Abstract
To investigate how intracellular parasites manipulate their host cell environment at the molecular level, we undertook a quantitative proteomic study of cells following infection with the apicomplexan parasite Toxoplasma gondii. Using conventional two-dimensional electrophoresis, difference gel electrophoresis (DIGE), and mass spectrometry, we identified host proteins that were consistently modulated in expression following infection. We detected modification of protein expression in key metabolic pathways, including glycolysis, lipid and sterol metabolism, mitosis, apoptosis, and structural-protein expression, suggestive of global reprogramming of cell metabolism by the parasite. Many of the differentially expressed proteins had not been previously implicated in the response to the parasite, while others provide important corroborative protein evidence for previously proposed hypotheses of pathogen-cell interactions. Significantly, over one-third of all modulated proteins were mitochondrial, and this was further investigated by DIGE analysis of a mitochondrion-enriched preparation from infected cells. Comparison of our proteomic data with previous transcriptional studies suggested that a complex relationship exits between transcription and protein expression that may be partly explained by posttranslational modifications of proteins and revealed the importance of investigating protein changes when interpreting transcriptional data. To investigate this further, we used phosphatase treatment and DIGE to demonstrate changes in the phosphorylation states of several key proteins following infection. Overall, our findings indicate that the host cell proteome responds in a dramatic way to T. gondii invasion, in terms of both protein expression changes and protein modifications, and reveal a complex and intimate molecular relationship between host and parasite.
The obligate intracellular protozoan Toxoplasma gondii has evolved an intimate relationship with its host that extends to the cellular and molecular levels (82, 83). The absolute requirement for invasion of an appropriate host cell in the replication of the parasite suggests that modification of host functions is central to pathogenesis. Intuitively, this subversion of the cell must be a complex process, since host cells are not inherently programmed to provide an environment conducive to pathogens. Host cells have evolved primary lines of defense as countermeasures to pathogen invasion, establishment, and replication. These include elaborate systems, such as the phagolysosomal fusion, reactive oxygen and nitrogen intermediates, the sequestration of nutrients, and even cell suicide (apoptosis), as defenses to limit pathogen growth. The implementation of these systems and the ability of successful pathogens to mitigate their effects are ultimately mediated by changes in both the levels and activities of key proteins. While some of these changes may be transcriptionally regulated and thus potentially revealed by microarray analysis, the true effectors are proteins, the activities of which are governed by both their absolute levels and their “activation” statuses. The latter are often the consequence of posttranslational modifications (PTMs) that are not apparent in transcriptional analyses.
The extent to which there is convergence between different intracellular pathogens with regard to changes instituted in the host is not entirely clear, but the dissection of these changes is critical to our understanding of both the microbial and host contributions to infection, and also to the disease process. Studies have shown that changes in the host cell transcriptome accompany viral (7, 99), bacterial (5, 21), and protozoan (6, 79, 93) infections. Despite these studies, how these mRNA changes manifest at the level of the proteome, in the end the final effector of physiological change, remains an open question. In this study, we used a systematic approach to examine the changes in the host cell proteome in response to infection with T. gondii. We exploited quantitative gel-based proteomic approaches, including high-resolution two-dimensional electrophoresis (2-DE) and difference gel electrophoresis (DIGE), in combination with mass spectrometry (MS) to interrogate simultaneously and to identify hundreds of proteins whose expression is altered in response to parasite infection. This study represents the first proteomic analysis of the modulation of host cells by an intracellular protozoan parasite. We chose the system of the T. gondii-infected cell, as published microarray data (6) provide a unique resource against which to compare the proteomic data generated.
T. gondii is an apicomplexan parasite; other members of the phylum include Plasmodium, the causative agent of malaria; Eimeria; Neospora; and Cryptosporidium. The parasite is remarkable, not just for its high prevalence, making it an important disease of humans and animals, but also because of its ubiquitous nature (90). Toxoplasma actively invades host cells and, remarkably, can infect and replicate within almost any nucleated cell (80). The parasite establishes itself in a unique compartment, the parasitophorous vacuole (PV), that is separated from the host cytoplasm by the parasite-modified PV membrane (PVM) (58). Key to the invasion process is the apical complex of the parasite, which contains a mixture of specialized proteins, including those released from rhoptries (ROP), which are club-shaped organelles defining the Apicomplexa (8). Many of the secreted ROP proteins interact directly with the host cell, potentially affecting host function (for a review, see reference 95). For example, the putative protein kinase ROP16 has recently been shown to affect the activation of signal transducer and activator of transcription (STAT) signaling pathways, leading to a downstream modulation of the host interleukin 12 cytokine (74). In addition, a rhoptry-derived protein phosphatase activity targets the host nucleus (31), while the protein ROP2 is secreted to the PVM, where it anchors host mitochondria (84). How these and other parasite activities manifest change at the level of host transcription has been previously investigated (6, 26, 27). This analysis, while valuable, is incomplete, as it is at least one step removed from the true effectors of cellular function—the proteins. Furthermore, transcriptional analysis fails to reveal the impact of translational regulation and PTMs that are critical to cellular homeostasis (2, 17, 36, 55).
To overcome the limitations of transcriptional analysis, here, we have adopted a quantitative proteomics approach to study changes in the host proteome and to determine the complexity of protein changes during invasion and intracellular replication of T. gondii. In taking this approach, our analysis of the proteome is unbiased with respect to preconceived hypotheses and previously available reagents. We also analyzed the phosphoproteomes of infected cells alongside steady-state protein using an adaptation of DIGE in which native and dephosphorylated protein populations were compared by differential fluorescent labeling. Our results showed substantial and reproducible changes in the steady-state expression and PTM states of proteins not previously implicated in the response to T. gondii infection. Although technological constraints mean that proteomics methods do not necessarily sample all protein classes equally, relative changes in protein expression are nonetheless highly tractable despite these limitations. Our approach is therefore a powerful hypothesis-generating tool: as well as providing corroborating evidence for previous specific functional studies, it also raises some questions about conclusions derived from exclusively transcriptional analyses.
MATERIALS AND METHODS
Cell culture, parasite preparation, and infection experiments.
Human foreskin fibroblasts (HFFs) were grown in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% heat-inactivated fetal calf serum (Gibco BRL), 2 mM glutamine (Sigma), and penicillin/streptomycin (Gibco BRL) at 5% CO2 and 37°C. The RH strain of T. gondii was maintained in HFFs (72), and the parasites were routinely checked for Mycoplasma and found to be negative using a Mycoplasma PCR detection kit (Stratagene). The flasks were then scraped, and the entire material was harvested and passed through a 3-μm filter to rupture intact HFFs and release any remaining intracellular parasites. The resulting material was pelleted at 2,900 × g and washed in Dulbecco's modified Eagle's medium, and the parasites were counted.
For the infection experiments, parasites were added to confluent HFF monolayers (7 to 10 days old; passage ≤ 20) at an infection ratio of 2:1. Typically, approximately 50% of the cells appeared heavily infected under these conditions at 24 h. The cells were harvested by washing them once in filter-sterile (FS) HEPES-buffered saline solution and then leaving the cells in versine-trypsin solution (4:1) for approximately 6 min. To the noninfected cells, an equal number of parasites were added. The cells were then pelleted and washed three times in sterile phosphate-buffered saline (PBS) and stored at −70°C or used immediately in sample preparation. For the phosphoproteome experiments, the cells were washed in FS HEPES and not PBS. Infected HFFs were imaged by growing HFFs on transwell slides and infecting the cells with T. gondii tachyzoites for 24 h. The cells were stained with Giemsa stain and viewed using an Axiovert light microscope at 400× magnification.
Sample preparation for 2-DE.
HFFs were lysed in an 8 M urea, 4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 40 mM Tris base (Sigma) lysis buffer containing DNase/RNase (Sigma) and protease inhibitors (Roche) complemented with 10 μl of EDTA, EGTA, and phenylmethylsulfonyl fluoride solution (Sigma). The sample was frozen in liquid nitrogen, heated to 29°C, and vortexed for 20 s. This was repeated five times. The sample was then pelleted, and the protein concentration was determined.
Conventional 2-DE.
A 24-cm immobilized pH gradient (IPG) strip (Amersham) was rehydrated overnight with 300 μg of protein in 450 μl of rehydration buffer (8 M urea, 2% CHAPS, 0.002% bromophenol blue, 0.01% dithiothrietol, and 0.02% IPG buffer) (Amersham) that contained 300 μg of the solubilized proteins. Isoelectric focusing was conducted for a maximum of 80,000 V·h total at a maximum of 8,000 V using the IPG Phor (Amersham). For the second dimension, the IPG strip was equilibrated for 15 min by rocking it in equilibration buffer (2% sodium dodecyl sulfate [SDS], 50 mM Tris-HCl, pH 8.8, 6 M urea, 30% [vol/vol] glycerol, 0.002% bromophenol blue) containing 10 mg/ml dithiothreitol and then a second 15-min wash with equilibration buffer containing 25 mg/ml iodoacetamide (Sigma). The IPG strip was then embedded in a precast gel and sealed into place using agarose sealing solution. The gels were run in Dalt tanks (Amersham) using a discontinuous buffer system of 1× SDS electrophoresis buffer (25 mM Tris-HCl, pH 8.3, 192 mM glycine, 0.1% SDS) in the bottom chamber and 2× electrophoresis buffer in the top chamber at 120 V overnight. The gels were stained with colloidal Coomassie. The gels were fixed for 2 h in 40% ethanol, 10% acetic acid and then washed twice, for 10 min each time, in double-distilled H2O (ddH2O). The gel was then left in the stain (4:1 colloidal stock-methanol) for 1 to 7 days (Coomassie stock, 5% Coomassie G250 [Sigma] in ddH2O; colloidal stock, 50 g ammonium sulfate, 500 ml ddH2O, 6 ml phosphoric acid, 10 ml Coomassie stock). The gel was rinsed with water to remove excess stain. Gel images were acquired using an Amersham-Pharmacia Biotech Labscan. Images were acquired at 300 dots per inch using Labscan version 3.0 software on a Umax flatbed scanner with an integrated transparency adapter.
Image analysis.
Spots were detected on the gel by eye and using the 2DElite automatic spot detection program. The software calculated the spot volumes relative to background and normalization, and the infected and noninfected gels were compared. Any spots present on one gel and not on the other or any spots that matched across the two gels whose volumes differed by 30% or more were chosen for further analysis by MS.
DIGE.
The Amersham DIGE protocol was followed for all steps (Ettan DIGE system user manual, ed. AA). In summary, 50 μg of the infected or noninfected cell sample was minimally labeled with 400 pmol of a CyDye fluorophore (Cy3 or Cy5). Five infected and noninfected samples were compared. An internal standard was generated by combining equal amounts of extracts from all the infected and noninfected samples included in the study. The standard was labeled with Cy2 and included in all the gels run. The labeling reaction was quenched using 10 mM lysine. Following the labeling reaction, the infected and noninfected samples, together with a standard aliquot, were mixed together and separated using 2-DE. One of the gels included 300 μg of standard protein sample to be used as the preparative gel. The proteins were visualized by using a fluorescence scanner at appropriate wavelengths for Cy2, Cy3, and Cy5 dyes (Typhoon 9400; GE Healthcare). Total protein on the preparative gel was detected by poststaining of the gel with Sypro Orange dye (Molecular Probes). The gel was fixed in 40% methanol, 7.5% acetic acid overnight; incubated in 0.05% SDS for 2 h; and then stained in Sypro Orange. After the gel was washed briefly in 7.5% acetic acid, proteins were visualized by fluorescence scanning. Image analysis was carried out with DeCyder 5.01 software (GE Healthcare). The DeCyder differential in-gel analysis (DIA) module was used for pairwise comparison of each infected and noninfected sample to the mixed standard present in each gel and for the calculation of normalized spot volumes/protein abundances. The 15 spot maps corresponding to the five gels were used to calculate average abundance changes and paired Student's t test P values for each protein across the five gels. This was done using the DeCyder biological variation analysis (BVA) module and the Cy3/Cy2 and the Cy5/Cy2 ratios for each individual protein. Changes detected by DIGE analysis were matched with the Sypro orange-stained preparative gel protein pattern, and spots were selected for picking according to the poststained image.
