Abstract
Mycoplasma pneumoniae is a leading cause of pneumonia and is associated with asthma. Evidence links M. pneumoniae respiratory disease severity with interleukin-12 (IL-12) concentrations in respiratory secretions. We evaluated the effects of IL-12 therapy on microbiologic, inflammatory, and pulmonary function indices of M. pneumoniae pneumonia in mice. BALB/c mice were inoculated with M. pneumoniae or SP4 broth. Mice were treated with intranasal IL-12 or placebo daily for 8 days, starting on day 1 after inoculation. Mice were evaluated at baseline and on days 1, 3, 6, and 8 after therapy. Outcome variables included quantitative bronchoalveolar lavage (BAL) M. pneumoniae culture, lung histopathologic score (HPS), BAL cytokine concentrations determined by enzyme-linked immunosorbent assay (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], IL-1b, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, and granulocyte-macrophage colony-stimulating factor), and plethysmography, both before and after methacholine treatment. M. pneumoniae-infected mice treated with IL-12 (MpIL12 mice) were found to have significantly higher BAL M. pneumoniae concentrations than those of M. pneumoniae-infected mice treated with placebo (MpP mice) (P < 0.001). MpIL12 mice had higher BAL concentrations of IL-12, IFN-γ, TNF-α, and IL-6, with differences in IL-12 and IFN-γ concentrations reaching statistical significance (P < 0.001). Airway obstruction was statistically elevated in MpIL12 mice compared to that in MpP mice (P = 0.048), while airway hyperreactivity was also elevated in MpIL12 mice but did not reach statistical significance (P = 0.081). Lung parenchymal pneumonia subscores were significantly higher in MpIL12 mice (P < 0.001), but no difference was found for overall HPS, even though a strong trend was noticed (P = 0.051). Treatment of experimental M. pneumoniae pneumonia with intranasal IL-12 was associated with more severe pulmonary disease and less rapid microbiologic and histological resolution.
Interleukin-12 (IL-12) is a heterodimeric cytokine composed of two subunits (p40 and p35) that is produced by antigen-presenting cells (phagocytes, dendritic cells, and Langerhans cells). IL-12 has a regulatory effect on the innate and adaptive immune responses targeting natural killer and T cells inducing the production of cytokines, most importantly gamma interferon (IFN-γ; the Th1 effector cytokine) (16, 24, 39). Recent studies indicate that IL-12 has an important role in increasing mucosa-associated immune defenses, and it has been investigated therapeutically in mice for the treatment of infection with various respiratory pathogens, such as Klebsiella pneumoniae, Francisella tularensis, and Mycobacterium avium. The therapeutic use of cytokines alone or in combination with antimicrobials for infectious disease has shown promising results by promoting the clearance of the involved pathogen and increasing survival, especially for intracellular microorganisms (10, 11, 15, 32, 33, 37). Immunologic treatment strategies that directly target the respiratory mucosa may improve and potentiate the host response.
Mycoplasma pneumoniae is an atypical bacterium that lacks a cell wall and is recognized as an important etiologic agent of acute lower respiratory infection in children and adults; approximately 20 to 30% of community-acquired pneumonia cases in the general population are due to M. pneumoniae (2, 7, 29, 47). In the last decades, M. pneumoniae respiratory infection has drawn increasing attention for its association with reactive airway disease and asthma (25-27). However, the involved microbial, immunologic, and pathogenic pathways have yet to be defined clearly for respiratory infection with M. pneumoniae. Murine model studies have shown that M. pneumoniae infection induces a Th1 cytokine response in the lungs, manifested by elevated concentrations of IL-12 and IFN-γ with associated airway obstruction (AO), airway hyperreactivity (AHR), and lung histologic inflammation (12, 18). In a recent study utilizing IL-12 (p35) knockout (KO) mice in our established murine model of M. pneumoniae respiratory infection, we demonstrated, somewhat surprisingly, that the absence of IL-12 significantly reduced disease severity, as assessed by pulmonary histopathology, airway function, and quantitative cultures (36).
