 88 (28.18) | 3.1 (2.5-3.8) |
| Positive | 172 (60) | 34% (59/172) | 87 (45.46) | 1.9 (1.6-2.4) |
| Total | 286 | 41% (116/286) | 175 (73.64) | 2.4 (2.05-2.76) |
Relationship between antibody level and age.
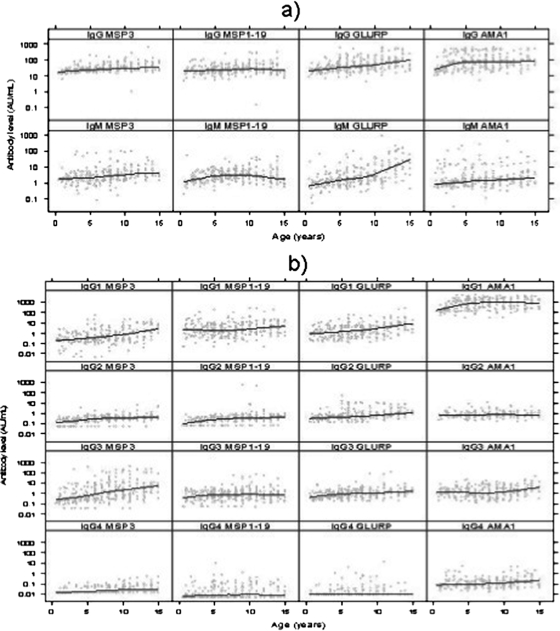
The relationship between antibody level and age differed for the four antigens and for IgG and IgM (Fig. 2a). Levels of IgG to MSP3, MSP1-19, and GLURP increased with increasing age, most steeply in the case of GLURP (Spearman correlation: r = 0.25, P < 0.001 for MSP3; r = 0.16, P = 0.007 for MSP1-19; and r = 0.33, P < 0.001 for GLURP). IgG to AMA1 increased in young children but did not increase in children above the age of 5 (r = 0.05, P = 0.42). The geometric mean levels of IgG were higher for AMA1 and GLURP than for MSP3 and MSP1-19. The levels of IgM to GLURP and MSP1-19 increased with age (r = 0.23, P < 0.001; r = 0.13, P = 0.03, respectively).
FIG. 2.
(a) IgG and IgM levels at baseline in relation to age. The line shows the LOESS smoothed estimate of the geometric mean. (b) IgG subclass levels at baseline in relation to age. The line shows the LOESS smoothed estimate of the geometric mean.
Several of the subclass responses were also found to be associated with age; these included the IgG subclass responses against MSP3 (r = 0.23, P < 0.001; r = 0.27, P < 0.001; r = 0.20, P < 0.001; and r = 0.30, P < 0.001 for IgG1, IgG2, IgG3, and IgG4, respectively), GLURP (r = 0.24, P < 0.001; r = 0.12, P = 0.03 for IgG1 and IgG3, respectively), IgG3 against MSP1-19 (r = 0.13, P = 0.01), and IgG1 (r = 0.20, P < 0.001) and IgG3 (r = 0.23, P < 0.001) against AMA1 (Fig. 2b). The levels of IgG1 to MSP3 and GLURP were at least twice as high in children older than 11 years as in children younger than 10 years. To a lesser extent, the same was true for IgG3. In general, the cytophilic IgGs (IgG1 and IgG3) were predominant over the noncytophilic IgGs (IgG2 and IgG4).
Relationship between levels of total IgG, IgG subclasses, and IgM to MSP3, MSP1-19, GLURP, and AMA1 and subsequent P. falciparum malaria episodes.
A total of 116/286 children (41%) had one or more episodes of malaria. Sixty-four children had one episode, 46 had two episodes, 5 had three episodes, and 1 child had four episodes (Fig. 1). In total, there were 175 episodes of clinical malaria, an incidence rate of 2.4 episodes per child per year (95% confidence interval [CI], 2.0 to 2.8) (Table 2).