Spot picking.
Spots of interest were excised from the gel using the Amersham Biosciences Ettan Spot Handling Work Station. The proteins were digested into their component peptides with trypsin and eluted from the gel plugs. One-tenth of the sample was then mixed with matrix (α-cyano hydroxycinnamic acid; Sigma) and spotted onto a matrix-assisted laser desorption ionization (MALDI) target plate. The remaining sample was dried in 96-well plates for use with electrospray liquid chromatography-tandem MS (LC-MS-MS).
Protein identification.
All protein spots were first subjected to MALDI-time of flight-MS analysis, and if they were not identified, then LC-MS-MS analysis was undertaken. MALDI (PerSeptive Biosystems; Voyager-DE Pro biospectrometry workstation) analysis involved peptides that were crystallized in matrix consisting of a saturated solution of α-cyano-4-hydroxycinnamic acid prepared in 50% (vol/vol) acetonitrile containing 1% (vol/vol) aqueous trifluoroacetic acid (Sigma) and applied directly to the MALDI plate. Three sets of 150 shots/spectrum were collected for each spot. The spectra from the MALDI were first baseline corrected, smoothed, calibrated against internal trypsin autolysis peptides, and deisotoped. For LC-MS-MS, the tryptic peptides were solubilized in 0.5% formic acid and were separated by nanoflow high-performance LC on a C18 reverse-phase column (Amersham Biosciences), and elution was performed with a continuous linear gradient of 40% acetonitrile for 20 min. The eluates were analyzed by online LC-MS-MS by use of a QStar Pulsar mass spectrometer (Applied Biosystems). A 3-s survey scan preceded each MS-MS data collection cycle of four product ion scans of 3 s each, and this gave a duty cycle of 115 s. The resulting MALDI-time of flight-MS and LC-MS-MS spectra were interpreted using MASCOT software by querying the present nonredundant National Center for Biotechnology Information (NCBI) database and a locally installed version of the Toxoplasma database (ToxoDB, release 4; http://www.toxodb.org/). Partial carboxymethylation and oxidation of methionine residues were considered in the search.
Enrichment of PVM-associated organelles from uninfected and T. gondii-infected cells.
Immortalized wild-type mouse embryonic fibroblasts and Vero cells (ATCC CCL-81) were maintained as described previously (61). The RH strain of T. gondii was used in these studies and was serially passaged as described previously (85).
In preparation for the isolation of the PVM-associated organelles from uninfected and infected cells, mouse embryonic fibroblasts were seeded in 10-cm dishes and incubated at 37°C for approximately 48 h. Following this incubation, the cells were infected with five T. gondii RH tachyzoites per cell and allowed to incubate at 37°C for 24 h.
Organelle isolation was performed using a modification of the protocol reported by Eskes et al. (24). Prior to being harvested, the cells were washed with 10 ml of PBS (137 mM NaCl, 2.7 mM KCl, 10 mM NaH2PO4, adjusted to pH 7.4) to remove any remaining medium. The cells were harvested and resuspended in 10 ml (five dishes/10 ml) isotonic mitochondrial buffer (210 mM mannitol, 70 mM sucrose, 10 mM HEPES [pH 7.5]) (MB) with complete protease inhibitor cocktail (Roche). The cells were pelleted via centrifugation for 10 min at 2,000 × g and resuspended in 3 ml MB prior to being mechanically lysed in a ball bearing homogenizer (18-μm clearance). Parasites and intact cells were removed from the lysate via low-speed centrifugation (2,000 × g for 10 min). The resulting supernatant was centrifuged at 10,000 × g for 10 min to pellet the mitochondria (and the endoplasmic reticulum [ER]).
In an effort to further purify the mitochondria from the membrane fraction resulting from the previous centrifugation, in two of the experiments, the resulting pellet was combined with 750 μl MB and transferred to a sucrose step gradient (1 ml 55% sucrose, 1.75 ml 40% sucrose, and 1.75 ml 30% sucrose). The gradient was centrifuged at 27,100 rpm for 40 min. In the third experiment, the membrane fraction resulting from the first 10,000 × g centrifugation was homogenized using a tight-fitting Dounce homogenizer prior to transfer to the sucrose gradient.
Following the high-speed centrifugation, fractions representing the interfaces of the gradient were collected, diluted in MB, and further centrifuged at 10,000 × g for 10 min. An aliquot was removed from each of these gradient fractions and used to assess mitochondrial enrichment via immunoblot assay. Immunoblotting was performed as previously described by Carmen et al. (11) using antibodies recognizing cytochrome c (BD Pharmingen; catalog no. 556433) and calnexin (1:3,000) (40). The resulting pellets from each gradient fraction were lysed, using the DIGE lysis method as described above, and the infected and noninfected samples from the three gradients were combined. DIGE analysis was performed as described above.
Western blot analysis.
Infected and mock-infected HFFs were harvested as described above. Equal amounts of infected HFF protein extract, noninfected HFF protein extract, and T. gondii protein extract were loaded onto one-dimensional electrophoresis (1-DE) gels. The proteins were transferred from the gel to a Hybond membrane (Amersham) using a wet-transfer system. The transfer was carried out at 100 V for 45 min in 10% transfer buffer (10× transfer buffer is 24.22 g Tris, 112.5 g glycine in 1 liter ddH2O), 20% methanol, and 70% ddH2O. The membrane was then stained with Ponceau S solution (2% Ponceau S concentrate [Sigma catalog no. P7767] diluted 1:100 with ddH2O) to check the transfer efficiency and to confirm that equal amounts of starting material had been loaded onto the 1-DE gel. The blot was blocked in 5% milk solution (Marvel) in 1× Tris-buffered saline (TBS) (10× TBS is 20 mM Tris, 137 mM NaCl in 1 liter ddH2O with the pH adjusted to 7.6 using HCl) plus 0.1% Tween 20 for 2 h at 37°C. Antibody was applied in the appropriate dilution in 1% milk solution in 1× TBS plus 0.1% Tween 20 and left overnight with shaking at 4°C. Mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Calbiochem; mouse immunoglobulin G isotype) was used at a concentration of 2 μg/ml (as recommended by the antibody data sheet) for Western blotting. The blots were then washed four times in 1% milk solution in 1× TBS for 15 min each time at room temperature. The secondary antibody was horseradish peroxidise-conjugated rabbit anti-mouse immunoglobulin G (Pierce; catalog no. 31450), used at a concentration of 1 in 10,000 in 1% milk solution in 10× TBS for 2 h at room temperature. The blot was then washed three times in 1% milk solution in 1× TBS for 15 min each time at room temperature. The blot was briefly rinsed in 10× TBS, and the bound antibody was visualized using Supersignal West Pico chemiluminescent substrate from Pierce (according to the manufacturer's instructions) and developed on film (Hyperfilm ECL from Amersham Biosciences). The films were developed and fixed using Kodak GBX developer/replenisher and fixer/replenisher (Sigma), respectively. Western blotting was repeated several times for each antibody to confirm the patterns observed.
Immunofluorescence and electron microscopy.
The detection of host mitochondria was performed using a monoclonal anti-cytochrome c antibody (1:1,500 dilution; Pharmingen) with the parasites detected using rabbit anti-SAG1 (1:7,000) (kindly provided by John Boothroyd, Stanford University) in HFFs infected for 24 h. The infected monolayer was fixed with 3% paraformaldehyde and permeabilized using acetone at −20°C. Images were acquired on a Leica confocal microscope system. Electron microscopy was performed exactly as described previously (86).
Phosphoproteomics.
Protein lysates were prepared from 24-h-infected and noninfected cells as previously described. The cells were washed in FS HEPES/saline solution during harvesting and not in PBS, as it might have interfered with the natural phosphorylation status of the proteins. The cells were lysed as previously described, except that the urea concentration was diluted to 2 M. Cell lysates (infected and noninfected) were halved, and half of each sample was treated with 10 units (in 10 μl) of shrimp alkaline phosphatase (SAP) (Promega). The remainder of each sample was treated with an equal volume of enzyme storage buffer (25 mM Tris-HCl, 1 mM MgCl2, 0.1 mM ZnCl2, and 50% glycerol; Promega) to be used as a control. The DeCyder DIA module was used to analyze the protein expression differences between the phosphorylated and dephosphorylated samples from T. gondii-infected and noninfected cells. The Cy3-labeled SAP-treated infected sample was compared with the Cy5-labeled, infected, and mock-treated sample. A twofold or greater difference in protein spot volume was marked as significant. The same analysis was also used to compare the Cy3-labeled, SAP-treated, noninfected cells with the Cy5-labeled, noninfected, mock-treated sample. Proteins of interest were excised from the gel, subjected to trypsin digestion, and analyzed by MS.
RESULTS AND DISCUSSION
Differential proteome analysis by conventional 2-DE gel-gel comparison.
Proteome comparisons of infected and noninfected cells were made at 6, 12, 16, and 24 h postinfection (p.i.). At 24 h p.i., 11 replicates were performed to ensure a high degree of reproducibility. Proteins from infected and noninfected cells were separated by 2-DE and stained with colloidal Coomassie blue. Since it was not possible to remove parasites from the infected cells before 2-DE analysis, an equivalent number of tachyzoites was added to the noninfected cell cultures at the point of harvest to ensure that the same amount of parasite protein was present in both extracts. This “mock-infection” approach minimized the number of false-positive differentially expressed spots that were due to parasite rather than host proteins. Moreover, parasite proteins represented a relatively small proportion of the total protein, and any proteins that were of parasite origin were easily detected by MS identification, thereby ensuring that they were not erroneously included in the subsequent analysis.
Differentially expressed proteins were picked from the gel and identified using MS. Figure 1 shows a typical example of some of the differentially expressed proteins identified by 2-DE. The function, gene name, and gene ontology category of each protein was then determined using the Harvester search engine from EMBL (http://www.harvester.embl.de) and the Human Protein Reference Database (http://www.hprd.org). All of the data from the conventional gel-to-gel experiments are summarized in Table 1, along with the DIGE analysis (see below).
FIG. 1.
Changes in the proteomes of HFFs infected with T. gondii compared with mock-infected cells. (A) Proteins were separated by 2-DE using immobilized pH gradient strips (pH 4 to 7), stained with colloidal Coomassie, and identified by MS. MW, molecular weight. (B) Selected areas (boxed) of difference between the two samples in panel A are enlarged (rows 1 to 4). The proteins are annotated by abbreviated gene names. Full protein names are given in Table 1.
TABLE 1.
