Abstract
Trichomonas vaginalis is a protist that causes the most common human sexually transmitted infection. A T. vaginalis cDNA expression library was screened with pooled sera from patients with trichomoniasis. A highly reactive cDNA clone of 1,428 bp encoded a trichomonad protein of 472 amino acids with sequence identity to α-enolase (tv-eno1). The sequence alignment confirmed the highly conserved nature of the enzyme with 65% to 84% identity among organisms. The expression of tv-eno1 was up-regulated by contact of parasites with vaginal epithelial cells, and this is the first report demonstrating up-regulation by cytoadherence of a plasminogen-binding α-enolase in T. vaginalis. Immunofluorescence with monoclonal antibody of nonpermeabilized trichomonads showed tv-ENO1 on the surface. The recombinant tv-ENO1 was expressed in Escherichia coli as a glutathione S-transferase (GST)::tv-ENO1 fusion protein, which was cleaved using thrombin to obtain affinity-purified recombinant tv-ENO1 protein (tv-rENO1) detectable in immunoblots by sera of patients. Immobilized tv-rENO1 bound human plasminogen in a dose-dependent manner, and plasminogen binding by tv-rENO1 was confirmed in a ligand blot assay. The plasminogen-specific inhibitor ɛ-aminocaproic acid blocked the tv-rENO1-plasminogen association, indicating that lysines play a role in binding to tv-rENO1. Further, both parasites and tv-rENO1 activate plasminogen to plasmin that is mediated by tissue plasminogen activator. These data indicate that as with other bacterial pathogens, tv-ENO1 is an anchorless, surface-associated glycolytic enzyme of T. vaginalis.
Trichomonas vaginalis is responsible for the number one, nonviral sexually transmitted disease worldwide (56). There are 250 million new cases of trichomoniasis worldwide and ∼9 million cases in the United States (15, 55, 57). Trichomoniasis is known to increase the portal of entry and exit for human immunodeficiency virus (51). A recent study showed serological evidence linking trichomoniasis with prostate cancer (52). Given the significant human morbidity caused by T. vaginalis, there is a need for identifying virulence factors and elucidating the mechanisms of disease pathogenesis.
A prerequisite for T. vaginalis infection is its ability to colonize the vaginal epithelium. Surface-associated adhesin proteins were shown to be involved in parasite adherence to vaginal epithelial cells (VECs) (4, 19, 20). There is a direct relationship between the amount of surface adhesin that binds to host cells in a ligand-receptor type interaction (8, 20) and the level of cytoadherence (8, 20). Contact with VECs produces a dramatic change in morphology accompanied by synthesis of adhesins (9, 26). A recent study using antisense RNA reaffirmed the importance of AP65 and AP33 in parasite associations with VECs (37, 38). In addition, heterologous expression of AP65 and AP33 on the Tritrichomonas foetus surface provided evidence that both are bona fide adhesins of T. vaginalis (25, 38). Interestingly, these adhesins show sequence identity to metabolic enzymes found in the double-membrane organelle called hydrogenosomes (4, 19). Finally, coordinated up-regulated synthesis and compartmentalization of adhesins outside the hydrogenosomes are modulated by iron (20).
Metabolic enzymes are known to possess alternative functions in addition to glycolysis and play an important role in several biological and pathophysiological processes (48, 49). For example, surface glyceraldehyde-3-phosphate dehydrogenase and α-enolase are without signal sequences and membrane-anchoring motifs and are known to be secreted before reassociation with surfaces of prokaryotic and eukaryotic cells (11, 12, 22, 41). These enzymes exhibit ligand-binding, nonenzymatic functions that play important roles in colonization and invasion (10, 11, 13, 22, 41).
This is the first report demonstrating the surface-associated nature of T. vaginalis α-enolase (tv-ENO1) and showing that tv-ENO1 binds human plasminogen. Synthesis of tv-ENO1 is increased in trichomonads after contact with VECs, and tv-ENO1 is released during normal growth and multiplication of the parasites. Further, plasminogen binds to tv-ENO1, and bound plasminogen is activated to plasmin by tissue plasminogen activator (tPA). These findings suggest a heretofore unknown role of tv-ENO1 during infection. Finally, it is clear that T. vaginalis is a member of the growing list of microbial pathogens and parasites with anchorless, surface-associated enzymes that possess alternative functions.
MATERIALS AND METHODS
Parasite and host cell culture.
T. vaginalis isolate T016 was grown in Trypticase-yeast extract-maltose (TYM) medium with 10% heat-inactivated donor horse serum (18) at 37°C. Trichomonads were labeled with [3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) for 18 h. Immortalized MS-74 human VECs (23) were used for adherence experiments and were grown in Dulbecco's modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum at 37°C in the presence of 5% CO2 as described before (23). For experiments involving contact by trichomonads with host cells, as before (26), parasites at the mid-logarithmic phase of growth (∼18 h) were added to confluent monolayer of MS-74 VECs (10:1 parasite/VEC ratio) and incubated for 30 min at 37°C.
cDNA library screening and analysis of sequence data.
A T. vaginalis isolate T016 cDNA library was constructed in the λ Zap II vector. The library was screened (47) with pooled sera (1:100) from patients with trichomoniasis. After two rounds of screening and plaque purification, phagemids were excised with Exassist interference-resistant helper phage according to the manufacturer's instructions. Sequencing was performed at the Advanced Nucleic Acid Core Facility of the University of Texas Health Science Center at San Antonio. The nucleotide sequence of the cDNA clone was translated into the corresponding amino acid sequence with BioEdit program. The BLAST program was used to find related proteins (7). Sequences were aligned using Clustal W program (53).
RNA isolation and RT-PCR.
Total RNA was isolated from parasites using the Trizol reagent (Invitrogen). For reverse transcription-PCR (RT-PCR), 1 μg of total RNA was reverse transcribed using Superscript II RNase H− reverse transcriptase (Invitrogen) followed by 100 ng of the reverse-transcribed cDNA used as template for the PCRs. The primers used for PCR amplifications of the tv-ENO1 transcript (tv-eno1) were as follows: tv-eno1-sense (S) primer, 5′-CTGGCAAGGACATCATGATC-3′; tv-eno1-antisense (AS) primer, 5′-CAGCGAGATCAGCGATGACG-3′. The α-tubulin-S primer was 5′-ACTCTGCTGCCTCGAGCACGGTATC-3′, and the AS primer was 5′-GAAATGACTGGTGCATAAAGAGC-3′.
Precipitation of released proteins.