In crude (unadjusted) analyses, levels of total IgG to MSP3, GLURP, AMA1, and MSP1-19 were associated with reduced risk of malaria; the association was less marked for AMA1 and MSP1-19 than for MSP3 and GLURP. IgMs to MSP3 and GLURP were associated with reduced malaria incidence, the association for MSP1−19 was weak, and there was no evidence of an association for AMA1 (Table 3).
TABLE 3.
IRRs for the association between total IgG or IgM level and malaria incidence
| Antibody | Antigen | Crude IRR (95% CI)a | IRR adjusted for age (95% CI)a | P value for adjusted RR |
|---|---|---|---|---|
| IgG | MSP3 | 0.42 (0.29-0.59) | 0.77 (0.58-1.01) | 0.062 |
| MSP1-19 | 0.82 (0.65-1.04) | 0.99 (0.82-1.20) | 0.897 | |
| GLURP | 0.56 (0.46-0.69) | 0.79 (0.66-0.94) | 0.009 | |
| AMA1 | 0.86 (0.74-0.99) | 0.96 (0.86-1.08) | 0.532 | |
| IgM | MSP3 | 0.84 (0.72-0.98) | 0.99 (0.87-1.12) | 0.878 |
| MSP1-19 | 0.85 (0.71-1.01) | 1.03 (0.88-1.19) | 0.721 | |
| GLURP | 0.69 (0.61-0.78) | 0.90 (0.80-1.01) | 0.069 | |
| AMA1 | 0.89 (0.79-1.00) | 0.94 (0.86-1.04) | 0.225 |
RRs indicate the ratio of malaria incidence rates associated with a twofold increase in antibody level.
After adjustment for age, the level of total IgG to GLURP was strongly associated with malaria incidence (RR, 0.79; 95% CI, 0.66 to 0.94; P < 0.009); for MSP3 the association was of borderline statistical significance (RR, 0.77; 95% CI, 0.58 to 1.01; P = 0.062); and there was no evidence of an association for total IgG to AMA1 or to MSP1-19 (Table 3).
IgG2 and IgG4, which had large proportions of zero values, were treated as categorical variables, and IgG1 and IgG3 were treated as continuous variables. When these IgG subclasses were considered, none of the subclasses of IgG to MSP3 were significantly associated with malaria incidence; of the subclasses of IgG to GLURP, the association was strongest for IgG3 (RR, 0.82; 95% CI, 0.72 to 0.91; P = 0.004) and IgG4. There was also a significant association for IgG1 to AMA1 (RR, 0.87; 95% CI, 0.78 to 0.97; P = 0.013). When IgM was considered, there was no evidence of an association with malaria incidence for MSP3, MSP1-19, or AMA1, and for GLURP, the association was moderate, with borderline statistical significance (Tables 4 and 5).
TABLE 4.
Age-adjusted IRRs for the association of cytophilic IgGs (IgG1 and IgG3) with malaria incidence
| Antibody | Antigen | IRR adjusted for age (95% CI)a | P value |
|---|---|---|---|
| IgG1 | MSP3 | 0.94 (0.87-1.02) | 0.162 |
| MSP1-19 | 1.05 (0.97-1.14) | 0.233 | |
| GLURP | 0.92 (0.83-1.01) | 0.069 | |
| AMA1 | 0.87 (0.78-0.97) | 0.013 | |
| IgG3 | MSP3 | 0.94 (0.88-1.01) | 0.113 |
| MSP1-19 | 0.94 (0.85-1.05) | 0.293 | |
| GLURP | 0.82 (0.72-0.94) | 0.004 | |
| AMA1 | 1.08 (0.97-1.20) | 0.144 |
Each ratio indicates the change in malaria incidence associated with a twofold increase in antibody level.
TABLE 5.