Changes in the proteomes of HFFs following infection with T. gondiia
| Gene name and function | Protein name | Proteomicsb | Microarrayb |
|---|---|---|---|
| Metabolism | |||
| PGLS | 6-Phosphogluconolactonase | ↓ | ↓ |
| ACAT1 | Acetyl coenzyme A acyltransferase 2 | M | ↑ |
| AK2c,d | Adenylate kinase 2 isoform a | ↑ | ↑ |
| ALDH1A3d | Aldehyde dehydrogenase 1A3 | ↑ | ↓ |
| ALDH1B1 | Aldehyde dehydrogenase X | ↓ | ↓ |
| AKR1B1d | Aldose reductase | ↑ | ↑ |
| CBR1 | Carbonyl reductase 1 | ↓ | NA |
| M6PRBP1c | Cargo selection protein TIP47 | ↓ | − |
| CTSBc | Cathepsin B | ↓ | ↓ |
| CTSDc,d | Cathepsin D preprotein | M | − |
| CPOXd | Coproporphyrinogen oxidase | ↑ | − |
| ECHS1d | Enoyl-coenzyme A hydratase 1 | ↓ | ↑ |
| GSS | Glutathione synthetase | ↑ | NA |
| GSTA2 | Glutathione-S-transferase chain A | ↓ | ↓ |
| HNRPC | hnRNP C-like protein | ↓ | NA |
| HNRPF | hnRNP F | M | ↑ |
| HNRPH2 | hnRNP H2 | ↓ | NA |
| HNRPA2B1 | hnRNP A2/B1 isoform A2 | ↑ | ↑ |
| HDLBP | Vigilin | ↓ | NA |
| LRP1c | lrp protein | ↓ | − |
| MTHFD2 | MTHFD2 protein | ↑ | ↑ |
| HEXB | N-Acetyl-beta-glucosaminidase polypeptide | ↓ | NA |
| NDUFs5d | NDUFS5 | ↑ | NA |
| n aE1 | Nucleoside diphosphate kinase1-n a23 | M | ↑ |
| PSPC1 | Paraspeckle protein 1 | ↓ | − |
| pck1 | Phosphoenolpyruvate carboxykinase | ↓ | NA |
| PCMT1 | Protein-l-isoaspartate O-methyltransferase | ↑ | NA |
| PNPO | Pyridoxine 5′-phosphate oxidase | ↓ | − |
| PPA1 | Pyrophosphatase (inorganic) | ↓ | NA |
| RPS12 | RPS12 | ↑ | NA |
| Glycolysis | |||
| ALDOAd,e | Aldolase A | ↑ | NA |
| ALDOB | Aldolase B | ↑ | − |
| ATP5Dd | ATP synthase beta subunit | ↑ | − |
| COX6Bd | Cytochrome c oxidase subunit Vib | ↓ | ↑ |
| DDAH1 | Dimethyl arginine dimethyl aminohydrolase | ↓ | ↓ |
| ENO1 | Enolase 1 | M | ↑ |
| GAPDHd | Glyceraldehyde 3 phosphate dehydrogenase | ↑ | ↑ |
| PGK1d | Phosphoglycerate kinase 1 | M | ↑ |
| P4HBd | Protein disulfide isomerase | ↑ | ↑ |
| PKM2d | Pyruvate kinase M2 isozyme | M | NA |
| TXNDC5c | Thioredoxin domain 5 isoform 2 | ↑ | − |
| TPI1d | Triose phosphate isomerase | M | ↑ |
| Cell cycle | |||
| DCTN2 | Dynactin 2 | M | ↓ |
| NEDD5 | NEDD5 | ↓ | NA |
| LMNB2 | Lamin B2 | ↑ | ↓ |
| NSFL1C | p47 | ↑ | − |
| PRDX1c,d | Peroxiredoxin 1 | ↓ | ↑ |
| PHBc,d | Prohibitin | M | − |
| S100A11 | S100 calcium binding protein A11 | ↓ | NA |
| TPTE2 | TPTE2 | ↓ | − |
| TUBB5 | Tubulin beta 5 | ↓ | ↑ |
| UBQLN2 | Ubiquilin 2 | ↑ | − |
| Transcription | |||
| BASP1 | Brain abundant membrane signal protein | ↑ | ↓ |
| C14orf166 | CLE7-C14orf166 | ↑ | − |
| HNRPK | hnRNP K isoform a | ↑ | NA |
| PIAS2c,e | PIAS-Ny protein | ↑ | NA |
| CSEN | Presenelin binding protein | ↑ | − |
| ZNF235 | Zinc finger protein | ↑ | ↑ |
| Translation | |||
| EEFIA2d | Elongation factor 1 alpha | ↑ | ↓ |
| UBA52 | Ubiquitin A-52 | ↑ | NA |
| WBSCR1d | WBSCR1 | ↑ | − |
| Protein fate | |||
| HSPA5d,e | BiP protein, HSPA5 precursor | ↑ | ↑ |
| CDC37 | CDC37 cell division cycle 37 homolog | ↑ | − |
| CCT2 | Chaperonin-containing TCP1 subunit 2 beta | M | − |
| HSPD1d | Chaperonin HSP60 | M | − |
| CALU | Crocalbin-like protein | ↑ | − |
| HSPA6 | DNAK-type molecular chaperone HSPA6 | M | NA |
| C12orf8 | ER protein 29 precursor | M | − |
| HSPA8c,d | HS70kd pro-42-kDa ATPase | M | ↑ |
| HSPA9Bd | HSP-MTHSP75 | M | − |
| HSPC054 | HSPC054 protein | ↑ | NA |
| PDIR | PDI-related protein 5 | ↑ | ↑ |
| PPIAd | Peptidylprolyl isomerase A (cyclophilin A) | ↓ | ↑ |
| TRA1 | Tumor rejection antigen (gp96) 1 | ↓ | NA |
| UCHL1 | Ubiquitin carboxyl-terminal esterase L1 | ↓ | NA |
| Protein binding | |||
| NPM1 | B23 nucleophosmin | ↑ | − |
| CALD1 | Caldesmon | ↓ | NA |
| CNN2 | Calponin isoform a | ↓ | ↓ |
| NS | E2IG3 (nucleotide binding protein) | ↑ | NA |
| HEBP1 | Heme binding protein 1 | ↓ | NA |
| PBPd | Neuropolypeptide h3 | ↓ | NA |
| THAP11 | THAP domain containing 11 | ↑ | − |
| TAGLN | Transgelin | ↓ | − |
| TAGLN2d | Transgelin 2 | ↓ | NA |
| TMOD3 | Tropomodulin 3 | ↓ | − |
| TPM3 | Tropomyosin 3 | ↓ | NA |
| UMP-CMPK | UMP-CMP kinase | ↓ | NA |
| Protein activity regulation | |||
| SERPINA1d | Visceral adipose-specific SERPIN | M | − |
| Cellular transport | |||
| ABCA13 | ATP binding cassette transporter A13 | ↑ | − |
| CLIC4 | Chloride intracellular channel 4 | ↓ | − |
| CLTA | Clathrin, light polypeptide A isoform a | ↓ | NA |
| SLC6A3 | DAT-1 gene human dopamine transporter | ↑ | ↑ |
| CLIC1d | Nuclear chloride ion channel protein | ↓ | − |
| TUBA1 | Tubulin alpha | M | ↑ |
| VPs33B | Vacuolar protein sorting 33B | ↑ | NA |
| Signal transduction | |||
| ANXA2d | Annexin 2 | ↑ | − |
| CLC | Cardiotrophin-like cytokine | M | NA |
| PLCL1 | Phospholipase C alpha | ↑ | − |
| PGRMC1 | Progesterone receptor membrane component | ↑ | ↑ |
| STK16e | Protein serine/threonine kinase | ↓ | NA |
| RANBP1 | Ran-binding protein 1 | ↓ | ↑ |
| RCN1 | Reticulocalbin 1 precursor; Rcal | ↓ | NA |
| PARK7 | RNA-binding protein regulatory subunit | ↓ | NA |
| ZYX | Zyxin | ↓ | − |
| Stress response | |||
| PDLIM1 | Carboxyl-terminal LIM domain protein | M | − |
| HSPB1c,d | Heat shock protein 27 | M | NA |
| CHIT1 | Methylumbelliferyl-acetate deacetylase | ↓ | − |
| PDIR | PDI-related protein 5 | ↑ | ↑ |
| PRDX2c,d | Peroxiredoxin 2 | ↓ | ↑ |
| PRDX3d | Peroxiredoxin 3 isoform a precursor | ↓ | NA |
| PRDX5d | Peroxiredoxin 5 | M | − |
| PRDX6 | Peroxiredoxin 6 | M | − |
| STIP1d | STIP1 | ↓ | NA |
| SOD2d | Superoxide dismutase | M | ↑ |
| PRDX4c,d | Thioredoxin peroxidise | ↑ | ↑ |
| RAD23B | UV excision repair protein RAD23 | ↑ | NA |
| Cell motility | |||
| MARCKS | Myristoylated alanine-rich C-kinase substrate | ↑ | − |
| Immune response | |||
| ANXA1d | Annexin 1 | ↑ | ↑ |
| Cell fate | |||
| LGALS1 | Beta galactosidase soluble lectin | ↓ | ↓ |
| VDAC1c,d | Porin 31HM | ↑ | ↑ |
| ALBd | Serum albumin precursor | M | NA |
| YWHAHc | Tyrosine 3-monooxygenase | ↓ | ↑ |
| Structural | |||
| ACTBe | Actin, beta | ↓ | ↓ |
| ACTG1d,e | Actin gamma 1 propeptide | ↓ | NA |
| ACTN1d | Actinin-1 alpha | ↓ | ↓ |
| CAPZA1 | Capping protein (actin filament) | ↓ | − |
| CFL1 | Cofilin 1 | ↓ | ↑ |
| COL7A1 | Collagen type 7 | ↓ | − |
| CKAP4d | Cytoskeleton-associated protein 4 | ↑ | − |
| DESd | Desmin | ↑ | NA |
| FILIP1 | Filamin | M | − |
| LMNAc,d,e | Lamin A/C, isoform 2, 70-kDa lamin | ↓ | ↓ |
| LMNB1 | Lamin B1 | ↑ | NA |
| LASP1 | Lasp-1 protein | M | NA |
| MAP1B | Microtubule-associated protein 1B | ↓ | ↑ |
| MSNd | Moesin | ↑ | ↑ |
| MYL4 | Myosin alkali light chain isoform1 | ↓ | NA |
| MYH9 | Myosin, nonmuscle, heavy chain | ↑ | NA |
| TPM2 | Tropomyosin 2 | M | NA |
| TUBB1 | Tubulin beta-1 chain | M | ↑ |
| TUBE1 | Tubulin epsilon 5 | ↓ | − |
| VIL2d | Vilin 2 | ↑ | ↑ |
| VIMd,e | Vimentin | M | ↓ |
| Interaction with environment | |||
| ANXA5d | Annexin 5 | M | NA |
| Development | |||
| AHNAK | Desmoyokin | ↓ | ↓ |
| Interaction with the cellular environment | |||
| LGALS3 | Galactose-specific lectin | ↑ | NA |
| MYH6d | Myosin alpha heavy chain | ↑ | NA |
| TPM1 | Tropomyosin 1 | ↓ | NA |
| TPM4 | Tropomyosin 4 | ↓ | NA |
| VCL | Vinculin | M | NA |
| Unclassified | |||
| FHL2 | 4 and a half LIM domains 2 isoform 2 | ↓ | ↓ |
| CHCHD3d | Coiled-coil-helix-coiled-coil-helix domain | ↑ | − |
| ITIH5 | Inter-alpha (globulin) inhibitor H5 | ↓ | NA |
| GRP58d | Protein disulfide-isomerase A3 precursor | ↑ | ↑ |
| MFAPL3e | Protein kinase NYD-Sp9 | ↑ | NA |
| snf7dc2 | snf7dc2 | ↓ | NA |
| ETHE1 | Thyroid hormone binding protein | ↓ | NA |
Data from conventional gel-gel and DIGE analyses are summarized. Included in the table are transcriptional data from a microarray experiment run under the same conditions (6) for each protein (where available).
Proteins are designated as being up-regulated in expression (↑), down-regulated in expression (↓), or modulated (M). Modulated proteins are described as those whose expression altered across several isoforms on the same gel, making a simple designation of either up- or down-regulated impossible. In many cases, this probably indicates a posttranslational modification event. A minus indicates no detectable change in expression. NA, not available, i.e., the gene was not present on the array, so the expression of the gene transcript in the infected host cell is unknown.