Trichomonads (107/ml) at 18 h growth were pelleted and resuspended in RPMI medium (Gibco Invitrogen Cell Culture) and incubated for an additional 1 h at 37°C. Parasites were monitored during growth and incubation periods to assure the absence of cell lysis (2). Supernatant was clarified by gentle centrifugation at 500 × g at 4°C as described before (2, 27). Despite the absence of lysis, the resulting supernatant was filtered through a 0.22-μm-pore-size filter to remove insoluble debris. Filtered supernatant was immediately precipitated using 10% trichloroacetic acid (TCA) (wt/vol) and incubated overnight (o/n) at 4°C. The precipitate was centrifuged for 10 min at 10,000 × g. The pellet was washed twice in 10 ml of acetone and air dried prior to resuspending in electrophoresis dissolving buffer. The protein sample was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% acrylamide followed by immunoblot analysis using the tv-ENO1 monoclonal antibody (MAb).
Autoligand assay.
The autoligand assay was carried out with slight modifications from earlier methods (8, 20). T. vaginalis parasites (2 × 106) were fixed with 3% glutaraldehyde in phosphate-buffered saline (PBS) for 1 h at room temperature (RT) followed by two washes with Ringer's solution. Parasites were blocked with 0.5 M glycine o/n at 4°C, which was followed by two washes with Ringer's solution. These parasites were incubated with whole-cell lysate prepared from 1 × 104 parasites. Another batch of similarly processed T. vaginalis parasites (2 × 106) was incubated with recombinant tv-ENO1 (tv-rENO1) (5 μg). Incubation was continued for 1 h at RT followed by two washes with Ringer's solution. The parasite-associated proteins were eluted by boiling the fixed cells in electrophoresis dissolving buffer for 3 min. Parasite-associated proteins were subjected to SDS-PAGE using 10% acrylamide gels. The proteins were blotted onto Hybond-P membranes (Amersham Pharmacia Biotech) and probed with the tv-ENO1 immunoglobulin G (IgG) MAb. The blots were further incubated with goat anti-mouse IgG secondary antibody. The bands were visualized by using the ECL (enhanced chemiluminescence) kit (Amersham Pharmacia Biotech).
Immunoblot analysis.
TCA-precipitated total proteins from 107 parasites, as before (1), were analyzed by SDS-PAGE (28) using 10% acrylamide gels prior to blotting onto Hybond-P membranes (Amersham Pharmacia Biotech). Blots were blocked in 0.1% Tween 20 and 5% milk powder before probing with tv-ENO1 IgG MAb and processing as described above.
Immunofluorescence detection.
Immunofluorescence of tv-ENO1 on nonpermeabilized trichomonads was carried out using a modification of a recent procedure (20). Briefly, 106 T. vaginalis parasites at the logarithmic phase of growth were washed twice with Ringer's solution and fixed with 2% paraformaldehyde for 10 min at RT. Trichomonads were blocked with 1% bovine serum albumin (BSA) for 1 h at RT prior to incubation for 1 h with hybridoma supernatants with tv-ENO1 MAb diluted 1:100. Parasites were washed with Ringer's solution and incubated for 1 h at 37°C with affinity-purified fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma Chemical Co., St. Louis, MO) diluted 1:1,000. Finally, parasites were washed twice with Ringer's solution and observed under ×100 magnification using the Olympus BX41 microscope.
Expression and purification of GST::tv-ENO1.
tv-rENO1 was expressed using pGEX-4T3 (Novagen, Madison, WI). The 1,416-bp coding region of tv-eno1 was PCR amplified from the cDNA clone T-en-1 using the en-S primer (5′-GATTATGGATCCAACGCCGAGCACGACGCTAT-3′) and en-AS primer (5′-CAT ATAGTCGACTCCTCAGCGAGCATATCG-3′). Gel-purified (Qiagen, Inc., Valencia, CA) PCR product with the BamHI and SalI sites was cloned in-frame with the glutathione S-transferase (GST) coding region of the pGEX-4T3 vector. The Escherichia coli strain DH5α competent cells transformed with expression construct pGEX-4T3-gst-en was screened on LB agar plates containing 100 μg/ml ampicillin. Finally, the correct construction of the expression plasmid referred to as pGEX-4T3-gst-en was confirmed before transformation of E. coli BL21(DE3) competent cells (Novagen). An o/n culture of transformed E. coli BL21(DE3) cells was inoculated at a 1:10 dilution into 200 ml of LB broth with 100 μg/ml ampicillin and cultured at 37°C until the A600 was 0.5 to 1.0. Expression was induced by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 4 h. Bacteria were harvested, and the pellet was washed with 20 ml of PBS-1 mM EDTA and 5 mM dithiothreitol. The pellet was resuspended in 10 ml of PBS-1% Triton X-100 and sonicated four times each at 60 seconds on ice. The GST::tv-ENO1 protein was affinity purified using glutathione Sepharose 4B (Amersham Pharmacia Biotech) per the manufacturer's protocol, and the GST was cleaved from eluted GST::tv-ENO1 by the addition of 80 U of thrombin (Sigma) for each milliliter of glutathione-Sepharose bed volume of eluted fusion protein. The cleavage reaction was stopped by freezing the sample at −20°C. The thawed mixture was subjected to SDS-PAGE using 10% acrylamide gel, and tv-rENO1 was then electroeluted. The purity of the tv-rENO1 was verified by SDS-PAGE and immunoblotting with tv-ENO1 MAb. Blots of tv-rENO1 were also probed using pooled sera from trichomoniasis patients (infected human sera [IHS]) compared to control, uninfected humans (normal human sera).
Plasminogen binding by organisms.
To determine binding to human plasminogen, wells of multiwell plates (Sigma) were each coated with 500 ng of plasminogen (Roche Diagnostic Co., Basel, Switzerland) diluted in carbonate buffer, pH 9.6, and incubated o/n at 4°C. The wells were blocked by PBS-1% BSA for 1 h at 37°C followed by three washes with PBS. Approximately 4 × 105 3H-labeled parasites were resuspended in DMEM-TYM and added to plasminogen-coated wells. After 20 min at 37°C, wells were washed with DMEM-TYM before scintillation counting. This assay was performed on four separate occasions with five replicates. This assay was very reproducible, and values did not vary by more than 5% of the mean.