Age-adjusted IRRs for the association of noncytophilic IgGs (IgG2 and IgG4) with malaria incidence
| Antibody and antigen | IRR (95% CI) at antibody level of:
|
P value | ||
|---|---|---|---|---|
| 0 | <Median (95% CI) | ≥Median (95% CI) | ||
| IgG2 | ||||
| MSP3 | 1 | 0.74 (0.45-1.22) | 0.75 (0.43-1.32) | 0.49 |
| MSP1-19 | 1 | 1.05 (0.67-1.64) | 0.77 (0.46-1.31) | 0.379 |
| GLURP | 1 | 1.05 (0.52-2.1) | 0.67 (0.32-1.40) | 0.079 |
| AMA1 | 1 | 0.91 (0.54-1.55) | 0.87 (0.50-1.52) | 0.74 |
| IgG4 | ||||
| MSP3 | 1 | 0.61 (0.34-1.09) | 0.75 (0.40-1.40) | 0.18 |
| MSP1-19 | 1 | 0.62 (0.39-0.99) | 1.04 (0.66-1.65) | 0.095 |
| GLURP | 1 | 0.51 (0.28-0.91) | 0.48 (0.27-0.85) | 0.004 |
| AMA1 | 1 | 0.61 (0.37-1.00) | 0.58 (0.35-0.98) | 0.102 |
In a combined model in which all immunological variables were considered, only IgG4 to GLURP remained, with a statistically significant association, in the final model; the associations for IgG3 to GLURP (RR, 0.87; 95% CI, 0.76 to 1.00; P = 0.051) and IgG1 to AMA1 (RR, 0.91; 95% CI, 0.82 to 1.01; P = 0.092) were weaker and did not reach statistical significance (Table 6).
TABLE 6.
Adjusted RRs for immunological variables independently associated with malaria risk in the final model
| Immunological variable | Antibody level or no. of yr | Crude IRR (95% CI) | Adjusted IRR (95% CI) | P value from likelihood ratio test | (IRRadj − IRRcrude)/(1 − IRRcrude) (%) |
|---|---|---|---|---|---|
| IgG4 to GLURP | 0 | 1 | 1 | 0.0164 | |
| <Median | 0.36 (0.18-0.70) | 0.57 (0.32-1.02) | |||
| ≥Median | 0.37 (0.19-0.72) | 0.53 (0.30-0.92) | |||
| IgG3 to GLURP | Values transformed to log2 | 0.68 (0.58-0.80) | 0.87 (0.76-1.00) | 0.051 | |
| IgG1 to AMA1 | Values transformed to log2 | 0.75 (0.66-0.85) | 0.91 (0.82-1.01) | 0.092 | |
| Age | 0-3.5 | 1 | 1 | <0.001 | |
| 3.6-5.9 | 0.47 (0.30-0.74) | 0.60 (0.38-0.95) | 25 | ||
| 6.0-7.9 | 0.37 (0.23-0.59) | 0.48 (0.30-0.76) | 18 | ||
| 8.0-10.9 | 0.07 (0.04-0.14) | 0.10 (0.05-0.20) | 3.2 | ||
| 11.0-15 | 0.046 (0.02-0.10) | 0.07 (0.03-0.15) | 2.5 |
To adjust for the effects of presumptive treatment with chloroquine, analyses were repeated with a binary variable, set to 1 for the period of 28 days after each recorded treatment and 0 otherwise, included in the model as a covariate. Treatment was significantly associated with a reduced incidence of clinical malaria in the following 28 days; adjustment did not significantly alter the estimates of the incidence rate ratios (IRRs) for the immunological variables, but attempts to adjust for treatment were limited by the fact that not all treatments were recorded.
The incidence of malaria decreased sharply with age; part of this decrease was explained by the immunological variables in the final model (Table 6), but age remained strongly associated with malaria incidence, indicating that, while the immunological variables explained some of the reduction in malaria incidence with increasing age, substantial unexplained variation remained. There was no evidence of interactions with baseline parasitemia for IgG or IgM to AMA1.
DISCUSSION
The aims of this immunoepidemiological study were to investigate whether the levels of antibodies to four different blood-stage antigens, associated with clinical protection in a number of separate studies (20, 30, 36, 42), were associated with protection from clinical malaria in an area of seasonal and stable malaria transmission in Burkina Faso; to identify the isotypes and/or subclasses of the antibodies most strongly associated with malaria incidence; and to estimate the independent effects of these responses when all were considered in a combined model.