To further study the role of IL-12, we evaluated the effect of intranasal IL-12 treatment on airway dysfunction and inflammation in our established murine model of M. pneumoniae pneumonia (12, 13, 18, 19).
MATERIALS AND METHODS
Organism and growth conditions.
M. pneumoniae (ATCC 29342) was reconstituted in SP4 broth and subcultured after 24 to 48 h in a flask containing 20 ml of SP4 medium at 37°C. Approximately 72 h later, the broth turned an orange hue, the supernatant was decanted, and 2 ml of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved an M. pneumoniae concentration in the range of 108 to 109 CFU/ml (determined by plating dilutions on SP4 agar). Aliquots were stored at −80°C. SP4 medium contained nystatin (50 units/ml) and ampicillin (1.0 mg/ml) to inhibit the growth of potential contaminants.
Animals and inoculation.
Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee. BALB/c mice were obtained from Charles River Laboratories, who confirmed their mycoplasma- and murine virus-free status. Mice were housed in the animal care facility of our institution in filter-top cages and allowed to acclimate to their new environment for 1 week. Isofluorane, an inhaled anesthetic, was used for sedation during inoculation. Two-month-old female BALB/c mice were inoculated intranasally to achieve aspiration once (day 0) with 107 CFU of M. pneumoniae in 50 μl SP4 broth. Control BALB/c mice were inoculated with sterile SP4 broth. All mice were housed in the same animal room and received identical daily care for the duration of the experiment.
Experimental design and sample collection.
Starting 1 day after M. pneumoniae or SP4 inoculation, isofluorane-anesthetized mice were treated intranasally to achieve aspiration once daily for 8 days with 100 ng of active recombinant murine IL-12 (p70) (endotoxin level of <1.0 endotoxin unit per 1 μg of cytokine, as determined by the Limulus amebocyte lysate method; R&D Systems, Inc., MN) in sterile phosphate-buffered saline (PBS) containing 1% normal BALB/c mouse serum (PBS-NMS) or, in the case of control mice, with PBS-NMS only. The intranasal route of IL-12 delivery was chosen to attain an increased local concentration of IL-12 at the site of infection compared with systemic administration. Groups of mice were evaluated 1, 3, 6, and 8 days after inoculation. Samples were obtained from four to eight mice per group (four groups, as follows: infected BALB/c mice treated with PBS-NMS, infected BALB/c mice treated with IL-12-PBS-NMS, SP4-inoculated BALB/c mice treated with PBS-NMS, and SP4-inoculated BALB/c mice treated with IL-12-PBS-NMS) at each time point; in addition, whole-body, unrestrained, nonsedated plethysmography was performed at each time point just prior to euthanizing the mice for sample collection. Results represent aggregate data from two separate experiments performed in identical fashion, as detailed above.
Mice were euthanized for sample collection by cardiac puncture after being anesthetized with an intraperitoneal injection of 75 mg of ketamine per kg of body weight and 5 mg of acepromazine per kg. Bronchoalveolar lavage (BAL) specimens were obtained by infusing 500 μl of SP4 broth through a 25-gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Whole-lung specimens (including the trachea and both lungs) were collected and fixed with a 10% buffered formalin solution for histologic evaluation.
Visual inspection.
Simple, nonquantitative visual inspection was performed daily on mice that were not anesthetized.
Culture.
Twenty-five microliters of undiluted BAL sample and serial 10-fold dilutions of BAL fluid in SP4 broth (50 μl of undiluted BAL sample in 450 μl SP4 broth was used for the initial dilution; subsequent dilutions were done in a similar fashion) were immediately cultured on SP4 agar plates at 37°C, while the remaining undiluted BAL fluid specimens were stored at −80°C. Quantification was performed by counting colonies on plated specimens, and data were expressed as log10 CFU per milliliter.
Histopathology.