Protein implicated in apoptosis.
Mitochondrial protein.
Protein that changed in expression at 12 h p.i. and 24 h p.i. (apart from PIAS2 and STK16, which were only modulated 12 h p.i.).
DIGE.
To increase the sensitivity and reproducibility of our differential proteome analysis, we compared infected and noninfected HFF proteomes using DIGE with a mixed internal standard containing protein extracts from five infected and five noninfected samples at 12 and 24 h p.i. After 2-DE, the analysis of the Cy2, Cy3, and Cy5 gel images with the BVA module of the DeCyder software revealed changes in the abundances of 362 protein spots, with the statistical variance of the infected versus the noninfected spot volume ratios lying within the 95th confidence interval (Student's t test; P < 0.05). The use of the mixed internal standard in this experimental design allowed the detection of significant abundance changes, based on the variance of the mean change within the cohort.
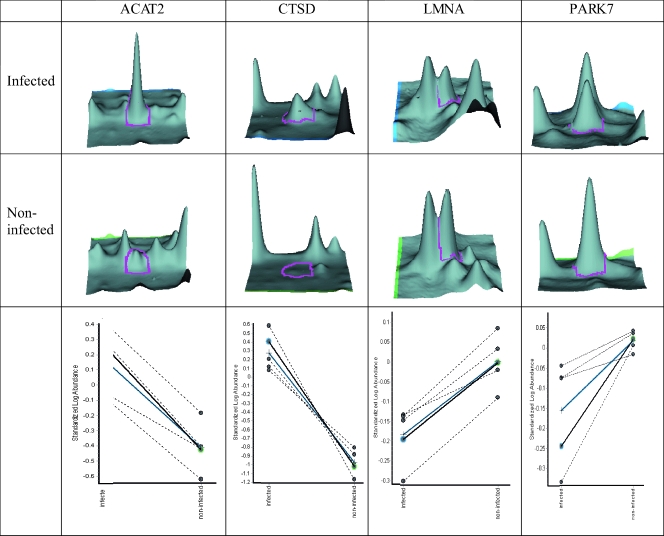
Figure 2 illustrates a three-dimensional view corresponding to the pixel volume distributions for the Cy5/Cy3 ratios of some of the proteins differentially expressed in infected cells. As shown, the expression levels of many host cell proteins were significantly increased upon infection with T. gondii (e.g., Fig. 2, acetyl coenzyme A acyltransferase 2 and cathepsin D), while other protein spots showed significantly lower levels of expression upon infection (e.g., Fig. 2, lamin A/C isoform 1 and RNA-binding protein regulatory subunit). The proteins of interest were excised from the gel, digested with trypsin, and analyzed by MS. Of these, 162 spots were unambiguously identified. Only 96 of these 162 protein spots represented individual human gene products, because some of the modulated proteins were identified at several different places on a single gel, indicating that multiple isoforms and/or posttranslationally modified forms might be present for that protein species. In these instances, it was not always possible to assign a simple up- or down-regulation to the protein. However, the shift in relative abundance from one isoform to another upon infection is likely to have functional significance, and where this occurred, the proteins were designated modulated. The combined data from all the conventional gel-to-gel experiments and the DIGE analysis are presented in Table 1 and represented in total 157 individual protein changes.
FIG. 2.
Three-dimensional view of differentially expressed proteins in T. gondii-infected and noninfected HFFs at 24 h p.i. as determined by DIGE analysis. The graphs show the pixel volume distribution for the Cy5/Cy3 ratios of the proteins differentially expressed in all five gels in the experiment. The dotted lines represent the standard log abundance of the volume of the selected spot from each gel in the experiment. The boldface line represents the three-dimensional spots shown above, and the blue line indicates the average standard log abundance across the five gel sets. ACAT1, acetyl coenzyme A acyltransferase 2; CTSD, cathepsin D; LMNA, lamin A/C isoform 1; PARK7, RNA-binding protein regulatory subunit.
Although many of the proteins seen in the conventional gel-to-gel comparisons were also seen in the DIGE experiment, the DIGE analysis provided increased sensitivity over the conventional approach. This was probably due to a number of factors, including the use of fluorescent dyes, which are more linear and sensitive than the colloidal Coomassie staining. Moreover, the DeCyder software used for the DIGE analysis allows univariate statistical testing, thus making analysis of the data more reliable than with conventional gel-to-gel comparisons.
In each experiment, the number of modulated proteins increased with time, so that most changes were seen at 24 h p.i. The earliest identified changes were at 12 h p.i., although a small number of differentially expressed protein spots were see at 6 h p.i., but no protein identity was obtained for these proteins. By 12 h p.i., approximately half the number of differentially expressed spots seen at 24 h p.i were observed. Proteins identified early during infection, at 12 h p.i., are indicated in Table 1. Changes in the steady-state levels of proteins in response to infection are influenced by both de novo synthesis and protein turnover. Accordingly, the levels of specific protein in the different phases of the infection may show a complex pattern that is temporally regulated. This is illustrated, for example, by proteins exhibiting a transient induction in expression levels postinfection, such as the cytoskeletal components beta and gamma actin, as well the intermediate filament protein vimentin. A similar pattern was observed for the STK16 kinase and a component of the transcriptional apparatus (PIAS) that may serve as a precursor to the large-scale induction of gene expression.
Functional distribution of modulated proteins.
To assign a functional category to each of the modulated proteins, the Harvester search engine at EMBL (http://harvester.embl.de/), the Human Protein Reference Database website (http://www.hprd.org/) and the gene ontology website (http://www.geneontology.org/) were searched, and proteins were classified into functional categories according to their assigned functions using the Munich Information Centre of Protein Sequences (http://mips.gsf.de). The functional category of each of the modulated proteins 24 h p.i. is shown in Table 1, and their relative distribution is summarized in Fig. 3. Interestingly, one-third of all modulated proteins in infected cells were mitochondrial (as determined using http://www.mitoproteome.org), significantly more than would be expected by chance; some predict that only approximately 4.25% of the human proteome is mitochondrial (51). This suggests that mitochondrial proteins are disproportionately modulated during infection and highlights the importance of this host organelle in the infection response.
FIG. 3.
Pie chart showing the distribution of modulated host cell proteins by functional category following infection with T. gondii. In total, 157 proteins were significantly (P ≤ 0.05) changed. The top pie chart shows the functional categories of proteins up-regulated in expression, and the bottom chart shows the functions of those proteins down-regulated during infection. Metabolic and structural proteins were the most heavily modulated categories, while over one-third of all modulated proteins were related to mitochondrial function.
Mitochondria and the ER have an intimate relationship with Toxoplasma due to their recruitment to and association with the PVM (86), as presented in Fig. 4. The central roles of both these organelle systems in virtually every aspect of cellular physiology points to the extent of reprogramming instituted in the infected cell. In discussing these changes, we use broad headings assigned by the gene ontology programs, although it should be noted that proteins can play multiple roles in distinct and sometimes seemingly unconnected categories. Thus, a higher degree of physiological connectivity may in fact be present.
FIG. 4.
The PVM of T. gondii associates intimately with host mitochondria and elements of rough ER. (A) Electron microscopy revealed an intracellular parasite with a mitochondrial cross section and ER lamellae in association with the PVM (the specific electron microscope image is for 4 h p.i.; the immunofluorescence assay is 24 h p.i.). (B) Identical to panel A, with the PVM-mitochondrial interface highlighted in red and the PVM-ER interactions highlighted in green. (C) Confocal microscopy depicting the rearrangement of host mitochondria visualized using anti-cytochrome c (green). (D) Visualization of 2 PVs decorated with anti-SAG1. (E) Merged image indicating both the PVM-associated and peripheral mitochondria. The scale bars are equal to 1 μm in panels A and B and 10 μm in panels C, D, and E.
Intermediary metabolism.
Metabolism was the largest modulated category in this study (30 proteins), indicating that modulation of the host metabolism is intrinsic to the cell response to infection. As such, metabolism can be linked not only to the anabolic and catabolic processes within the cell, but also intimately to energetics (52), the cell cycle (48, 50), and cell death (29). Overall, 16 proteins related to metabolism were down-regulated, with only 10 being up-regulated and the remaining 4 modulated, possibly due to PTM (Table 1).
Obligate intracellular parasitism is fundamentally linked to auxotrophies that necessitate a requirement to scavenge critical nutrients (25, 84). T. gondii is a purine auxotroph and must salvage nucleobases, nucleosides, or nucleotides, as it is unable to convert guanine- to adenine-based substituents (49). The parasite also takes up host hypoxanthine, xanthine, adenine, inosine, and guanine (81). In this study, we show that adenylate kinase 2 is up-regulated in the infected cell, while the nucleoside diphosphate kinase is modulated (Table 1). This protein, which is localized in the mitochondrial intermembrane space, regulates the homeostasis of the cellular adenine and guanine nucleotide pools by the phosphorylation of AMP to ADP, which is further phosphorylated to ATP (47). The up-regulation of adenylate kinase 2 following infection with T. gondii may be in response to demands placed on the cell by T. gondii adenine salvage. In addition, nucleoside diphosphate kinases represent a family of proteins with multiple functions in nucleic acid metabolism and are localized to the cytoplasm, nucleus, and mitochondria (42). The mitochondrion outer-membrane-associated forms play roles in the regulation of energetics (56, 57). In addition, four metabolic heterogeneous nuclear ribonucleoproteins (hnRNPs), which are involved in the regulation of mRNA splicing and transcription, were also found to be modulated in infected cells, suggesting a role in the regulation of parasite-induced changes in transcription (6).
T. gondii can synthesize lipids de novo (15, 35), as well as actively scavenge specific lipids and sterols from the extracellular milieu, lipid bodies, and mitochondria (13, 15, 16, 18). Each of the four proteins directly involved in lipid and sterol metabolism identified in this study (carbonyl reductase 1, vigilin, cargo selection protein TIP47, and LRP protein) was down-regulated in response to infection. That a larger number of proteins directly involved in lipid and sterol biosynthesis and metabolism are not involved is somewhat surprising, given their marked induction in transcriptional studies (6). What is clear, however, is that several proteins involved in glycolysis, amino acid metabolism, and other aspects of intermediary metabolism central to lipid synthesis exhibit marked changes (see below). Thus, an increase in the supply of materials required for lipid synthesis and modification appears to be sufficient to sustain parasite growth.
Other changes in metabolic processes have an impact on the protein-degradative capacity of the cell, including the activities of the lysosomal cathepsin proteases (Table 1, Metabolism) and changes in a serpin class protease inhibitor (Table 1, Protein activity regulation). While lysosomes were believed not to interact with the PV (reviewed in reference 83), recent evidence suggests they may in fact serve an important nutritional function as the source of sterols (14, 16). A reduction in the degradative capacity of lysosomal contents may therefore be important to sustain parasite growth. Of interest, several serpin class protease inhibitors are secreted by the parasite into the PV lumen (64, 70). The absence of a marked increase in the levels of enzymes directly involved in amino acid biosynthesis suggests that, like the situation observed for lipids, the changes instituted in other aspects of intermediary metabolism satisfy the needs for parasite growth. It should also be noted that the cells used in these studies were cultured in a rich medium replete with high levels of amino acids and serum components, all of which, by providing a pool of readily accessed nutrients, may mask some of the potential induction of biosynthetic pathways.
Six proteins involved in carbohydrate metabolism (aldose reductase, aldehyde dehydrogense 1A3, aldehyde dehydrogenase X, hexoaminidase B, phosphoenoylpyruvate carboxykinase, and 6-phosphogluconolactonase) were modulated in the infected cell. In addition to these proteins, carbohydrate metabolism was represented in the significant number of proteins involved in glycolysis, which tie together both anabolic processes and energy metabolism.