For MSP3, MSP1-19, and GLURP, the levels of total IgG antibodies increased with increasing age, reflecting cumulative exposure to malaria parasites and, possibly, gradual maturation of the immune system over time. These findings confirm those of earlier studies of blood-stage antigens carried out in areas where malaria is endemic (30, 32, 36). However, for IgM, an association with age was evident for GLURP and MSP1-19, but not for AMA1 and MSP3, showing that the induction of these antibodies might differ according to the nature of the antigen and the level of malaria transmission.
Previous studies using human sera from individual members of populations in areas of malaria endemicity have found evidence of an association between the levels of total IgG to MSP3, MSP1-19, and GLURP and a reduced subsequent risk of clinical malaria (5, 20, 30, 39, 42), although results have not been consistent across all studies (19, 42). For GLURP antigens, the IgG antibodies generated were shown to be associated with a lower risk of clinical malaria in the majority of studies, while for MSP3 and MSP1-19, the situation has remained controversial. Our data show that GLURP-specific IgGs were negatively correlated with P. falciparum clinical malaria, but we found no evidence for associations with MSP1-19 and AMA1. There was a borderline statistically significant association for MSP3; the wide CI for the RR indicates that we cannot exclude the possibility that the level of IgG to this antigen is associated with reduced risk of clinical malaria. Previous studies carried out in the same area found that the presence of a positive antibody response to MSP3 and GLURP long synthetic peptides at the beginning of the malaria high transmission season was associated with a reduced risk of clinical malaria (30). As with IgG, IgM antibodies have previously been shown to recognize and bind directly to trophozoite- or schizont-infected red blood cells (33). Although displaying lower affinity for antigen than IgG isotypes, IgM displays increased avidity because of its pentameric structure. Neutralization and agglutination of merozoites and parasitized red blood cells by antibodies have been shown to be possible protective mechanisms during Plasmodium infection (17). In this study, there was no evidence of an association of IgM to MSP3, MSP1-19, or AMA1 with malaria incidence and only weak evidence for IgM to GLURP. These findings conflict with those of previous reports that have suggested a protective role for IgM antibodies against malaria infection (9, 45) and disease severity (13).
Many studies have shown that MSP3, GLURP, and MSP1-19 contain B-cell epitopes that are targeted by cytophilic IgGs, such as IgG1 and IgG3, and, in conjunction with blood mononuclear cells via their FcγRII receptors, trigger the release of killing factors, such as tumor necrosis factor alpha (11, 35, 36, 43). AMA1 is a target of antibodies that prevent parasites from invading red blood cells in vitro (23, 25). Our data showed that antibody responses to these four antigens are predominantly cytophilic, of the IgG1 and IgG3 subclasses; however, only IgG3 to GLURP and IgG1 to AMA1 were associated with a lower risk of subsequent malaria episodes in this study. These data are consistent with those reported by Dodoo et al. in Ghana, Oeuvray et al. in Senegal, and Soe et al. in east Asia, where cytophilic antibody responses were reported to be associated with a reduced risk of clinical malaria (20, 36, 42). In addition, we found a significant negative association between the levels of noncytophilic IgG4 against GLURP and clinical malaria incidence. These epidemiological results are in contrast with the in vitro observation that noncytophilic antibodies can inhibit the bridging of merozoites to human monocytes by cytophilic antibodies against the same antigenic target and thereby reduce the ability of the latter to control parasite multiplication by the ADCI mechanism (10). Furthermore, it has been shown that noncytophilic IgG4 antibodies against blood-stage antigens (GLURP, MSP1, MSP2, and RESA) are associated with enhanced risk of infection and with a high risk of malaria attack (6). IgG4 responses can develop as a result of repeated exposure, as suggested by Aalberse and colleagues, who showed that prolonged immunization results in an IgG4-dominated antibody response (1). Nevertheless, we cannot exclude the possibility that this isotype may be an indicator of a specific cytokine response responsible for protection. This is consistent with previous findings showing a genetic linkage of parasitemia to chromosome 5q31-q33, which contains genes encoding cytokines involved in isotype switching toward IgG4 and in the proliferation, differentiation, and activation of immune system cells (16, 31, 38).