Lung tissue was fixed in buffered formalin, and whole-mount sections of paraffin-embedded lungs were stained with hematoxylin and eosin. The histopathologic score (HPS) was determined by a single pathologist who was unaware of the infection status of the animals from which specimens were taken. The HPS was based on individual grading of peribronchiolar/bronchial infiltrate (percentage of sites and quality), bronchiolar/bronchial luminal exudate, perivascular infiltrate (percentage of sites), and parenchymal pneumonia (neutrophilic alveolar infiltrate); after each of these was graded, the subscores were compiled for the HPS. This HPS system assigns values from 0 to 26: the higher the score, the greater the inflammatory changes in the lung (8). The parenchymal pneumonia subscore was based on grading of the neutrophilic alveolar infiltrate (0, no parenchymal pneumonia; 3, patchy parenchymal pneumonia; and 5, patchy and confluent parenchymal pneumonia).
Plethysmography.
Whole-body, unrestrained, nonsedated plethysmography (Buxco Electronics, Wilmington, NC) was used to monitor the respiratory dynamics of mice in a quantitative manner at baseline (AO) and after methacholine exposure (AHR). Prior to methacholine exposure, mice were allowed to acclimate to the chamber, and then plethysmography readings were recorded to establish baseline values. Next, the mice were exposed to aerosolized methacholine (100 mg/ml); after exposure, plethysmography readings were recorded again. Enhanced pause (Penh) is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh measured by plethysmography has previously been validated with animal models of AHR (14, 17, 38, 42). AO and AHR were measured in four to eight mice per group 1, 3, 6, and 8 days after inoculation.
Measurement of cytokines in BAL fluid.
Concentrations of cytokines in BAL specimens were assessed using multiplex bead immunoassays (BioSource International, Camarillo, CA) in conjunction with a Luminex LabMAP system, following the manufacturer's instructions. The cytokines examined and the levels of sensitivity of the assays were as follows: tumor necrosis factor alpha (TNF-α), 5 pg/ml; IFN-γ, 1 pg/ml; IL-1β, 10 pg/ml; IL-2, 15 pg/ml; IL-4, 5 pg/ml; IL-5, 10 pg/ml; IL-6, 10 pg/ml; IL-10, 15 pg/ml; IL-12, 15 pg/ml; and granulocyte-macrophage colony-stimulating factor, 10 pg/ml.
Statistics.
For all statistical analysis, Sigma Stat 2003 software (SPSS Science, San Rafael, CA) was used. Two-way analysis of variance (ANOVA) was used to compare values for the different groups of animals over 1, 3, 6, and 8 days if the data were normally distributed. A comparison was considered statistically significant if the P value was <0.05.
RESULTS
Visual inspection.
By simple visual inspection, BALB/c mice inoculated with M. pneumoniae and treated with IL-12 (MpIL12 mice) had more ruffled fur than did infected BALB/c mice treated with PBS-NMS (MpP mice) for the first 2 to 3 days after inoculation. They were also slightly less active. There was no other visible difference among the groups.
BAL culture.
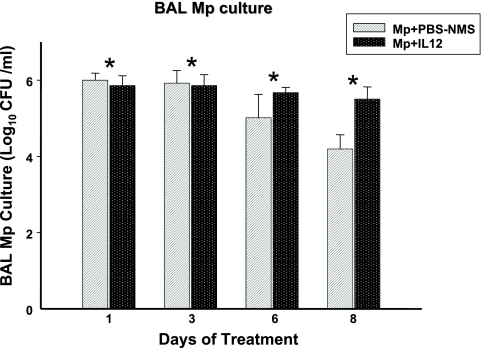
BAL cultures were positive for all mice infected with M. pneumoniae. MpIL12 mice had significantly higher BAL M. pneumoniae concentrations than did MpP mice (P < 0.001) (Fig. 1). All control mice had negative BAL cultures.
FIG. 1.
Quantitative M. pneumoniae (Mp) cultures of BAL fluid samples of BALB/c mice inoculated with M. pneumoniae and treated with intranasal IL-12 (MpIL12) and BALB/c mice inoculated with M. pneumoniae and treated with intranasal PBS-NMS (MpP). Values shown are means ± standard deviations (error bars). Data represent results from two separate experiments with four to eight mice in each group per time point. *, P < 0.001 between MpIL12 and MpP mice by two-way ANOVA.
Histopathology.