Energy metabolism.
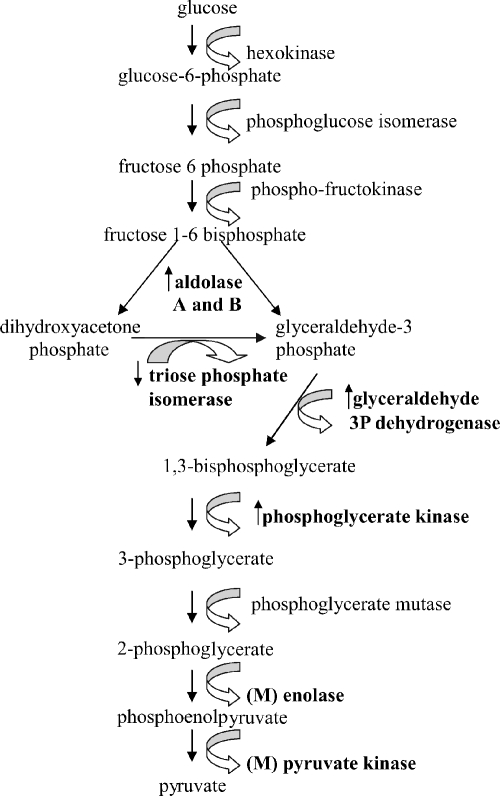
The glycolytic pathway showed considerable modification during infection, with seven enzymes showing evidence of modulation in expression, six of which showed either an increase or modulation (aldolase A and B, enolase, glyceraldehyde 3 phosphate dehydrogenase, phosphoglycerate kinase, and pyruvate kinase) and only one of which showed a decrease in expression (triose phosphate isomerase) (Fig. 5). The up-regulation of a key glycolytic enzyme (GAPDH) in the infected cell was confirmed by immunoblot analysis (Fig. 6). Together, these data provide evidence of an up-regulation of glycolysis following parasite invasion, a result that is in agreement with previous transcriptional studies (6).
FIG. 5.
Proteins modulated in the glycolytic pathway in T. gondii-infected cells. ↑, up-regulation in protein expression in the pathogen-infected cell;↓, down-regulation in protein expression in the pathogen-infected cell; (M), protein is modulated in a more complex way during infection. Six of the 10 enzymes involved in the glycolytic pathway were altered in expression in the parasite-infected cell as determined by proteomic analysis.
FIG. 6.
Western blot analysis to determine levels of GAPDH protein expression during infection. Extracts from T. gondii-infected (Inf) and noninfected (Non) cells were separated on 1-DE gels. Pure parasite extract was also run as a control, but no signal was detected, so the area has been cropped from the image. The proteins were transferred onto Hybond membranes and incubated with GAPDH antibody. The blot shows a band of the predicted mass of 36 kDa for GAPDH protein. This protein was identified as up-regulated in infected cells by proteomics. The Western blot signal was stronger in the infected lanes, confirming the proteomic results.
Increased glycolysis results in the production of both ATP and pyruvate, which directly or indirectly enters most biosynthetic processes in the cell and may feed oxidative phosphorylation (1). Since the yield of ATP from glycolysis is small relative to that by oxidative phosphorylation, the induction of the glycolytic cascade may be a means of providing resources for anabolic processes as opposed to energy. This decision would be influenced by pyruvate kinase, which is modulated in our study (Table 1, Glycolysis). In this context, one would expect a shift in energy production between glycolysis in the cytoplasm and the mitochondrion-localized oxidative phosphorylation. The observation that clearly identifies the last three components of this pathway as being modulated (ATP synthase beta subunit, cytochrome c oxidase subunit Vib, and inorganic pyrophosphatase) suggests changes in oxidative phosphorylation. However, this analysis of the whole cell does not distinguish between the bulk mitochondria and those associated with the PVM, both populations of which are active, as noted from the accumulation of membrane potential-sensitive dyes (86, 89).
Many of the metabolic changes identified in the transcriptional study of Blader et al. (6) and in our work here suggest a global reprogramming of cell metabolism. Such a reprogramming of metabolism has been described in cytomegalovirus-infected cells, where metabolomic analyses have indicated a shift toward anabolic function as opposed to energy production (65). The marshalling of resources and the building blocks of macromolecules are consistent with entry into the cell cycle, suggesting the activation of this pathway. We have recently obtained evidence that T. gondii infection promotes the G1-to-S transition in infected HFFs (R. E. Molestina, E. I. Guendy, and A. P. Sinai, unpublished data). Toxoplasma infection does not promote the replication of the infected cell, unlike the related Apicomplexa of Theileria species (22), but clearly is influenced by cell cycle modulators, as seen with the overexpression of the CDA1 gene promoting cystogenesis (71).
Cell cycle.
Ten proteins with key functions in mitosis and cell proliferation, including dynactin 2, lamin B2, and prohibitin, were modulated during infection (Table 1, Cell cycle). The connection of mitosis and its machinery is particularly intriguing in light of the fact that the Toxoplasma vacuole appears to recruit the host centrosomes/microtube organizing centers to a juxtavacuolar position in the infected cell, where they appear to be physically connected to the PVM (15, 77). This physical association would be expected to interfere with the initiation and progression of mitosis—an event that is generally not observed in T. gondi-infected cells. Furthermore the susceptibility of cells to infection with T. gondii appears to be linked to their location in the cell cycle (23). Of the cell cycle-related proteins modulated by infection, prohibitin exhibits dynamic modulation across the cell cycle check points (73) while possessing additional activities linked to mitochondria (60).
Cell fate and apoptosis.
Toxoplasma-infected cells are profoundly resistant to apoptosis along both the intrinsic mitochondrion-dependent pathway and the extrinsic death receptor pathway (11, 12, 32, 33, 66, 67). Transcriptional data indicate that apoptosis-related genes activated by infection and regulated by NF-κB play a critical role in the inhibition of apoptosis (62, 63); however, posttranscriptional mechanisms are also involved, as seen with the Bcl2 family of proteins. Parasite infection results in an infection-dependent selective degradation of proapoptotic members of the Bcl2 family (Bad and Bax), but not the antiapoptotic Bcl2 (11).
While it did not detect the modulation of Bcl2 family proteins (modulation is observed only at infection rates much higher than those used here [11]), our proteomic study revealed the modulation of 16 proteins implicated in apoptosis in the infected cell (indicated in Table 1). These proteins impinge on diverse cellular pathways, with several focused on mitochondrial activities. Among these, VDAC (for voltage-dependent anion channel, also known as the mitochondrial porin) presents an example of a duality of function (Table 1, Cell fate). VDAC in normal cells forms a component of the adenine nucleotide transport mechanism across the mitochondrial outer membrane while contributing to cell death by being a component of the permeability transition pore leading to cytochrome c release, triggering apoptosis (19).
Heat shock protein 27 (HSP27) and HSP70 were both modulated in the infected host cell (Table 1). These proteins inhibit key effectors of the apoptotic pathway and play roles in the proteasome-mediated degradation of apoptosis-regulatory proteins (30). HSP27 can prevent the formation of the apoptosome and the subsequent activation of caspases (10), while HSP70 protects cells from stress-induced caspase-dependent apoptosis, both upstream and downstream of the death-associated mitochondrial events (30).
Transcription, translation, and protein fate.
Six transcriptional proteins (brain abundant membrane signal protein, c14orf166, hnRNP K isoform a, protein inhibitor of activated STAT2, presenelin binding protein, and zinc finger protein) and two translational proteins (elongation factor 1 alpha and ubiquitin A-52) were identified as modulated in expression in the infected cell, with the general trend being strongly biased (eight out of nine) toward up-regulation of these transcriptional and translational proteins (Table 1, Transcription and Translation). Interestingly, our data appear to be in direct contrast to results with some virus infections, which found that the host translational mechanism is shut down in herpes simplex virus-, poliovirus-, influenza virus-, and enterovirus 71-infected cells (28, 53, 54). We also observed that HSPs were modulated during infection, which may be linked to an increase in transcription and translation, since HSPs are important in protein processing (30). HSPs are present in all cells under normal conditions and during stress (e.g., heat/cold/oxygen deprivation) and can make up to 15% of the total cellular protein content (68). HSPs ensure the correct conformation of proteins and facilitate the degradation of selected proteins by the ubiquitin/proteasome system, as well as their own inherent activities (30). An increase in proteolytic activity is also predicted to increase the pools of peptides that could be important in the presentation of antigens driving the immune response and, by extension, parasite differentiation (30, 96). Notably, host HSPs have been implicated in immunity to T. gondii (41). Another regulatory cascade known to be impacted by HSPs is the stress hypoxia pathway induced in parasite-infected cells (87). Seven HSPs, including HSP60, HSP70, and MTHSP75, were modulated in the parasite-infected cell, with a general trend of up-regulation.
Cellular transport.
Tubulin alpha (TUBA1) was shown to be modulated in infected cells. While providing structural function in the cell, microtubules are vital for intracellular transport and distribution of diverse organelles. Mitochondria, the ER, lysosomes, and components of both the endocytic and exocytic pathways are redistributed around the PV and within the infected cell following infection (15, 83). The transport of specific molecules across lipid membranes is an essential function of all cells, and the largest transporter gene family is the ATP-binding cassette transporter superfamily. Here, the ATP-binding cassette transporter A13 was shown to be up-regulated in infected cells. This anion transporter is a key gatekeeper influencing intracellular cholesterol transport (20) and is perhaps up-regulated during infection as part of the mechanism used to scavenge host cell lipids by the parasite (as discussed in “Intermediary metabolism” above).
Immune and stress responses.
Several proteins involved in oxidative stress were modulated during infection, including superoxide dismutase, HSP27, and protein disulfide isomerase (PDI)-related protein 5. Six isoforms of the antioxidant protein peroxiredoxin (PRDX) were identified as modulated in the infected host cell, indicating strong involvement in the cell response to infection. The NF-κB family of transcription factors participates in the regulation of many aspects of innate and adaptive immunity to T. gondii (59). Thioredoxin peroxidase (PRDX4), which was up-regulated during infection, has a regulatory role in the activation of NF-κB (44, 98). Annexin 1, which is thought to have anti-inflammatory activity (2), was also up-regulated, as were other proteins previously associated with the gene ontology category “response to pathogens and parasites,” including annexin 1, methylumbelliferyl acetate deacetylase, cardiotrophin-like cytokine, PRDX5, visceral adipose-specific serpin, and superoxide dismutase.
Structural proteins.
Structural proteins were the second-largest group of proteins to undergo modulation in expression following infection. As previously noted, infection with Toxoplasma causes extensive remodeling of the host cell at the level of the cytoskeleton. The up-regulation of alpha-tubulin, a component of the microtubule cytoskeleton, is discussed above. Notably, actin and actin-related proteins representing key components of microfilaments are down-regulated, while the microfilament-dependent motor protein myosin heavy chain is induced. Together, these results suggest infection has a global impact on the two main cytoskeletal systems within the cell. A third class of cytoskeletal structures is the intermediate filaments, typified by the protein vimentin (94). Vimentin has been implicated in diverse functions, from structural roles to roles in the cell cycle and mitosis (94), as well as lipid metabolism, particularly in regard to the biology of lipid droplets (76), whose roles in lipid storage and metabolism are the likely link to energy metabolism and mitochondrial function (3). More relevant to this study, vimentin is reorganized around the PV in T. gondii-infected cells (15, 38). Vimentin was found to be heavily modulated in the infected cell. As discussed below, vimentin is phosphorylated and is present in several isoforms in the cell (2), and our data suggest that the PTM of host vimentin has an important function in the context of T. gondii infection (see below).