However, the levels of IgG4 and IgG2 detected were low, suggesting a need for further investigations of noncytophilic antibody specificities and their role in protection against malaria.
Antibodies against MSP1-19 were also predominantly of the IgG1 and IgG3 isotypes, in agreement with the results of previous studies from countries in Africa where malaria is endemic (14, 22). In some studies, scientists have found that high levels of anti-MSP1 IgG1 antibodies are associated with protection against malaria attacks (22), whereas in other studies, the scientists failed to observe such an association (19). In our study, cytophilic isotypes against MSP1-19 were not associated with malaria incidence. In east Asia, Soe et al. reported that MSP1-19 IgG and IgG1 subclass responses were predominant in individuals who did not develop malaria (42).
In our study, children were kept under daily active surveillance and were treated presumptively with chloroquine if they had a fever. This reduces malaria incidence and, hence, the power of the study, but is also a potential source of bias if the frequency of treatment is associated with baseline immune responses. Not all treatments were recorded, which limited our attempts to adjust for these effects. Exposure to malaria is an important potential confounder in immunoepidemiological studies, and inadequate measurement and adjustment for differences in exposure may lead to underestimation of the strength of associations between immunological variables and malaria incidence.
In this paper, we have shown that antibody responses against GLURP (IgG3 and IgG4) and AMA1 (IgG1) were associated with reduced clinical malaria incidence. Currently included in malaria vaccine formulations for clinical trials in humans, these two blood-stage antigens offer good prospects for a vaccine since epidemiological and laboratory data suggest that immune responses targeting these antigens are associated with a reduced risk of clinical malaria in many areas with different malaria endemicities. A series of ongoing studies using the same standardized methods will verify this hypothesis in different epidemiological settings. Nevertheless, the best option to confirm whether these observed associations reflect functional protection from malaria remains efficacy trials of the vaccine candidates based on these antigens.
Acknowledgments
We thank the Ministry of Health, Burkina Faso, and the population of the study village for their cooperation. We also acknowledge Shirley Longacre for the kind donation of the recombinant MSP1-19 and Ramadani Noor for helpful comments and critical review of the manuscript. We are grateful to the staff of CNRFP whose participation has made this study possible.
This investigation received support from AMANET and The Netherlands Ministry of Foreign Affairs (DGIS).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Aalberse, R. C., R. van der Gaag, and J. van Leeuwen. 1983. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J. Immunol. 130722-726. [PubMed] [Google Scholar]
- 2.Abdulhadi, N. H. 2003. Protection against severe clinical manifestations of Plasmodium falciparum malaria among sickle cell trait subjects is due to modification of the release of cytokines and/or cytoadherence of infected erythrocytes to the host vascular beds. Med. Hypotheses 60912-914. [DOI] [PubMed] [Google Scholar]
- 3.Aidoo, M., D. J. Terlouw, M. S. Kolczak, P. D. McElroy, F. O. ter Kuile, S. Kariuki, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2002. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 3591311-1312. [DOI] [PubMed] [Google Scholar]
- 4.Allison, A. C. 1954. Protection afforded by sickle-cell trait against subtertian malareal infection. Br. Med. J. 1290-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.al-Yaman, F., B. Genton, K. J. Kramer, S. P. Chang, G. S. Hui, M. Baisor, and M. P. Alpers. 1996. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am. J. Trop. Med. Hyg. 54443-448. [DOI] [PubMed] [Google Scholar]