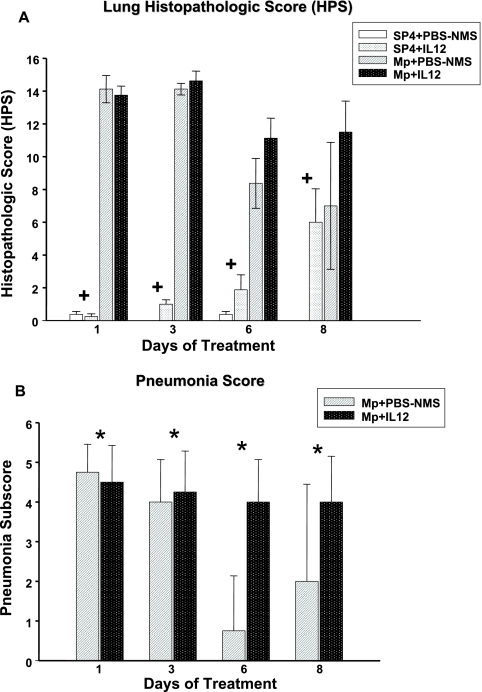
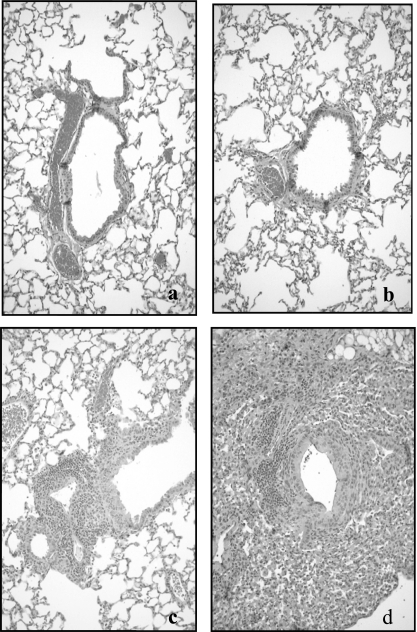
MpIL12 mice had higher lung HPS, which was not statistically significant but showed a strong trend (P = 0.051) compared with MpP mice (Fig. 2). The parenchymal pneumonia subscore (neutrophilic alveolar infiltrate) of the overall HPS was statistically higher for MpIL12 mice than for MpP mice (P < 0.001) (Fig. 2). Other subscores of the overall HPS were not significantly different for the infected mice. Uninfected mice treated with IL-12 displayed significantly higher HPS than did uninfected mice treated with PBS-NMS (P < 0.001), but there was no difference in the parenchymal pneumonia subscore. Figure 3 shows the histopathologic appearance of a representative mouse lung inoculated with SP4 broth (treated with intranasal PBS-NMS or IL-12) compared with that of a representative mouse lung inoculated with M. pneumoniae (treated with intranasal PBS-NMS or IL-12) on day 6 of treatment.
FIG. 2.
Lung HPS (A) and pneumonia subscores (B) of BALB/c mice inoculated with M. pneumoniae (Mp) or sterile SP4 broth (controls) and then treated with IL-12 or PBS-NMS. Values shown are means ± standard deviations (error bars). Data represent results from two separate experiments with four to eight mice in each group per time point. +, P < 0.001 between BALB/c mice inoculated with SP4 and treated with IL-12 or PBS-NMS (controls), by two-way ANOVA; *, P < 0.001 for pneumonia subscore between BALB/c mice inoculated with M. pneumoniae and treated with intranasal IL-12 or PBS-NMS (controls), by two-way ANOVA. All uninfected mice had a pneumonia subscore of zero.
FIG. 3.
Comparative histopathological appearance of the lungs of uninfected control BALB/c mice treated with PBS-NMS (a) (HPS = 0) or intranasal IL-12 (b) (HPS = 1) and of M. pneumoniae-infected BALB/c mice treated with PBS-NMS (c) (HPS = 6) or intranasal IL-12 (d) (HPS = 15) on day 6 of treatment. Magnification, ×20.
Plethysmography.