An enzyme-linked immunosorbent assay (ELISA) was performed to determine the plasminogen-binding ability of tv-rENO1. Wells of multiwell plates (Nunc) were each coated with 1 μg of tv-rENO1 prepared in carbonate buffer, pH 9.6. Where necessary, control wells were each coated with 1 μg of BSA. The plates were incubated o/n at 4°C. The wells were blocked by PBS-1% BSA followed by three washes with PBS-0.1% Tween 20. Different concentrations (1 μg to 4 μg) of the human plasminogen (Roche Diagnostic) diluted with PBS-1% BSA were added to the tv-rENO1-coated wells and incubated for 1 h at 37°C. Competition experiments were also performed by the addition of the 40 mM lysine analogue ɛ-aminocaproic acid (ɛ-ACA) (Sigma) prepared in water or 1% BSA in PBS as a control for different concentrations of plasminogen before the addition to wells in the binding assay. Further, different concentrations of ɛ-ACA were used in experiments with wells coated with 1 μg tv-rENO1. In this case, ɛ-ACA was incubated for 1 h followed by the addition of plasminogen (2 μg). All reactions were carried out at 37°C for 1 h after which wells were washed twice in PBS-0.1% Tween 20. Binding was determined by incubation for 1 h at 37°C with anti-plasminogen IgG antibody. After the wells were washed twice with PBS-0.1% Tween 20, they were then incubated with horseradish peroxidase for 30 min at 37°C, and color was measured at A450. The concentrations of ɛ-ACA used in our experiments are consistent with those used for other bacterial pathogens (40) and parasites (45). After the wells were washed three times with PBS-0.1% Tween 20, 100 ng horseradish peroxidase-conjugated mouse anti-human plasminogen IgG (R&D Systems, Inc., Minneapolis, MN) was added to each well and incubated at 37°C for 1 h. ELISA was read at A450 using a microplate reader (Bio Tek Instruments Inc., Winooski, VT).
Ligand blot analysis.
To study the binding ability of tv-ENO1 to human plasminogen, tv-rENO1 and plasminogen were electrophoresed by SDS-PAGE with 10% acrylamide gels and blotted onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The nitrocellulose membrane was blocked o/n at 4°C with 5% (wt/vol) nonfat dry milk in PBS-0.1% Tween 20. After the membrane was washed extensively with PBS-0.1% Tween 20, it was incubated o/n at 4°C with human plasminogen (35 μg/ml) in PBS-1% BSA. After the blot was washed, it was incubated with mouse anti-human plasminogen MAb (1 μg/ml) (R & D Systems) prepared in PBS-1% BSA for 1 h at RT, followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG (Sigma) (1:2,000 [vol/vol]), and bands were detected by using the ECL kit (Amersham Pharmacia Biotech).
Plasminogen activation assay.
Plasminogen activation was performed by measuring the amidolytic activity of generated plasmin. The reaction mixture in each well of 96-well plates contained 2 μg human plasminogen (Roche Diagnotics), 1 μg of pure tv-ENO1, or 1 × 105 parasites and 3 μg of plasmin substrate (d-valyl-l-lysyl-p-nitroaniline hydrochloride) (Sigma), 15 ng tPA (Sigma), and PBS to a final volume of 150 μl. In parallel control experiments, generation of the plasmin was determined in either the absence of tPA or in the presence of ɛ-ACA. The effects of trichomonad cysteine proteinases were determined by the addition of 1 mM each of inhibitors Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) and E64 (both from Sigma), as before (20, 50). Plates were incubated at RT for 2 h before the measurement of A405.
RESULTS
Isolation of α-enolase cDNA and sequence comparisons.
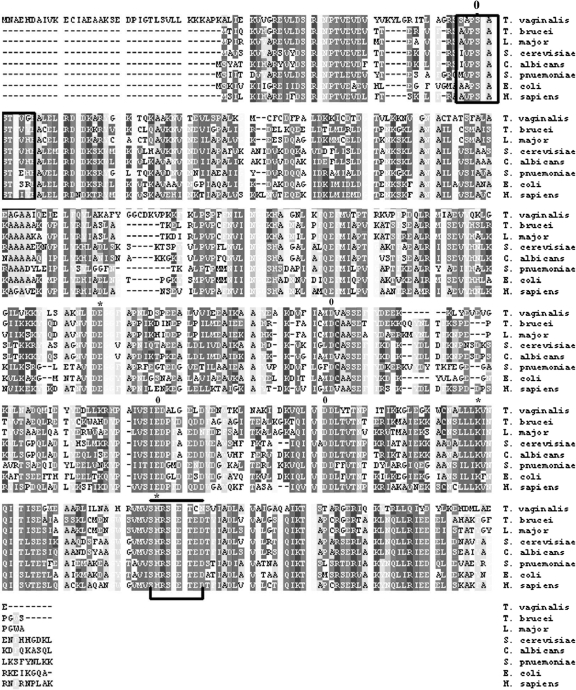
We screened a cDNA expression library with sera from patients. Two clones with overlapping sequence were highly reactive and characterized further. One full-length cDNA clone consisted of 1,428 bp with an open reading frame encoding 472 amino acids with a calculated molecular mass of 52 kDa. This protein showed a high degree of amino acid sequence identity with the proteins encoded by many other enolase genes available in GenBank. A comparison of the amino acid sequence of the T. vaginalis α-enolase with the sequences of similar enzymes of other organisms is shown in Fig. 1. The highest level of identity (84%) was scored with Saccharomyces cerevisiae followed by 76% with Streptococcus pneumoniae. The crucial amino acid residues for α-enolase activity have been identified in S. cerevisiae as Glu 211, Lys 345, and His 373 (59). The corresponding residues in tv-ENO1 are Glu 255, Lys 389, and His 417. In S. cerevisiae enolase, Ser 39, Asp 246, Glu 295, and Asp 320 are complexed to magnesium ions, corresponding to tv-ENO1 Ser 78, Asp 292, Glu 337, and Asp 364. Analysis of the sequence revealed a domain similar to the internal plasminogen-binding site found in S. pneumoniae α-enolase (FYDKERKVY) that is also found in the T. vaginalis sequence (FYDEEKKLY) between positions 298 and 306. This indicates that tv-ENO1 binds to plasminogen.
FIG. 1.
Sequence analyses of T. vaginalis α-enolase. T. vaginalis tv-ENO1 was compared to the enolase enzymes of parasites, fungi, bacteria, and humans. The amino acid sequence identity values of T. vaginalis tv-ENO1 to the enolase enzymes of Trypanosoma brucei, Leishmania major, Saccharomyces cerevisiae, Candida albicans, Streptococcus pneumoniae, E. coli, and Homo sapiens as listed vertically were 74%, 69%, 84%, 65%, 76%, 65%, and 67%, respectively. Active sites are marked with an asterisk (amino acids E255, K389, and H417), and the positions of metal-binding residues are labeled zero (amino acids S78, D292, E337, and D364). The enolase finger print motif is boxed within amino acids 75 to 84, and the domain for hydrophobic putative signal peptides is indicated by a bar and a bracket (amino acids 416 to 423). Gaps introduced to maximize alignment are indicated by dashes.
Parasite contact with VEC signals for elevated tv-eno1 expression and tv-ENO1 synthesis.