- 6.Aucan, C., Y. Traore, F. Tall, B. Nacro, T. Traore-Leroux, F. Fumoux, and P. Rihet. 2000. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 681252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audran, R., M. Cachat, F. Lurati, S. Soe, O. Leroy, G. Corradin, P. Druilhe, and F. Spertini. 2005. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect. Immun. 738017-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet, S., S. Petres, I. Holm, T. Fontaine, S. Rosario, C. Roth, and S. Longacre. 2006. Soluble and glyco-lipid modified baculovirus Plasmodium falciparum C-terminal merozoite surface protein 1, two forms of a leading malaria vaccine candidate. Vaccine 245997-6008. [DOI] [PubMed] [Google Scholar]
- 9.Boudin, C., B. Chumpitazi, M. Dziegiel, F. Peyron, S. Picot, B. Hogh, and P. Ambroise-Thomas. 1993. Possible role of specific immunoglobulin M antibodies to Plasmodium falciparum antigens in immunoprotection of humans living in a hyperendemic area, Burkina Faso. J. Clin. Microbiol. 31636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Antibodies in falciparum malaria: what matters most, quantity or quality? Mem. Inst. Oswaldo Cruz 87(Suppl. 3)229-234. [DOI] [PubMed] [Google Scholar]
- 11.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58211-219. [DOI] [PubMed] [Google Scholar]
- 13.Brasseur, P., J. J. Ballet, and P. Druilhe. 1990. Impairment of Plasmodium falciparum-specific antibody response in severe malaria. J. Clin. Microbiol. 28265-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavanagh, D. R., D. Dodoo, L. Hviid, J. A. Kurtzhals, T. G. Theander, B. D. Akanmori, S. Polley, D. J. Conway, K. Koram, and J. S. McBride. 2004. Antibodies to the N-terminal block 2 of Plasmodium falciparum merozoite surface protein 1 are associated with protection against clinical malaria. Infect. Immun. 726492-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chippaux, J. P., A. Massougbodji, J. C. Boulard, and M. Akogbeto. 1992. Morbidity and severity of malaria attacks in carriers of sickle-cell trait. Rev. Epidemiol. Sante Publique 40240-245. (In French.) [PubMed] [Google Scholar]
- 16.Chomarat, P., and J. Banchereau. 1998. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int. Rev. Immunol. 171-52. [DOI] [PubMed] [Google Scholar]
- 17.Cohen, S., and G. A. Butcher. 1971. Serum antibody in acquired malarial immunity. Trans. R. Soc. Trop. Med. Hyg. 65125-135. [DOI] [PubMed] [Google Scholar]
- 18.Cohen, S., I. A. McGregor, and S. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192733-737. [DOI] [PubMed] [Google Scholar]
- 19.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 672131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodoo, D., M. Theisen, J. A. Kurtzhals, B. D. Akanmori, K. A. Koram, S. Jepsen, F. K. Nkrumah, T. G. Theander, and L. Hviid. 2000. Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J. Infect. Dis. 1811202-1205. [DOI] [PubMed] [Google Scholar]
- 21.Druilhe, P., and J. L. Perignon. 1997. A hypothesis about the chronicity of malaria infection. Parasitol. Today 13353-357. [DOI] [PubMed] [Google Scholar]
- 22.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173765-769. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 706948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khusmith, S., and P. Druilhe. 1983. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect. Immun. 41219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kocken, C. H., C. Withers-Martinez, M. A. Dubbeld, A. van der Wel, F. Hackett, A. Valderrama, M. J. Blackman, and A. W. Thomas. 2002. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect. Immun. 704471-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunel, F., and P. Druilhe. 1989. Effector cells involved in nonspecific and antibody-dependent mechanisms directed against Plasmodium falciparum blood stages in vitro. Infect. Immun. 572043-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lusingu, J. P., L. S. Vestergaard, M. Alifrangis, B. P. Mmbando, M. Theisen, A. Y. Kitua, M. M. Lemnge, and T. G. Theander. 2005. Cytophilic antibodies to Plasmodium falciparum glutamate rich protein are associated with malaria protection in an area of holoendemic transmission. Malar. J. 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83293-303. [DOI] [PubMed] [Google Scholar]