PVM-associated organelle isolation.
Organelles, such as the mitochondria and the ER, are known to associate with the PVM (Fig. 4) (86). Interestingly, over one-third of all the proteins detected as being differentially expressed in the whole-cell analysis were determined to be related to mitochondrion function based on the ontogeny programs employed (Table 1), with a number of additional proteins known to be localized to the ER (BiP and PDI). In order to investigate this phenomenon in more detail, a subproteome enriched in the organelles known to associate with the PVM was prepared by sucrose flotation gradient from both infected and noninfected cells, and differential protein expression was investigated using DIGE analysis. Western blots were run to determine the enrichment of the organelle preparations (Fig. 7). Probing with anti-cytochrome c and anti-calnexin confirmed enrichment for both mitochondria and the ER.
FIG. 7.
Immunoblot analysis to determine organelle enrichment. Aliquots of the pellets resulting from the centrifugation (10,000 × g) of the fractions collected from the sucrose gradient were analyzed via immunoblotting to determine the levels of mitochondrial and ER enrichment. This analysis, performed using antibodies recognizing the mitochondrial protein cytochrome c and the ER marker calnexin, showed that the subcellular fractionation protocol described above resulted in enrichment of PVM-associated organelles. Note the change in the distribution of the densities of these organelles in infected cells, which potentially reflects the significant changes in their physiologies and activities possibly contributed to by changes in their protein compositions and protein/lipid ratios.
Twenty-three proteins were identified by DIGE as being differentially expressed between the PVM organelle-enriched fractions from infected and noninfected cells (Table 2). Twelve of these are recognized as being of mitochondrial origin as determined by MitoProteome (http://www.mitoproteome.org). Three were identified as ER proteins, and the remaining eight proteins were from other origins. Nine proteins were identified as modulated that had not been previously identified in the whole-cell proteome analysis (Table 2). Of interest was modulation of the energy metabolism enzyme pyruvate dehydrogenase, which is necessary for condensation to citrate and required for the tricarboxylic acid metabolic cycle resulting in energy production in the cell (4). Two metabolic proteins (glutamate dehydrogenase and 3-hydroxyisobutyrate dehydrogenase) and a second isoform of the oxidative phosphorylation protein ATP synthase (V1 subunit A) were identified as modulated, again highlighting the dramatic metabolic changes occurring in the host cell. Further work using more specific organelle enrichment techniques will eventually allow a more precise proteomic analysis of the roles of these host cell organelles during parasite infection.
TABLE 2.
Proteins modulated in the PVM-associated organelles from T. gondii-infected and noninfected cells
| Protein name | Gene name | Localization | Matches to HFF dataa | Functional category |
|---|---|---|---|---|
| Glutamate dehydrogenase | GLUD1 | Mitochondria | No match | Metabolism |
| ATPase, H+ transporting, lysosomal 70 kDa, V1 subunit Ab | ATP6V1A | Mitochondria | No match | Metabolism |
| 3-Hydroxyisobutyrate dehydrogenase | HIBADH | Mitochondria | No match | Metabolism |
| ATP synthase, H+ transporting, mitochondrial F1 complex beta | ATP5B | Mitochondria | Glycolysis | |
| Protein disulfide isomerase | P4HB | Mitochondria | Glycolysis | |
| Pyruvate dehydrogenase | PDHB | Mitochondria | No match | Glycolysis |
| Polymerase I and transcript release factor | PTRF | Mitochondria | No match | Transcription |
| Heat shock 70-kDa protein 5 | HSPA5 | ER | Protein fate | |
| Heat shock 70-kDa 9B | HSPA9B | Mitochondria | Protein fate | |
| Heat shock protein 60 | HSPD1 | Mitochondria | Protein fate | |
| Tumor rejection antigen (Gp96) 1 | HSP901B | Mitochondria | Protein fate | |
| ES/130 ribosome receptor | RRBP1 | ER | No match | Protein binding |
| Protein kinase C, delta binding protein | PRKCDBP | Not clear | No match | Signal transduction |
| Proliferation-inducing gene 20 protein | RCN1 | ER | Signal transduction | |
| Human serum albumin 1 | ALB | Secreted protein | Cell fate | |
| Actin gamma | ACTG | Cytoskeleton | Structural | |
| Capping protein (actin filament) muscle Z-line, alpha 1 | CAPZA1 | Cytoskeleton | Structural | |
| Cytoskeleton-associated protein | CKAP1 | Cytoskeleton | No match | Structural |
| Lamin c | LMNA | Mitochondria | Structural | |
| Moesin (membrane-organizing extension spike protein) | MSN | Mitochondria | Structural | |
| Restin protein | RSN | Cytoskeleton | No match | Structural |
| Vimentin | VIM | Mitochondria | Structural | |
| Tropomyosin kappa sarcomeric (tropomyosin 3) | TPM3 | Cytoskeleton | Interaction with cellular environment |
Proteins that were not identified in the whole-cell DIGE experiment are denoted by “no match.”
Note that ATPase is likely to represent lysosomal contamination rather than being of mitochondrial origin.
Gene-protein correlation.
Our proteomic study was designed to complement as far as possible a previously published analysis of differential gene expression at the transcriptional level using microarrays (6). In order to determine the extent to which our data were compatible with the published transcriptional data, 96 modulated proteins that matched genes on the array were compared (Table 1). Of these, 35% agreed in the direction of change, i.e., both gene and protein expression were up-regulated due to the presence of the parasite or both gene and protein expression were down-regulated after infection. Fifteen percent of the matched genes and proteins differed in terms of expression direction. The remaining 50% of the modulated proteins matched clones from the microarray experiment that had a volume change of less than twofold and that were therefore considered not to be significantly modulated.
A second, more quantitative comparison involved the actual volume differences in gene and protein expression. The proteins that were modulated in the DIGE experiment and matched modulated genes on the array were compared by volume using the Spearman's rank correlation coefficient to quantify the association between the two variables. The calculated r value was 0.40, showing a weak positive correlation between protein and transcriptional expression. There are several reasons that might explain the apparently weak correlation between mRNA and protein: (i) proteomic bias (e.g., proteins with masses greater than 100,000 and those with pI values of less than 4 and greater than 9 are often not detectable on 2-DE gels) (39), (ii) mRNAs differ in their rates of translation into protein (69), and (iii) proteins differ in their in vivo half-lives (34, 69). Finally, the capacity of T. gondii to multiply within experimentally enucleated cells (46, 78) suggests that posttranscriptional events and steady-state protein levels no longer replenished by de novo transcription are sufficient to sustain growth.
Investigation into the phosphoproteome of T. gondii-infected cells.
Our 2-DE analyses demonstrated that many of the protein changes following parasite infection were likely to result from PTMs, since alteration in the mobility of the same protein species or identification of multiple isoforms of the same protein were relatively common (Fig. 2). In order to study one of the common PTMs, that of phosphorylation of proteins, we developed a modification of the DIGE technique (SAP-DIGE) in which SAP was used to dephosphorylate proteins from T. gondii-infected and noninfected cells. The phosphorylated and dephosphorylated fractions were then differentially labeled with Cy dyes and analyzed by DIGE. Loss of the phosphate groups alters the mobility of proteins in the 2-DE gel, which in combination with DIGE analysis enables phosphorylated proteins to be identified. Dephosphorylation of proteins has previously been used in 1-DE and MS studies (45, 88). T. gondii-infected and noninfected cell extracts were divided, and one half of each sample was treated with SAP. The four extracts were then separated on two DIGE gels, the spot maps were compared, and differentially expressed proteins were identified using MS (Table 3). Caldesmon, calreticulin, nucleobindin, PDI, and thyroid hormone binding protein precursor were shown to be dephosphorylated in infected cells. Interestingly, parasite infection also causes down-regulation of caldesmon (as seen in the previous conventional and DIGE 2-DE experiments). It is possible that the additional dephosphorylation of this protein may also lead to its inactivation in the infected cell. Caldesmon is an actin and myosin binding protein involved in mitosis (37); therefore, it is possible that dephosphorylation of this protein following infection may be related to an inhibition of mitosis and subsequent cell division. Hai and Gu suggested that phosphorylation of caldesmon causes regulation of the protein and inhibition of its activity (37). Vimentin, chaperonin, and lamin A/C isoform 1 protein were shown to undergo phosphorylation in the infected cell, but not in the noninfected cell. Vimentin is known to be one of the most prominent phosphoproteins in the cell and is associated with filament reorganization (92). Its activation may be related to the structural reorganization that takes place during infection or, alternatively, may reflect physiological changes consistent with the alteration of the cell cycle (91). Consistent with this theme, changes in the phosphorylation of the lamins occurs during different phases of the cell cycle (9). Lamin proteins are thought to be involved in nuclear stability, chromatin structure, and gene expression (75).
TABLE 3.
Proteins undergoing a change in phosphorylation state following infection with T. gondii as identified using SAP-DIGE
| Protein | Phosphorylation status in infected cells | Functional category |
|---|---|---|
| Caldesmon | Unphosphorylated | Mitosis |
| Calreticulin | Unphosphorylated | Protein folding |
| Nucleobindin | Unphosphorylated | Protein fate |
| Protein disulfide isomerase | Unphosphorylated | Structural |
| Thyroid hormone binding protein | Unphosphorylated | Signal transduction/cellular communication |
| Chaperonin (HSP60) | Phosphorylated | Energy metabolism |
| LMNA protein | Phosphorylated | Unknown |
| Vimentin | Phosphorylated | Structural |
Concluding remarks.
This study represents the first quantitative analysis of the modulation of a host cell proteome by an intracellular parasite, providing evidence for global reprogramming of cell metabolism and demonstrating the intimate relationship between pathogen and host. Spear et al. (87) previously classified genes modulated in response to Toxoplasma infection into three categories based on their roles in the pathogen-infected cell: (i) prohost, i.e., genes required by the host cell for defense against the invading pathogen; (ii) proparasite, i.e., host genes required for parasite survival; and (iii) bystander, i.e., genes that are differentially regulated as a consequence of modulations in the first two categories. Potential proparasite response proteins identified in our study include the large number of apoptotic proteins modulated in the host cell and also the up-regulation of glycolytic enzymes. Many prohost proteins were also identified, including up-regulation of the metabolic protein AKR1B1, which protects cells from toxic products, and down-regulation of hnRNP K, a protein that represses the induction of immune response genes.
Only a few comparative proteomic analyses of the host response to infection have been published to date, and these have focused on bacterial and viral pathogens (43, 97). A key question that arises from this study that demonstrates such an active and apparently specific response from the cell is to what degree the response is shared with all intracellular pathogens and which processes are specific to T. gondii (or Apicomplexa in general). Several groups have employed microarrays to analyze the host response to infection. A study by Vaena de Avalos investigated the host cell transcriptional response to infection with Trypanosoma cruzi (93). If these results are compared to transcriptional changes in Toxoplasma (6), very different expression profiles are apparent, indicating that at the transcriptional level at least, the host cell has a highly specific response to the two different parasites. We performed a preliminary DIGE study to investigate the proteomic response of HFFs infected with the nonapicomplexan intracellular parasite Leishmania major (data not shown). In this experiment, infection with L. major revealed significant down-regulation of 34 protein spots and up-regulation of 19 spots. This compares to down-regulation of 141 protein spots and up-regulation of 213 protein spots in the equivalent T. gondii experiment. Moreover, most of the differentially expressed proteins identified during L. major infection were structural proteins, including down-regulation of actin beta and tubulin beta. Importantly, however, apart from structural proteins, very few of the differentially expressed proteins were common to both experiments, suggesting that the response of the cell proteome to T. gondii may be quite specific. Further work is now needed to examine host cell proteome responses in a range of Apicomplexa and other intracellular protozoa.