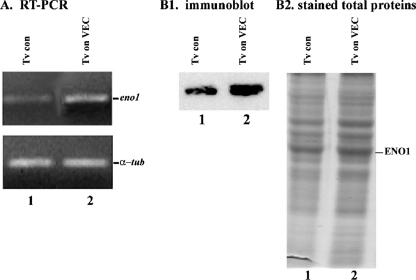
Contact of T. vaginalis with VECs results in increased synthesis and surface placement of adhesins (9, 20, 26). We, therefore, tested whether contact by trichomonads with MS-74 VECs influenced tv-eno1 transcription. As seen in Fig. 2A (lane 2), T. vaginalis organisms after contact had increased amounts of tv-eno1, as evidenced by larger amounts of transcript as detected by RT-PCR compared to parasites alone as a control (lane 1). Using primers specific to α-tubulin as a control, we showed equal amounts of RNA added to each sample. Further, we showed increased amounts of tv-ENO1 detected by tv-ENO1 MAb in total proteins of trichomonads after contact with VECs (Fig. 2, panel B1, lane 2) compared to proteins of control organisms handled similarly except without contact (lane 1). The increased amount of tv-ENO1 is also evident in stained total proteins in duplicate protein preparations as seen in Fig. 2, panel B2. The total protein patterns were derived from identical numbers of parasites.
FIG. 2.
Analysis of T. vaginalis α-enolase expression. (A) Increased amounts of tv-eno1 transcript by RT-PCR from trichomonads after contact with immortalized MS-74 VECs (Tv on VEC) (lane 2) were compared to parasites without contact (Tv control [Tv con]) (lane 1), as evidenced by ethidium bromide staining of gels after 1% agarose gel electrophoresis. Equal quantities of α-tubulin (α-tub) RT-PCR product showed equal amounts of RNA in the PCR. (B) Increased amount of tv-ENO1 detected by tv-ENO1 MAb in total proteins of trichomonads after contact with VECs (panel B1, lane 2) compared to control organisms (lane 1). The increased amount of tv-ENO1 is also evident in stained total proteins in duplicate protein preparations as seen in panel B2. The total protein patterns were derived from identical numbers of parasites.
tv-ENO1 released from trichomonads reassociates with parasites.
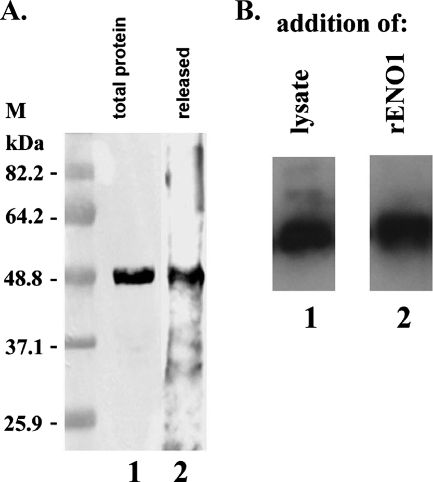
From our earlier studies (27), we found that trichomonad metabolic enzymes, including α-enolase, were released. To affirm earlier results, we performed immunoblot analysis of the TCA-precipitated proteins in spent medium using tv-ENO1 MAb. The results once again revealed that tv-ENO1 is released from the parasite (Fig. 3A, lane 2). We next verified the association of the released tv-ENO1 with trichomonads in an autoligand assay. Glutaraldehyde-fixed T. vaginalis was incubated with both parasite lysates and tv-rENO1, and unbound proteins were removed by washing. Results in Fig. 3B show that tv-ENO1 MAb reacted strongly both with protein of lysate (lane 1) and tv-rENO (lane 2) that bound to fixed trichomonads used in the assay. These data demonstrate that tv-ENO1 is associated with the surfaces of parasites.
FIG. 3.
The tv-ENO1 is released and parasite associated. (A) Total proteins from T. vaginalis parasites and TCA-precipitated released proteins were electrophoresed on an SDS-polyacrylamide gel with 10% acrylamide, and the proteins were blotted onto a Hybond-P membrane. Immunoblot analysis using tv-ENO1 MAb detected tv-ENO1 from TCA-precipitated proteins released under conditions where no lysis was evident (lane 2) (27). Parasite total TCA-precipitated proteins were used as a control (lane 1). The positions of molecular mass markers (M) (in kilodaltons) are indicated to the left of the gel. (B) Glutaraldehyde-fixed T. vaginalis parasites were incubated with a lysate of total proteins from parasites (lane 1) or with tv-rENO1 (lane 2). Immunoblotting using tv-ENO1 MAb of the protein bound to and removed from these fixed parasites was performed. Association of tv-ENO1 was detected in fixed parasites incubated with both whole-cell lysate (lane 1) and tv-rENO1 (lane 2), indicating association of ENO1 with the parasite surface.
tv-ENO1 is on the T. vaginalis surface.
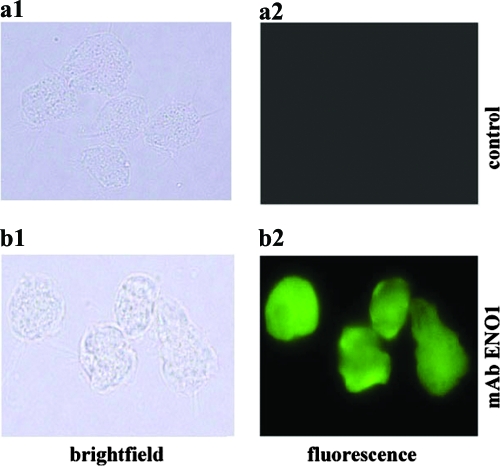
Next we performed immunofluorescence on washed, mid-logarithmic trichomonads from o/n batch culture. As shown in the representative experiments in Fig. 4 (panel b2), the tv-ENO1 MAb reacted strongly with the surfaces of nonpermeabilized organisms. No fluorescence and immunoreactivity was ever detected by secondary antibody incubation with parasites (panel a2).
FIG. 4.
Fluorescence detection of tv-ENO1 on the surface of nonpermeabilized T. vaginalis. Paraformaldehyde-fixed, nonpermeabilized cells were treated with tv-ENO1 MAb, which was detected with FITC-conjugated anti-mouse IgG (panel b2). For controls, nonpermeabilized T. vaginalis parasites were treated with secondary antibody only, and no fluorescence was detected (panel a2). Bright-field microscopy shows the trichomonads in the field of fluorescence (panels a1 and b1).
Expression of GST::tv-ENO1, purification of tv-rENO1, and immunogenicity.