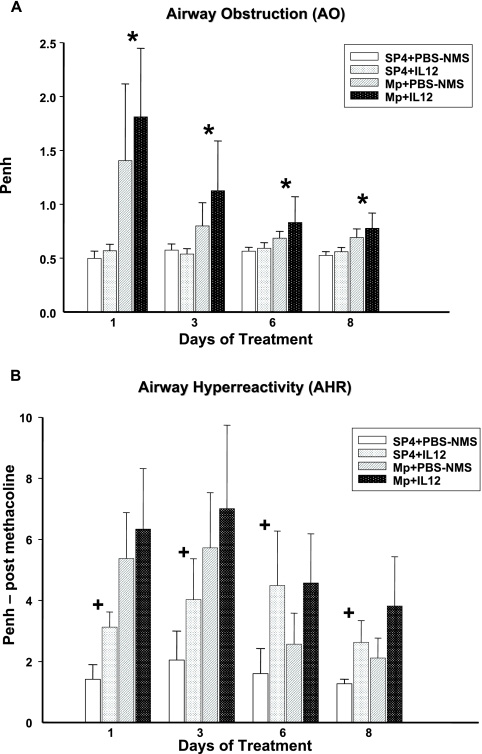
AO, measured by baseline Penh, was significantly higher in MpIL12 mice than in MpP mice (P = 0.048) (Fig. 4A). Penh values after standardized methacholine challenge (AHR) were not statistically different between MpIL12 and MpP mice, even though a trend was demonstrated (P = 0.081). No difference in AO was seen between uninfected mice treated with IL-12 and uninfected mice treated with PBS-NMS. In contrast, uninfected mice treated with IL-12 displayed significantly higher AHR than did uninfected mice treated with PBS-NMS (P < 0.001) (Fig. 4B).
FIG. 4.
AO (A) and AHR (B) were assessed by whole-body plethysmography by measuring Penh in BALB/c mice inoculated with M. pneumoniae (Mp) or sterile SP4 broth (controls) and then treated with IL-12 or PBS-NMS. Values shown are means ± standard deviations (error bars). Data represent results from two separate experiments with four to eight mice in each group per time point. *, P = 0.048 between BALB/c mice inoculated with M. pneumoniae and treated with IL-12 or PBS-NMS, by two-way ANOVA; +, P < 0.001 between BALB/c mice inoculated with SP4 and treated with IL-12 or PBS-NMS (controls), by two-way ANOVA.
BAL cytokines.
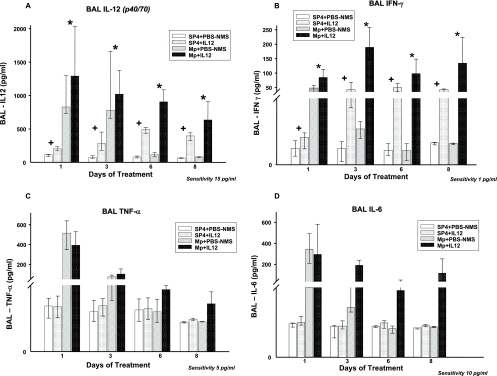
MpIL12 mice had significantly greater BAL concentrations of IL-12 and IFN-γ than did MpP mice. TNF-α and IL-6 concentrations in BAL samples tended to be higher for the MpIL12 mice than the MpP mice; however, no statistically significant difference was found between the two groups (P = 0.06 and 0.098, respectively) (Fig. 5). No significant differences in the BAL concentrations of IL-1β, IL-2, IL-4, IL-5, IL-10, and granulocyte-macrophage colony-stimulating factor were found in the MpIL12 mice compared with the MpP mice. IL-12 and IFN-γ levels were significantly higher in uninfected mice treated with IL-12 than in uninfected mice treated with PBS-NMS (P < 0.001 for both cytokines) (Fig. 5).
FIG. 5.