Acknowledgments
This work was supported by BBSRC funding to J.M.W. The contributions to this work from the Sinai laboratory were supported by NIH grant AI062826 to A.P.S.
We are grateful for discussions with I. Blader and J. Boothroyd (Stanford University Medical School) of the interpretation of published microarray data. We thank G. H. Coombs, University of Strathclyde, and J. McGregor, University of Glasgow, for the provision of Leishmania parasites. We thank P. H. Whitfield, University of Liverpool, for helpful comments on the manuscript.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Ainscow, E. K., and M. D. Brand. 1999. Internal regulation of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur. J. Biochem. 266737-749. [DOI] [PubMed] [Google Scholar]
- 2.Alfonso, P., A. Nunez, J. Madoz-Gurpide, L. Lombardia, L. Sanchez, and J. I. Casal. 2005. Proteomic expression analysis of colorectal cancer by two-dimensional differential gel electrophoresis. Proteomics 52602-2611. [DOI] [PubMed] [Google Scholar]
- 3.Annunen-Rasila, J., S. Ohlmeier, H. Tuokko, J. Veijola, and K. Majamaa. 2007. Proteome and cytoskeleton responses in osteosarcoma cells with reduced OXPHOS activity. Proteomics 72189-2200. [DOI] [PubMed] [Google Scholar]
- 4.Arjunan, P., N. Nemeria, A. Brunskill, K. Chandrasekhar, M. Sax, Y. Yan, F. Jordan, J. R. Guest, and W. Furey. 2002. Structure of the pyruvate dehydrogenase multienzyme complex E1 component from Escherichia coli at 1.85 Å resolution. Biochemistry 415213-5221. [DOI] [PubMed] [Google Scholar]
- 5.Belcher, C. E., J. Drenkow, B. Kehoe, T. R. Gingeras, N. McNamara, H. Lemjabbar, C. Basbaum, and D. A. Relman. 2000. The transcriptional responses of respiratory epithelial cells to Bordetella pertussis reveal host defensive and pathogen counter-defensive strategies. Proc. Natl. Acad. Sci. USA 9713847-13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blader, I. J., I. D. Manger, and J. C. Boothroyd. 2001. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J. Biol. Chem. 27624223-24231. [DOI] [PubMed] [Google Scholar]
- 7.Bosinger, S. E., K. A. Hosiawa, M. J. Cameron, D. Persad, L. Ran, L. Xu, M. R. Boulassel, M. Parenteau, J. Fournier, E. W. Rud, and D. J. Kelvin. 2004. Gene expression profiling of host response in models of acute HIV infection. J. Immunol. 1736858-6863. [DOI] [PubMed] [Google Scholar]
- 8.Bradley, P. J., C. Ward, S. J. Cheng, D. L. Alexander, S. Coller, G. H. Coombs, J. D. Dunn, D. J. Ferguson, S. J. Sanderson, J. M. Wastling, and J. C. Boothroyd. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 28034245-34258. [DOI] [PubMed] [Google Scholar]
- 9.Bridger, J. M., N. Foeger, I. R. Kill, and H. Herrmann. 2007. The nuclear lamina. Both a structural framework and a platform for genome organization. FEBS J. 2741354-1361. [DOI] [PubMed] [Google Scholar]
- 10.Bruey, J. M., C. Ducasse, P. Bonniaud, L. Ravagnan, S. A. Susin, C. Diaz-Latoud, S. Gurbuxani, A. P. Arrigo, G. Kroemer, E. Solary, and C. Garrido. 2000. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2645-652. [DOI] [PubMed] [Google Scholar]
- 11.Carmen, J. C., L. Hardi, and A. P. Sinai. 2006. Toxoplasma gondii inhibits ultraviolet light-induced apoptosis through multiple interactions with the mitochondrion-dependent programmed cell death pathway. Cell. Microbiol. 8301-315. [DOI] [PubMed] [Google Scholar]
- 12.Carmen, J. C., and A. P. Sinai. 2007. Suicide prevention: disruption of apoptotic pathways by protozoan parasites. Mol. Microbiol. 64904-916. [DOI] [PubMed] [Google Scholar]
- 13.Charron, A. J., and L. D. Sibley. 2002. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 1153049-3059. [DOI] [PubMed] [Google Scholar]
- 14.Coppens, I. 2006. Contribution of host lipids to Toxoplasma pathogenesis. Cell. Microbiol. 81-9. [DOI] [PubMed] [Google Scholar]
- 15.Coppens, I., J. D. Dunn, J. D. Romano, M. Pypaert, H. Zhang, J. C. Boothroyd, and K. A. Joiner. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125261-274. [DOI] [PubMed] [Google Scholar]
- 16.Coppens, I., A. P. Sinai, and K. A. Joiner. 2000. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 149167-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox, B., T. Kislinger, and A. Emili. 2005. Integrating gene and protein expression data: pattern analysis and profile mining. Methods 35303-314. [DOI] [PubMed] [Google Scholar]
- 18.Crawford, M. J., N. Thomsen-Zieger, M. Ray, J. Schachtner, D. S. Roos, and F. Seeber. 2006. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO J. 253214-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crompton, M. 1999. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341233-249. [PMC free article] [PubMed] [Google Scholar]
- 20.Dean, M., Y. Hamon, and G. Chimini. 2001. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 421007-1017. [PubMed] [Google Scholar]
- 21.Detweiler, C. S., D. B. Cunanan, and S. Falkow. 2001. Host microarray analysis reveals a role for the Salmonella response regulator phoP in human macrophage cell death. Proc. Natl. Acad. Sci. USA 985850-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobbelaere, D. A., and S. Rottenberg. 2003. Theileria-induced leukocyte transformation. Curr. Opin. Microbiol. 6377-382. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak, J. A., and M. S. Crane. 1981. Vertebrate cell cycle modulates infection by protozoan parasites. Science 2141034-1036. [DOI] [PubMed] [Google Scholar]
- 24.Eskes, R., S. Desagher, B. Antonsson, and J. C. Martinou. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forst, C. V. 2006. Host-pathogen systems biology. Drug Discov. Today 11220-227. [DOI] [PubMed] [Google Scholar]
- 26.Fouts, A. E., and J. C. Boothroyd. 2007. Infection with Toxoplasma gondii bradyzoites has a diminished impact on host transcript levels relative to tachyzoite infection. Infect. Immun. 75634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gail, M., U. Gross, and W. Bohne. 2001. Transcriptional profile of Toxoplasma gondii-infected human fibroblasts as revealed by gene-array hybridization. Mol. Genet. Genomics 265905-912. [DOI] [PubMed] [Google Scholar]
- 28.Garfinkel, M. S., and M. G. Katze. 1992. Translational control by influenza virus. Selective and cap-dependent translation of viral mRNAs in infected cells. J. Biol. Chem. 2679383-9390. [PubMed] [Google Scholar]
- 29.Garland, J. M. 2000. Regulation of apoptosis by cell metabolism, cytochrome c and the cytoskeleton. Symp. Soc. Exp. Biol. 5281-118. [PubMed] [Google Scholar]
- 30.Garrido, C., E. Schmitt, C. Cande, N. Vahsen, A. Parcellier, and G. Kroemer. 2003. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2579-584. [PubMed] [Google Scholar]
- 31.Gilbert, L. A., S. Ravindran, J. M. Turetzky, J. C. Boothroyd, and P. J. Bradley. 2007. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot. Cell 673-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goebel, S., U. Gross, and C. G. Luder. 2001. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J. Cell Sci. 1143495-3505. [DOI] [PubMed] [Google Scholar]
- 33.Goebel, S., C. G. Luder, and U. Gross. 1999. Invasion by Toxoplasma gondii protects human-derived HL-60 cells from actinomycin D-induced apoptosis. Med. Microbiol. Immunol. 187221-226. [DOI] [PubMed] [Google Scholar]
- 34.Greenbaum, D., N. M. Luscombe, R. Jansen, J. Qian, and M. Gerstein. 2001. Interrelating different types of genomic data, from proteome to secretome: 'oming in on function. Genome Res. 111463-1468. [DOI] [PubMed] [Google Scholar]
- 35.Gupta, N., M. M. Zahn, I. Coppens, K. A. Joiner, and D. R. Voelker. 2005. Selective disruption of phosphatidylcholine metabolism of the intracellular parasite Toxoplasma gondii arrests its growth. J. Biol. Chem. 28016345-16353. [DOI] [PubMed] [Google Scholar]
- 36.Gygi, S. P., Y. Rochon, B. R. Franza, and R. Aebersold. 1999. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 191720-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hai, C. M., and Z. Gu. 2006. Caldesmon phosphorylation in actin cytoskeletal remodeling. Eur. J. Cell Biol. 85305-309. [DOI] [PubMed] [Google Scholar]
- 38.Halonen, S. K., and E. Weidner. 1994. Overcoating of Toxoplasma parasitophorous vacuoles with host cell vimentin type intermediate filaments. J. Eukaryot. Microbiol. 4165-71. [DOI] [PubMed] [Google Scholar]
- 39.Harry, J. L., M. R. Wilkins, B. R. Herbert, N. H. Packer, A. A. Gooley, and K. L. Williams. 2000. Proteomics: capacity versus utility. Electrophoresis 211071-1081. [DOI] [PubMed] [Google Scholar]
- 40.Hebert, J. L., Y. Lecarpentier, K. Zamani, C. Coirault, G. Daccache, and D. Chemla. 1995. Relation between aortic dicrotic notch pressure and mean aortic pressure in adults. Am. J. Cardiol. 76301-306. [DOI] [PubMed] [Google Scholar]
- 41.Hisaeda, H., and K. Himeno. 1997. The role of host-derived heat-shock protein in immunity against Toxoplasma gondii infection. Parasitol. Today 13465-468. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa, N., N. Shimada, Y. Takagi, Y. Ishijima, M. Fukuda, and N. Kimura. 2003. Molecular evolution of nucleoside diphosphate kinase genes: conserved core structures and multiple-layered regulatory regions. J. Bioenerg. Biomembr. 357-18. [DOI] [PubMed] [Google Scholar]
- 43.Jiang, X. S., L. Y. Tang, J. Dai, H. Zhou, S. J. Li, Q. C. Xia, J. R. Wu, and R. Zeng. 2005. Quantitative analysis of severe acute respiratory syndrome (SARS)-associated coronavirus-infected cells using proteomic approaches: implications for cellular responses to virus infection. Mol. Cell Proteomics 4902-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin, D. Y., H. Z. Chae, S. G. Rhee, and K. T. Jeang. 1997. Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation. J. Biol. Chem. 27230952-30961. [DOI] [PubMed] [Google Scholar]
- 45.Jonas, B. A., and M. L. Privalsky. 2004. SMRT and N-CoR corepressors are regulated by distinct kinase signaling pathways. J. Biol. Chem. 27954676-54686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones, T. C. 1973. Multiplication of toxoplasmas in enucleate fibroblasts. Proc. Soc. Exp. Biol. Med. 1421268-1271. [DOI] [PubMed] [Google Scholar]
- 47.Kohler, C., A. Gahm, T. Noma, A. Nakazawa, S. Orrenius, and B. Zhivotovsky. 1999. Release of adenylate kinase 2 from the mitochondrial intermembrane space during apoptosis. FEBS Lett. 44710-12. [DOI] [PubMed] [Google Scholar]
- 48.Koivunen, P., M. Hirsila, A. M. Remes, I. E. Hassinen, K. I. Kivirikko, and J. Myllyharju. 2007. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem. 2824524-4532. [DOI] [PubMed] [Google Scholar]