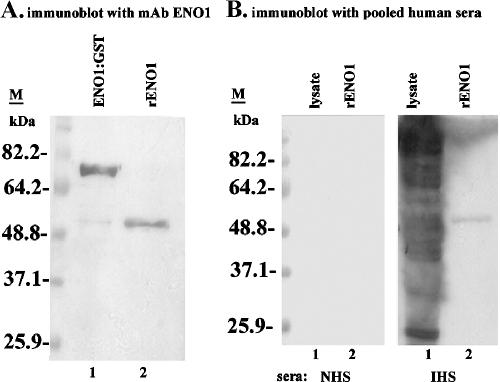
We then expressed GST::tv-ENO1 in E. coli. The fusion protein was affinity purified, and tv-rENO1 was obtained by digestion with thrombin. Figure 5A shows the results of immunoblot analysis using tv-ENO1 MAb as a probe to confirm expression of the fusion protein (lane 1) and purification of the tv-rENO1 after thrombin cleavage of tv-rENO1 (lane 2). Availability of purified tv-rENO1 permitted us to test directly for antibody in sera of patients. As shown in Fig. 5B, the tv-rENO1 was recognized by IgG antibodies from a preparation of pooled sera derived from 10 patients (IHS blot, lane 2). Similar reactivity was seen with the individual serum samples from patients (not shown). Sera from normal human subjects (NHS blot, lane 1) with no known history of T. vaginalis infection neither detected purified tv-rENO1 (lane 2) nor native ENO1 in lysate (lane 1). As expected, IHS reacted with numerous trichomonad proteins in total lysate after SDS-PAGE and immunoblotting of total TCA-precipitated proteins.
FIG. 5.
Purification of tv-rENO1 and antibody for tv-ENO1 in patient sera. (A) Recombinant GST::tv-ENO1 fusion protein (lane 1) and fusion protein digested with thrombin (lane 2) were electrophoresed on an SDS-polacrylamide gel with 10% acrylamide and blotted onto a Hybond-P membrane. The fusion protein and purified tv-rENO1 were detected using tv-ENO1 MAb. No detection of protein is evident in controls with secondary antibody alone. The positions of molecular mass markers (M) (in kilodaltons) are presented to the left of the gel. (B) Total proteins (lane 1) from T. vaginalis parasites and tv-rENO1 (lane 2) were electrophoresed by SDS-PAGE using 10% acrylamide. Blots were probed with pooled sera from patients (IHS) versus control sera of uninfected humans (normal human sera [NHS]).
T. vaginalis surface tv-ENO1 and tv-rENO1 binds plasminogen.
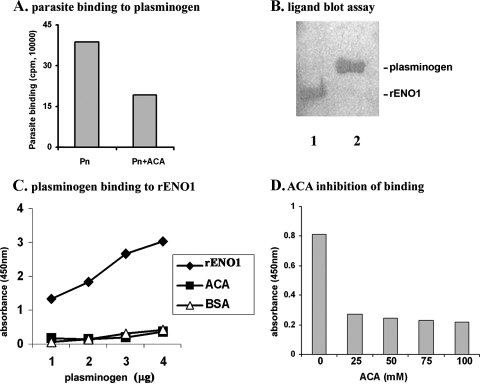
We next wanted to know whether parasites with surface tv-ENO1 bound to immobilized plasminogen. In this assay, optimal associations were obtained by 105 organisms, and Fig. 6A presents binding of 105 3H-labeled cells to wells coated with 500 ng of plasminogen (bar Pn). Further, there was a reduction in parasite binding upon incubation with ɛ-ACA (bar Pn+ACA), suggesting that binding to plasminogen is lysine dependent. We next performed a ligand blot assay and incubated blotted tv-rENO1 with plasminogen followed by detection with MAb to plasminogen. Figure 6B (lane 1) shows plasminogen bound to immobilized tv-rENO1 as detected with MAb. For a control and as expected, lane 2 shows MAb detection to blotted purified plasminogen.
FIG. 6.
Plasminogen binding assay. (A) T. vaginalis parasites bind to human plasminogen. 3H-labeled T. vaginalis cells (1 × 105) were incubated with immobilized plasminogen (Pn) for 20 min at 37°C as mentioned in Materials and Methods. (B) Plasminogen binds to tv-rENO1. The tv-rENO1 (lane 1) and human plasminogen as a control (lane 2) were electrophoresed by SDS-PAGE on a 10% acrylamide gel. After electrophoresis, proteins were blotted onto a Hybond-P membrane and incubated with plasminogen (35 μg/ml). The blot was probed with anti-plasminogen IgG antibody followed by secondary antibody. The bands indicate plasminogen bound to tv-rENO1 immobilized on the membrane (lane 1). (C) Plasminogen binds to tv-rENO1 immobilized on microtiter well plates in a concentration-dependent manner. ELISA was performed in a multiwell plate coated with tv-rENO1 (1 μg/well) and increasing amounts of human plasminogen (1 μg to 4 μg). In a competition assay, 40 mM ɛ-ACA was included during incubation with plasminogen. Control wells lacked tv-rENO1 and were coated with only BSA (1 μg well). The assay was performed at A450 using antibody to plasminogen. (D) Effects of different ɛ-ACA concentrations on plasminogen binding. Various concentrations of ɛ-ACA (25 mM to 100 mM) were added to wells containing 1 μg immobilized tv-rENO1, followed by the addition of plasminogen, and ELISA was performed as described in Materials and Methods. The results represent the averages from four independent experiments with quadruplicate samples. These experiments were very reproducible and never varied by more than 5% of the average shown here.
An ELISA was performed using immobilized tv-rENO1 and increasing amounts of plasminogen. Figure 6C shows the expected concentration-dependent binding of plasminogen to tv-rENO1-coated wells. For controls, wells coated only with BSA showed negligible, nonspecific binding of plasminogen, and importantly, the addition of 40 mM ɛ-ACA inhibited plasminogen binding to tv-rENO1, suggesting strongly that binding of plasminogen to tv-rENO1 is lysine dependent. Further, wells coated with tv-rENO1 were incubated with 2 μg plasminogen and increasing amounts of ɛ-ACA. As seen in Fig. 6D, ɛ-ACA effectively inhibited binding of plasminogen to tv-rENO1, as evidenced by decreased A450 at 25 mM, and only slightly greater inhibition was seen with a higher concentration of ɛ-ACA (Fig. 6D). These data suggest that surface tv-ENO1 of T. vaginalis and the tv-rENO1 bind in a specific fashion to plasminogen.
Plasminogen is activated by parasites and tv-rENO1.