Cytokine concentrations in BAL fluid specimens from BALB/c mice inoculated with M. pneumoniae (Mp) or sterile SP4 broth (controls) and then treated with IL-12 or PBS-NMS. Values shown are means ± standard deviations (error bars). Data represent results from two separate experiments with four to eight mice in each group per time point. *, P < 0.001 between BALB/c mice inoculated with M. pneumoniae and treated with IL-12 or PBS-NMS, by two-way ANOVA; +, P < 0.001 between BALB/c mice inoculated with SP4 and treated with IL-12 or PBS-NMS (controls), by two-way ANOVA.
DISCUSSION
This investigation shows that the administration of exogenous IL-12 during M. pneumoniae lower respiratory tract infection significantly increases disease severity by both microbiologic and pulmonary parameters. In the last decade, several studies have shown that Th1 cytokines have a pivotal role in the pathogenic mechanisms of M. pneumoniae infection (12, 18, 36, 43, 44). The Th1 host immune response, in particular IL-12 production, is considered an essential prerequisite for protection against intracellular organisms (35, 41). After infection occurs, IL-12, through the activation of NK and T cells, stimulates the production of IFN-γ, among other cytokines, which activates macrophage activity and promotes the production of cytotoxic CD8+ and T-helper CD4+ lymphocytes (40). In mice and humans, the deficiency of IL-12 or its receptor increases susceptibility to intracellular pathogens, denoting the protective effect of IL-12 (1, 9, 28, 45, 46).
The role of innate and adaptive immunity in Mycoplasma respiratory infection has been explored (6, 12, 22); however, the immunopathogenic mechanisms of action involved in M. pneumoniae respiratory tract infection remain to be defined completely (6, 20, 22). In a previous investigation of M. pneumoniae pulmonary infection, we showed that IL-12 (p35) KO mice infected with M. pneumoniae had a significantly better outcome than did infected wild-type mice. The IL-12 (p35) KO mice demonstrated improved bacterial clearance, decreased pulmonary histological inflammation, particularly in the neutrophilic alveolar infiltrates, and decreased AO (36). These findings were counterintuitive to most prior IL-12 investigations. The purpose of the current study was to evaluate whether the administration of IL-12 would produce opposite results to those seen when IL-12 (p35) KO mice were infected with M. pneumoniae.
We observed significantly higher concentrations of M. pneumoniae in quantitative BAL cultures from MpIL12 mice than in those from MpP mice. This may indicate that IL-12 decreases the ability of the immune system to efficiently clear M. pneumoniae infection. A similar finding was noted by Carter et al. for a murine model infected with Bordetella pertussis and treated with escalating doses of intraperitoneal IL-12. They found that higher IL-12 doses were associated with an increase in bacterial colonization of the lungs (5). The mechanism by which IL-12 decreases Bordetella pertussis and Mycoplasma pneumoniae clearance is unclear; a hypothesis that could explain the decreased bacterial clearance derives from oncologic reports that evidence a paradoxical transient immunosuppression induced by higher doses of IL-12, in particular for CD8+ T cells, and this immunosuppression could explain the reduced ability to clear the infection (3, 31). Another possibility may be that IL-12 promotes the production of Th1 cytokines and, in our model, also the proinflammatory cytokines TNF-α and IL-6, which results in increased inflammation in the lungs and could result in decreased bacterial clearance.
In addition, we were able to demonstrate that MpIL12 mice had greater pulmonary histologic inflammation, particularly in neutrophilic alveolar infiltrates; interestingly, in the control mice treated with IL-12, the overall lung histologic inflammation was also significantly elevated without a specific effect on neutrophilic alveolar infiltrates. This may be explained by the finding that IL-12 significantly induced pulmonary cytokine production. As expected, the BAL concentrations of IFN-γ in IL-12-treated mice were significantly elevated, attesting to the validity of our therapeutic strategy; however, a strong trend for increased concentrations of the proinflammatory cytokines TNF-α and IL-6 was seen only for the infected mice. In previous studies, TNF-α has been associated directly with M. pneumoniae disease severity (12, 13, 18). Consistent with the increased inflammation, we found significantly higher AO in MpIL12 mice. While AHR was also higher in MpIL12 mice, this finding did not reach significance (P = 0.081). Again of note is the fact that uninfected mice treated with IL-12 had AHR that was significantly greater than that of the untreated mice.