- 49.Krug, E. C., J. J. Marr, and R. L. Berens. 1989. Purine metabolism in Toxoplasma gondii. J. Biol. Chem. 26410601-10607. [PubMed] [Google Scholar]
- 50.Krylov, S. N., Z. Zhang, N. W. Chan, E. Arriaga, M. M. Palcic, and N. J. Dovichi. 1999. Correlating cell cycle with metabolism in single cells: combination of image and metabolic cytometry. Cytometry 3714-20. [DOI] [PubMed] [Google Scholar]
- 51.Kumar, M., R. Verma, and G. P. Raghava. 2006. Prediction of mitochondrial proteins using support vector machine and hidden Markov model. J. Biol. Chem. 2815357-5363. [DOI] [PubMed] [Google Scholar]
- 52.Kushmerick, M. J. 2005. From crossbridges to metabolism: system biology for energetics. Adv. Exp. Med. Biol. 565171-182, 379-395. [DOI] [PubMed] [Google Scholar]
- 53.Kuyumcu-Martinez, N. M., M. E. Van Eden, P. Younan, and R. E. Lloyd. 2004. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: a novel mechanism for host translation shutoff. Mol. Cell. Biol. 241779-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leong, W. F., and V. T. Chow. 2006. Transcriptomic and proteomic analyses of rhabdomyosarcoma cells reveal differential cellular gene expression in response to enterovirus 71 infection. Cell Microbiol. 8565-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Roch, K. G., J. R. Johnson, L. Florens, Y. Zhou, A. Santrosyan, M. Grainger, S. F. Yan, K. C. Williamson, A. A. Holder, D. J. Carucci, J. R. Yates III, and E. A. Winzeler. 2004. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 142308-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lipskaya, T. Y., and K. N. Plakida. 2003. In rat liver mitochondria all nucleoside diphosphate kinase of the outer compartment is associated with the outer surface of the outer membrane. Biochemistry (Moscow) 681136-1144. [DOI] [PubMed] [Google Scholar]
- 57.Lipskaya, T. Y., and V. V. Voinova. 2005. Functional coupling between nucleoside diphosphate kinase of the outer mitochondrial compartment and oxidative phosphorylation. Biochemistry (Moscow) 701354-1362. [DOI] [PubMed] [Google Scholar]
- 58.Martin, A. M., T. Liu, B. C. Lynn, and A. P. Sinai. 2007. The Toxoplasma gondii parasitophorous vacuole membrane: transactions across the border. J. Eukaryot. Microbiol. 5425-28. [DOI] [PubMed] [Google Scholar]
- 59.Mason, N. J., D. Artis, and C. A. Hunter. 2004. New lessons from old pathogens: what parasitic infections have taught us about the role of nuclear factor-κB in the regulation of immunity. Immunol. Rev. 20148-56. [DOI] [PubMed] [Google Scholar]
- 60.Mishra, S., L. C. Murphy, and L. J. Murphy. 2006. The prohibitins: emerging roles in diverse functions. J. Cell. Mol. Med. 10353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molestina, R. E., T. M. Payne, I. Coppens, and A. P. Sinai. 2003. Activation of NF-κB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IκB to the parasitophorous vacuole membrane. J. Cell Sci. 1164359-4371. [DOI] [PubMed] [Google Scholar]
- 62.Molestina, R. E., and A. P. Sinai. 2005. Detection of a novel parasite kinase activity at the Toxoplasma gondii parasitophorous vacuole membrane capable of phosphorylating host IκBα. Cell. Microbiol. 7351-362. [DOI] [PubMed] [Google Scholar]
- 63.Molestina, R. E., and A. P. Sinai. 2005. Host and parasite-derived IKK activities direct distinct temporal phases of NF-κB activation and target gene expression following Toxoplasma gondii infection. J. Cell Sci. 1185785-5796. [DOI] [PubMed] [Google Scholar]
- 64.Morris, M. T., A. Coppin, S. Tomavo, and V. B. Carruthers. 2002. Functional analysis of Toxoplasma gondii protease inhibitor 1. J. Biol. Chem. 27745259-45266. [DOI] [PubMed] [Google Scholar]
- 65.Munger, J., S. U. Bajad, H. A. Coller, T. Shenk, and J. D. Rabinowitz. 2006. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nash, P. B., M. B. Purner, R. P. Leon, P. Clarke, R. C. Duke, and T. J. Curiel. 1998. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J. Immunol. 1601824-1830. [PubMed] [Google Scholar]
- 67.Payne, T. M., R. E. Molestina, and A. P. Sinai. 2003. Inhibition of caspase activation and a requirement for NF-κB function in the Toxoplasma gondii-mediated blockade of host apoptosis. J. Cell Sci. 1164345-4358. [DOI] [PubMed] [Google Scholar]
- 68.Pockley, A. G. 2001. Heat shock proteins in health and disease: therapeutic targets or therapeutic agents? Exp. Rev. Mol. Med. 31-21. [DOI] [PubMed] [Google Scholar]
- 69.Pratt, J. M., J. Petty, I. Riba-Garcia, D. H. Robertson, S. J. Gaskell, S. G. Oliver, and R. J. Beynon. 2002. Dynamics of protein turnover, a missing dimension in proteomics. Mol. Cell Proteomics 1579-591. [DOI] [PubMed] [Google Scholar]
- 70.Pszenny, V., S. O. Angel, V. G. Duschak, M. Paulino, B. Ledesma, M. I. Yabo, E. Guarnera, A. M. Ruiz, and E. J. Bontempi. 2000. Molecular cloning, sequencing and expression of a serine proteinase inhibitor gene from Toxoplasma gondii. Mol. Biochem. Parasitol. 107241-249. [DOI] [PubMed] [Google Scholar]
- 71.Radke, J. R., R. G. Donald, A. Eibs, M. E. Jerome, M. S. Behnke, P. Liberator, and M. W. White. 2006. Changes in the expression of human cell division autoantigen-1 influence Toxoplasma gondii growth and development. PLoS Pathog. 2e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 4527-63. [DOI] [PubMed] [Google Scholar]
- 73.Roskams, A. J., V. Friedman, C. M. Wood, L. Walker, G. A. Owens, D. A. Stewart, M. S. Altus, D. B. Danner, X. T. Liu, and J. K. McClung. 1993. Cell cycle activity and expression of prohibitin mRNA. J. Cell. Physiol. 157289-295. [DOI] [PubMed] [Google Scholar]
- 74.Saeij, J. P., S. Coller, J. P. Boyle, M. E. Jerome, M. W. White, and J. C. Boothroyd. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445324-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schirmer, E. C., and L. Gerace. 2004. The stability of the nuclear lamina polymer changes with the composition of lamin subtypes according to their individual binding strengths. J. Biol. Chem. 27942811-42817. [DOI] [PubMed] [Google Scholar]
- 76.Schweitzer, S. C., and R. M. Evans. 1998. Vimentin and lipid metabolism. Subcell. Biochem. 31437-462. [PubMed] [Google Scholar]
- 77.Sehgal, A., S. Bettiol, M. Pypaert, M. R. Wenk, A. Kaasch, I. J. Blader, K. A. Joiner, and I. Coppens. 2005. Peculiarities of host cholesterol transport to the unique intracellular vacuole containing Toxoplasma. Traffic 61125-1141. [DOI] [PubMed] [Google Scholar]
- 78.Sethi, K. K., B. Pelster, G. Piekarski, and H. Brandis. 1973. Multiplication of Toxoplasma gondii in enucleated L cells. Nat. New Biol. 243255-256. [DOI] [PubMed] [Google Scholar]
- 79.Sexton, A. C., R. T. Good, D. S. Hansen, M. C. D'Ombrain, L. Buckingham, K. Simpson, and L. Schofield. 2004. Transcriptional profiling reveals suppressed erythropoiesis, up-regulated glycolysis, and interferon-associated responses in murine malaria. J. Infect. Dis. 1891245-1256. [DOI] [PubMed] [Google Scholar]
- 80.Sibley, L. D. 2004. Intracellular parasite invasion strategies. Science 304248-253. [DOI] [PubMed] [Google Scholar]
- 81.Sibley, L. D. 2002. No more free lunch. Nature 415843-844. [DOI] [PubMed] [Google Scholar]
- 82.Sibley, L. D. 2003. Toxoplasma gondii: perfecting an intracellular life style. Traffic 4581-586. [DOI] [PubMed] [Google Scholar]
- 83.Sinai, A. P., and K. A. Joiner. 1997. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu. Rev. Microbiol. 51415-462. [DOI] [PubMed] [Google Scholar]
- 84.Sinai, A. P., and K. A. Joiner. 2001. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J. Cell Biol. 15495-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinai, A. P., S. Paul, M. Rabinovitch, G. Kaplan, and K. A. Joiner. 2000. Coinfection of fibroblasts with Coxiella burnetii and Toxoplasma gondii: to each their own. Microbes Infect. 2727-736. [DOI] [PubMed] [Google Scholar]
- 86.Sinai, A. P., P. Webster, and K. A. Joiner. 1997. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci. 1102117-2128. [DOI] [PubMed] [Google Scholar]
- 87.Spear, W., D. Chan, I. Coppens, R. S. Johnson, A. Giaccia, and I. J. Blader. 2006. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell. Microbiol. 8339-352. [DOI] [PubMed] [Google Scholar]
- 88.Stensballe, A., S. Andersen, and O. N. Jensen. 2001. Characterization of phosphoproteins from electrophoretic gels by nanoscale Fe(III) affinity chromatography with off-line mass spectrometry analysis. Proteomics 1207-222. [DOI] [PubMed] [Google Scholar]
- 89.Tanabe, K., and K. Murakami. 1984. Reduction in the mitochondrial membrane potential of Toxoplasma gondii after invasion of host cells. J. Cell Sci. 7073-81. [DOI] [PubMed] [Google Scholar]
- 90.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 301217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tolstonog, G. V., R. L. Shoeman, U. Traub, and P. Traub. 2001. Role of the intermediate filament protein vimentin in delaying senescence and in the spontaneous immortalization of mouse embryo fibroblasts. DNA Cell Biol. 20509-529. [DOI] [PubMed] [Google Scholar]
- 92.Turowski, P., T. Myles, B. A. Hemmings, A. Fernandez, and N. J. Lamb. 1999. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell 101997-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaena de Avalos, S., I. J. Blader, M. Fisher, J. C. Boothroyd, and B. A. Burleigh. 2002. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J. Biol. Chem. 277639-644. [DOI] [PubMed] [Google Scholar]
- 94.Wang, N., and D. Stamenovic. 2002. Mechanics of vimentin intermediate filaments. J. Muscle Res. Cell. Motil. 23535-540. [DOI] [PubMed] [Google Scholar]
- 95.Wastling, J. M., and P. J. Bradley. 2007. Proteomic analysis of the rhoptry organelles of Toxoplasma gondii, p. 455-474. In J. W. Ajioka and D. Soldati (ed.), Toxoplasma: molecular and cellular biology. Horizon BioScience, Norfolk, United Kingdom.
- 96.Yap, G. S., and A. Sher. 2002. The use of germ line-mutated mice in understanding host-pathogen interactions. Cell. Microbiol. 4627-634. [DOI] [PubMed] [Google Scholar]
- 97.Zhang, C. G., B. A. Chromy, and S. L. McCutchen-Maloney. 2005. Host-pathogen interactions: a proteomic view. Exp. Rev. Proteomics 2187-202. [DOI] [PubMed] [Google Scholar]
- 98.Zhang, P., B. Liu, S. W. Kang, M. S. Seo, S. G. Rhee, and L. M. Obeid. 1997. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of bcl-2. J. Biol. Chem. 27230615-30618. [DOI] [PubMed] [Google Scholar]
- 99.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 9514470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]