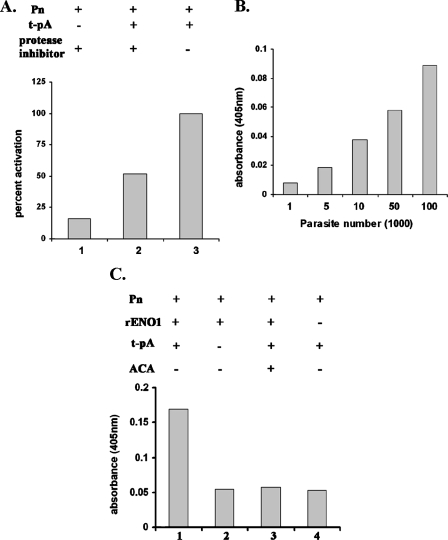
Finally, we performed an activation assay to determine the ability of trichomonad tv-ENO1 and tv-rENO1 to produce plasmin from plasminogen. In the absence of protease inhibitors, parasites mediated activation of plasminogen in the presence of tPA (Fig. 7A, bar 3), and the A405 value was normalized to 100%. Not surprisingly, addition of 1 mM each of TLCK and E64, inhibitors to the numerous cysteine proteinases of T. vaginalis (20, 50), gave a reduction in the amount of plasmin generated (bar 2), indicating a role for trichomonad proteases in plasminogen activation. Importantly, the absence of tPA yielded minimal detectable plasmin generation (bar 1). Further, the activation of plasminogen is directly proportional to the number of parasites in the reaction mixture (Fig. 7B). In addition, an experiment was done with plasminogen in the presence of tPA and tv-rENO1. Figure 7C (bar 1) shows that indeed tv-rENO1 activates plasminogen compared to reaction mixtures lacking tPA (bar 2) and tv-rENO1 (bar 4). Last, the addition of ɛ-ACA inhibited plasmin generation (bar 3). These results suggest strongly that live organisms and purified rENO1 mediate the activation of plasminogen to plasmin.
FIG. 7.
Activation of human plasminogen. (A) T. vaginalis parasites convert plasminogen into plasmin in the presence of tPA. Briefly, 1 × 105 parasites were incubated (+) with 2 μg of plasminogen (Pn) for 2 h at 37°C under different conditions as described in Materials and Methods. TLCK and E64 (each at 1 mM) were used to inhibit the cysteine proteinases of T. vaginalis. Plasmin generation was measured by reading at A405. (B) Plasminogen activation with various numbers of T. vaginalis parasites. The plasminogen activation assay was carried out with different parasite numbers (1 × 104 to 1 × 106) as mentioned in Materials and Methods. (C) The tv-rENO1 generates plasmin from plasminogen. Briefly, tv-rENO1 (1 μg) was incubated with plasminogen (2 μg), and plasmin formation was measured as mentioned in Materials and Methods. It is noteworthy that the results in panels A, B, and C are the averages of four different experiments with each experiment performed with quadruplicate samples. As mentioned in the legend to Fig. 6, readings never varied more than 5% of the mean as presented.
DISCUSSION
It is now appreciated that the non-self-limiting nature of trichomoniasis occurs in the exceedingly complex and constantly changing urogenital tracts of women, as evidenced by the menstrual cycle with fluctuations in pH, iron, and nutrients, the presence of blood components during menses, and the desquamation of the VECs. Preparatory to contact with VECs (8), trichomonads must go through the mucous layer (31), and specific associations with the extracellular matrix proteins fibronectin and laminin (6, 14, 17) may indicate penetration to the basement membrane. Trichomonal cytotoxicity of vaginal and cervical epithelial cells and the vaginal discharge following infection may be critical obstacles for successful host colonization by the parasite. It is known that secretions of patients contain numerous trichomonad proteins, including high-molecular-weight immunogenic proteins (3) and cysteine proteinases (5). Further, it is known that numerous proteins are readily released during growth, and multiplication without lysis of organisms has been established (2, 27).
In this report we readily obtained by screening a cDNA expression library with a pooled sera a full-length cDNA clone that encodes the T. vaginalis glycolytic enzyme α-enolase (tv-ENO1). The amino acid sequence has identity to α-enolase from a diverse group of organisms (Fig. 1). The enzyme contains the active site and conformation-determining metal ion-binding site residues necessary for dimerization and enzyme function. A unique feature of the T. vaginalis enzyme is a 38-amino-acid extension (amino acids 1 to 38) in the amino terminus that does not show homology to other known sequences. Analysis of the sequence revealed that a domain similar to the internal plasminogen-binding site found in S. pneumoniae α-enolase (FYDKERKVY) is also found in T. vaginalis (FYDEEKKLY) between positions 298 and 306.
There was an elevated expression of T. vaginalis tv-eno1 upon parasite contact. We showed earlier that brief contact of trichomonads with VECs produced dramatic changes in parasite morphology (9) in addition to synthesis of adhesins and other trichomonad proteins (26), suggesting host-specific signaling of parasites. More recently, we showed that parasite contact with VECs (24) and the released proteins of T. vaginalis (27), the majority of which were metabolic enzymes, including tv-ENO1, induced expression of numerous VEC genes. We now confirm that T. vaginalis tv-ENO1 is released and at the same time is present on the parasite surface. Further, in an autoligand assay, we demonstrate the ability of purified rENO1 to associate with the parasite surface, making tv-ENO1 another member of the family of anchorless, surface-associated trichomonad proteins and consistent with the detection by fluorescence with ENO1 MAb of nonpermeabilized organisms.
Enolase is a ubiquitous, glycolytic enzyme (2-phospho-d-glycerate hydrolase) that catalyzes the dehydration of 2-phospho-d-glycerate (PGA) to phosphoenolpyruvate (PEP) in glycolysis, and the same enzyme catalyzes hydration of PEP to PGA during gluconeogenesis. For many years, enolase was regarded solely as a soluble cytosolic glycolytic enzyme. However, recent studies have shown that enolase is a protein with diverse distribution and biological functions (41). For example, α-enolase is a heat shock protein and is capable of binding to cytoskeleton and chromatin structures to modulate transcription. It is known that α-enolase is also released in other microorganisms and yeast and is present on the surfaces of a diverse group of organisms, including bacteria, parasites, and mammalian cells (10, 12, 21, 22, 41). α-Enolase plays a role in invasion of host tissue by pathogens (41) and exhibits adhesive function (10, 12, 16, 41). Recent studies have indicated that α-enolase present in the cell walls of Staphylococcus aureus, S. pneumoniae, and Streptococcus pyogenes binds and activates plasminogen (10, 35, 40) and influences Streptomyces adherence to human pharyngeal cells (42). Therefore, it is not altogether inconceivable, given this precedence, that tv-ENO1 may play an adhesive role in trichomonads, in addition to that of known adhesins (4, 19, 20). α-Enolase has been implicated as playing a role in pathophysiological processes, especially through the presence of autoantibodies (40, 41), and although the exact role of these autoantibodies in disease is unclear, our finding of antibody to tv-ENO1 in the sera of patients merits careful attention.