- 29.McGregor, I. A. 1964. The passive transfer of human malarial immunity. Am. J. Trop. Med. Hyg. 13(Suppl.)237-239. [DOI] [PubMed] [Google Scholar]
- 30.Meraldi, V., I. Nebie, A. B. Tiono, D. Diallo, E. Sanogo, M. Theisen, P. Druilhe, G. Corradin, R. Moret, and B. S. Sirima. 2004. Natural antibody response to Plasmodium falciparum Exp. -1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol. 26265-272. [DOI] [PubMed] [Google Scholar]
- 31.Metcalf, D. 1991. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science 254529-533. [DOI] [PubMed] [Google Scholar]
- 32.Metzger, W. G., D. M. Okenu, D. R. Cavanagh, J. V. Robinson, K. A. Bojang, H. A. Weiss, J. S. McBride, B. M. Greenwood, and D. J. Conway. 2003. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol. 25307-312. [DOI] [PubMed] [Google Scholar]
- 33.Mota, M. M., K. N. Brown, A. A. Holder, and W. Jarra. 1998. Acute Plasmodium chabaudi chabaudi malaria infection induces antibodies which bind to the surfaces of parasitized erythrocytes and promote their phagocytosis by macrophages in vitro. Infect. Immun. 664080-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odegbemi, J. O., and A. I. Williams. 1995. Immunoglobulin levels in malaria infected Nigerians with and without abnormal haemoglobin. Afr. J. Med. Med. Sci. 2421-25. [PubMed] [Google Scholar]
- 35.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 841594-1602. [PubMed] [Google Scholar]
- 36.Oeuvray, C., M. Theisen, C. Rogier, J. F. Trape, S. Jepsen, and P. Druilhe. 2000. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect. Immun. 682617-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan, W., D. Huang, Q. Zhang, L. Qu, D. Zhang, X. Zhang, X. Xue, and F. Qian. 2004. Fusion of two malaria vaccine candidate antigens enhances product yield, immunogenicity, and antibody-mediated inhibition of parasite growth in vitro. J. Immunol. 1726167-6174. [DOI] [PubMed] [Google Scholar]
- 38.Rihet, P., Y. Traore, L. Abel, C. Aucan, T. Traore-Leroux, and F. Fumoux. 1998. Malaria in humans: Plasmodium falciparum blood infection levels are linked to chromosome 5q31-q33. Am. J. Hum. Genet. 63498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley, E. M., S. J. Allen, J. G. Wheeler, M. J. Blackman, S. Bennett, B. Takacs, H. J. Schonfeld, A. A. Holder, and B. M. Greenwood. 1992. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 14321-337. [DOI] [PubMed] [Google Scholar]
- 40.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45297-308. [DOI] [PubMed] [Google Scholar]
- 41.Sirima, S. B., I. Nebie, A. Ouedraogo, A. B. Tiono, A. T. Konate, A. Gansane, A. I. Derme, A. Diarra, I. Soulama, N. Cuzzin-Ouattara, S. Cousens, and O. Leroy. 2007. Safety and immunogenicity of the Plasmodium falciparum merozoite surface protein-3 long synthetic peptide (MSP3-LSP) malaria vaccine in healthy, semiimmune adult males in Burkina Faso, West Africa. Vaccine 252723-2732. [DOI] [PubMed] [Google Scholar]
- 42.Soe, S., M. Theisen, C. Roussilhon, K. S. Aye, and P. Druilhe. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theisen, M., S. Soe, C. Oeuvray, A. W. Thomas, J. Vuust, S. Danielsen, S. Jepsen, and P. Druilhe. 1998. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect. Immun. 6611-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theisen, M., J. Vuust, A. Gottschau, S. Jepsen, and B. Hogh. 1995. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin. Diagn. Lab. Immunol. 230-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahlgren, M., A. Bjorkman, H. Perlmann, K. Berzins, and P. Perlmann. 1986. Anti-Plasmodium falciparum antibodies acquired by residents in a holoendemic area of Liberia during development of clinical immunity. Am. J. Trop. Med. Hyg. 3522-29. [DOI] [PubMed] [Google Scholar]