Our study shows that both T. vaginalis parasites and tv-rENO1 bind human plasminogen and release plasmin. Previous studies in T. vaginalis have shown that parasites can bind to fibronectin and laminin, but not to collagen (14, 17). Plasminogen is an abundant single-chain glycoprotein of 92 kDa which is present in human plasma, milk, secretions, and saliva (36, 39, 54). Plasminogen binds to the carboxy-terminal lysines of the cell surface receptors via its lysine-binding sites (44). Plasminogen activation with subsequent generation of plasmin, a serine protease, is mediated by the proteolytic activity of urokinase-type plasminogen activator or tPA (33). The major biological function of plasmin is to degrade fibrin and extracellular matrices of basement membranes (44). It has been established that α-enolase is a receptor and activator of plasminogen in pathogenic eukaryotic and prokaryotic cells (10, 21, 22, 41). Recently, a secreted form of α-enolase in S. pneumoniae and the reassociation of secreted enolase with the bacterial cell surface, leading to increased plasminogen-binding activity, has been reported (10). Plasminogen activation is responsible for the degradation of intravascular clots and extracellular proteolysis in a wide variety of physiological and pathological processes (43, 44, 46). Interestingly, recent reports have pointed to an enhanced activation of plasminogen upon interaction with the pathogen-derived α-enolase (22, 28-30, 34, 58). The ability to bind plasminogen by pathogenic bacteria provides the microorganism with invasive properties (16, 30, 32, 34). Therefore, for T. vaginalis, plasminogen binding and plasmin generation could facilitate T. vaginalis penetration to the basement membrane to permit associations with fibronectin and laminin. This would ensure the parasite access to growth factors and nutrients via localized proteolytic activity and contribute to the non-self-limiting nature of infection (33). Finally, while a role for vascular damage by T. vaginalis plasmin generation is unknown, the existence of serum antibody to trichomonad proteins in the sera of patients may be the result of sufficient damage to vasculature that permits surveillance by the host immune system. This work now establishes the T. vaginalis α-enolase as a new surface-associated virulence factor.
Acknowledgments
This work was supported by Public Health Service grants AI43940 and AI45429 from the National Institutes of Health.
Members of the laboratory are also acknowledged for their suggestions and discussion of our work.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Alderete, J. F. 1983. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect. Immun. 391041-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete, J. F., and G. E. Garza. 1984. Soluble Trichomonas vaginalis antigens in cell-free culture supernatants. Mol. Biochem. Parasitol. 13147-158. [DOI] [PubMed] [Google Scholar]
- 3.Alderete, J. F., E. Newton, C. Dennis, and K. A. Neale. 1991. The women infected with Trichomonas vaginalis have numerous proteinases and antibody to trichomonal proteinases. Genitourin. Med. 67469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderete, J. F., J. L. O'Brien, R. Arroyo, J. A. Engbring, O. Musatovova, O. Lopez, C. Lauriano, and J. Nguyen. 1995. Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 1769-83. [DOI] [PubMed] [Google Scholar]
- 5.Alderete, J. F., and D. Provenzano. 1997. The vagina has reducing environment sufficient for activation of Trichomonas vaginalis cysteine proteinases. Genitourin. Med. 73291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alderete, J. F., M. Benchimol, M. W. Lehker, and M. L. Crouch. 2002. The complex fibronectin-Trichomonas vaginalis interactions and trichomonosis. Parasitol. Int. 51285-292. [DOI] [PubMed] [Google Scholar]
- 7.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arroyo, R., J. Engbring, and J. F. Alderete. 1992. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol. Microbiol. 6853-862. [DOI] [PubMed] [Google Scholar]
- 9.Arroyo, R., A. Gonzalez-Robles, A. Martinez-Palomo, and J. F. Alderete. 1993. Signaling of Trichomonas vaginalis for amoeboid transformation and adhesin synthesis follows cytoadherence. Mol. Microbiol. 7299-309. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 401273-1287. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2004. Glyceraledyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 722416-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernal, D., J. E. de la Rubia, A. M. Carrasco-Abad, R. Toledo, S. Mas-Coma, and A. Marcilla. 2004. Identification of enolase as a plasminogen-binding protein in excretory-secretory products of Fasciola hepatica. FEBS Lett. 563203-206. [DOI] [PubMed] [Google Scholar]
- 13.Boël, G., H. Jin, and V. Pancholi. 2005. Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties. Infect. Immun. 736237-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casta e Silva Filho, F., W. de Souza, and J. D. Lopes. 1988. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc. Natl. Acad. Sci. USA 858042-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cates, W., Jr., and The American Social Health Association Panel. 1999. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex. Transm. Dis. 26S2-S7. [DOI] [PubMed] [Google Scholar]
- 16.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Dengen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 891111-1119. [DOI] [PubMed] [Google Scholar]
- 17.Crouch, M. L., and J. F. Alderete. 1999. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology 1452835-2843. [DOI] [PubMed] [Google Scholar]
- 18.Diamond, L. S. 1957. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 43488-490. [PubMed] [Google Scholar]
- 19.Engbring, J. A., and J. F. Alderete. 1998. Characterization of Trichomonas vaginalis AP33 adhesin and cell surface interactive domains. Microbiology 1443011-3018. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, A. F., T. H. Chang, M. Benchimol, D. J. Klumpp, M. W. Lehker, and J. F. Alderete. 2003. Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis. Mol. Microbiol. 471207-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolodar, A., P. Fischer, S. Bergmann, D. W. Buttner, S. Hammerschmidt, and N. W. Brattig. 2003. Molecular cloning of an alpha-enolase from the human filarial parasite Onchocerca volvulus that binds human plasminogen. Biochim. Biophys. Acta 1627111-120. [DOI] [PubMed] [Google Scholar]
- 22.Jong, A. Y., S. H. Chen, M. F. Stins, K. S. Kim, T. L. Tuan, and S. H. Huang. 2003. Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J. Med. Microbiol. 52615-622. [DOI] [PubMed] [Google Scholar]
- 23.Klumpp, D. J., S. G. Forrestal, J. E. Karr, C. S. Mudge, B. E. Anderson, and A. J. Schaeffer. 2002. Epithelial differentiation promotes the adherence of type 1-piliated Escherichia coli to human vaginal cells. J. Infect. Dis. 1861631-1638. [DOI] [PubMed] [Google Scholar]
- 24.Kucknoor, A., V. Mundodi, and J. F. Alderete. 2005. Trichomonas vaginalis adherence mediates differential gene expression in human vaginal epithelial cells. Cell. Microbiol. 7887-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucknoor, A. S., V. Mundodi, and J. F. Alderete. 2005. Heterologous expression in Tritrichomonas foetus of functional Trichomonas vaginalis AP65 adhesin. BMC Mol. Biol. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kucknoor, A. S., V. Mundodi, and J. F. Alderete. 2005. Adherence to human vaginal epithelial cells signals for increased expression of Trichomonas vaginalis genes. Infect. Immun. 736472-6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucknoor, A. S., V. Mundodi, and J. F. Alderete. 2007. The proteins secreted by Trichomonas vaginalis and vaginal epithelial cell response to secreted and episomally expressed AP65. Cell. Microbiol. 92586-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lahteenmaki, K., P. Kuusela, and T. K. Korhonen. 2001. Bacterial plasminogen activators and receptors. FEMS Microbiol. Rev. 25531-552. [DOI] [PubMed] [Google Scholar]
- 30.Lähteenmäki, K., R. Virkola, R. Pouttu, P. Kuusela, M. Kukkonen, and T. K. Korhonen. 1995. Bacterial plasminogen receptors: in vitro evidence for a role in degradation of the mammalian extracellular matrix. Infect. Immun. 633659-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehker, M. W., and D. Sweeney. 1999. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex. Transm. Infect. 75231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leigh, J. A., and R. A. Lincoln. 1997. Streptococcus uberis acquires plasmin activity following growth in the presence of bovine plasminogen through the action of its specific plasminogen activator. FEMS Microbiol. Lett. 154123-129. [DOI] [PubMed] [Google Scholar]
- 33.Lijnen, H. R. 2001. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb. Haemost. 86324-333. [PubMed] [Google Scholar]
- 34.McCoy, H. E., C. C. Broder, and R. Lottenberg. 1991. Streptokinases produced by pathogenic group C streptococci demonstrate species-specific plasminogen activation. J. Infect. Dis. 164515-521. [DOI] [PubMed] [Google Scholar]
- 35.Molkanen, T., J. Tyynela, J. Helin, N. Kalkkinen, and P. Kuusela. 2002. Enhanced activation of bound plasminogen on Staphylococcus aureus by staphylokinase. FEBS Lett. 51772-78. [DOI] [PubMed] [Google Scholar]
- 36.Moody, G. H. 1982. Plasminogen in human saliva. Int. J. Oral Surg. 11110-114. [DOI] [PubMed] [Google Scholar]
- 37.Mundodi, V., A. S. Kucknoor, D. J. Klumpp, T. H. Chang, and J. F. Alderete. 2004. Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis. Mol. Microbiol. 531099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mundodi, V., A. S. Kucknoor, and J. F. Alderete. 2007. Antisense RNA decreases AP33 gene expression and cytoadherence by T. vaginalis. BMC Microbiol. 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myohanen, H., and A. Vaheri. 2004. Regulation and interactions in the activation of cell-associated plasminogen. Cell. Mol. Life Sci. 612840-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pancholi, V., and V. A. Fischetti. 1998. α-Enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 27314503-14515. [DOI] [PubMed] [Google Scholar]
- 41.Pancholi, V. 2001. Multifunctional alpha-enolase: its role in diseases. Cell. Mol. Life Sci. 58902-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pancholi, V., and G. S. Chhatwal. 2003. Housekeeping enzymes as virulence factors for pathogens. Int. J. Med. Microbiol. 293391-401. [DOI] [PubMed] [Google Scholar]
- 43.Plow, E. F., J. Felez, and L. A. Miles. 1991. Cellular regulation of fibronolysis. Thromb. Haemost. 6632-36. [PubMed] [Google Scholar]
- 44.Plow, E. F., T. Herren, A. Redlitz, L. A. Miles, and J. L. Hoover-Plow. 1995. The cell biology of the plasminogen system. FASEB J. 9939-945. [DOI] [PubMed] [Google Scholar]
- 45.Ramajo-Hernandez, A., R. Perez-Sanchez, V. Ramajo-Martin, and A. Oleaga. 2007. Schistosoma bovis: plasminogen binding in adults and the identification of plasminogen-binding proteins from the worm tegument. Exp. Parasitol. 11583-91. [DOI] [PubMed] [Google Scholar]
- 46.Redlitz, A., B. J. Fowler, E. F. Plow, and L. A. Miles. 1995. The role of an enolase-related molecule in plasminogen binding to cells. Eur. J. Biochem. 227407-415. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 48.Sirover, M. A. 1996. Emerging new functions of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in mammalian cells. Life Sci. 582271-2277. [DOI] [PubMed] [Google Scholar]
- 49.Sirover, M. A. 1999. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta 1432159-184. [DOI] [PubMed] [Google Scholar]
- 50.Solano-Gonzalez, E., M. E. Alvarez-Sanchez, L. Avila-Gonzalez, V. H. Rodriguez-Vargas, R. Arroyo, and J. Ortega-Lopez. 2006. Location of the cell-binding domain of CP65, a 65 kDa cysteine proteinase involved in Trichomonas vaginalis cytotoxicity. Int. J. Biochem. Cell Biol. 382114-2127. [DOI] [PubMed] [Google Scholar]
- 51.Sorvillo, F., L. Smith, P. Kerndt, and L. Ash. 2001. Trichomonas vaginalis, HIV, and African-Americans. Emerg. Infect. Dis. 7927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutcliffe, S., E. Giovannucci, J. F. Alderete, T. H. Chang, C. A. Gaydos, J. M. Zenilman, A. M. De Marzo, W. C. Willette, and E. A. Platz. 2006. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 15939-945. [DOI] [PubMed] [Google Scholar]
- 53.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 254876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, L., K. D. Hayes, and L. J. Mauer. 2006. Fluorescent labeling study of plasminogen concentration and location in simulated bovine milk systems. J. Dairy Sci. 8958-70. [DOI] [PubMed] [Google Scholar]
- 55.Weinstock, H., S. Berman, and W. Cates, Jr. 2004. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect. Sex. Reprod. Health 366-10. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. 1995. Three hundred, thirty-three million new, STD curable cases in 1995. AIDS Wkly. 199515-16. [PubMed] [Google Scholar]
- 57.World Health Organization. 2001. Global prevalence and incidence of selected curable sexually transmitted infections. Overview and estimates. World Health Organization, Geneva, Switzerland.
- 58.Yavlovich, A., A. A. Higazi, and S. Rotten. 2001. Plasminogen binding and activation by Mycoplasma fermentas. Infect. Immun. 691977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, E., J. M. Brewer, W. Minor, L. A. Carreira, and L. Lebioda. 1997. Mechanism of enolase: the crystal structure of asymmetric dimmer enolase-2-phospho-d-glycerate/enolase-phosphoenolpyruvate at 2.0 A resolution. Biochemistry 3612526-12534. [DOI] [PubMed] [Google Scholar]