Our demonstration of worsening of M. pneumoniae disease with the administration of IL-12 is in sharp contrast with what has been shown for many other infectious diseases, especially with intracellular organisms, such as Mycobacterium tuberculosis, Mycobacterium avium (10, 32), Brucella spp. (37), Francisella tularensis (11), and viruses such as coxsackieviruses (34). In particular, Duckett et al. investigated the survival rates of mice pretreated with intranasal IL-12 and then infected with Francisella tularensis and found that all of the untreated mice succumbed to the infection, while mice who received IL-12 therapy survived, demonstrating that IL-12 provides protection against lethal respiratory tularemia (11). In general, IL-12 therapy has been found to decrease the severity of infections. Notable exceptions include Bordetella pertussis, as discussed earlier, and now M. pneumoniae infections. Interestingly, Bordetella pertussis is an atypical respiratory pathogen that attaches to respiratory epithelium and possesses an ADP-ribosylating toxin similarly to M. pneumoniae (23, 30).
The different mechanisms by which M. pneumoniae interacts with the host in this regard are not completely clear; perhaps the fact that M. pneumoniae acts as an extracellular pathogen in the acute stages of infection differentiates its immunopathogenesis from that of these intracellular pathogens. IL-12 seems to potentiate the immunopathogenic mechanisms of M. pneumoniae. Another explanation for our results may be that IL-12 has a narrow window for therapeutic concentrations because of the induction of potentially toxic levels of IFN-γ; our results could represent side effects of IL-12. In other studies, mice treated with IL-12 developed pulmonary congestion and hepatic dysfunction after long-term use of high doses of IL-12, but similar findings were not found when the same mice infected with Mycobacterium tuberculosis were treated with IL-12 in a similar manner for just 1 week (4, 32). However, in these studies, IL-12 was administered parenterally, and this method of delivery could increase systemic side effects. The dose and length of therapy used in our experiment were significantly lower and shorter, respectively, than those of the above-mentioned studies, and we did not notice any pulmonary congestion in either group of mice, infected or controls, treated with intranasal IL-12. Moreover, the mirror opposite results obtained with our prior IL-12 KO mouse investigation sustain the validity of an immunopathogenic action of IL-12 in M. pneumoniae respiratory infection. The data obtained in the current study suggest that for M. pneumoniae respiratory infection, intranasal IL-12 therapy is not only nonprotective but may exacerbate the course of the disease. Possibly, IL-12 therapy potentiates the innate host response and directly contributes to the pulmonary disease of M. pneumoniae (6, 18, 21, 36).
Acknowledgments
Simple, nonquantitative visual inspections of mice were performed daily by C.M.S.
This work was funded by NIH/NIAID grant 5KO8 AI052262 (to R. D. Hardy).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 26 November 2007.
REFERENCES
- 1.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 2801432-1435. [DOI] [PubMed] [Google Scholar]
- 2.Block, S., J. Hedrick, M. R. Hammerschlag, G. H. Cassell, and J. C. Craft. 1995. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr. Infect. Dis. J. 14471-477. [DOI] [PubMed] [Google Scholar]
- 3.Brunda, M. J., L. Luistro, R. R. Warrier, R. B. Wright, B. R. Hubbard, M. Murphy, S. F. Wolf, and M. K. Gately. 1993. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 1781223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Car, B. D., V. M. Eng, B. Schnyder, M. LeHir, A. N. Shakhov, G. Woerly, S. Huang, M. Aguet, T. D. Anderson, and B. Ryffel. 1995. Role of interferon-gamma in interleukin 12-induced pathology in mice. Am. J. Pathol. 1471693-1707. [PMC free article] [PubMed] [Google Scholar]
- 5.Carter, C. R., B. M. Dagg, K. M. Whitmore, J. R. Keeble, C. Asokanathan, D. Xing, and K. B. Walker. 2004. High dose interleukin-12 exacerbates Bordetella pertussis infection and is associated with suppression of cell-mediated immunity in a murine aerosol challenge model. Clin. Exp. Immunol. 135233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartner, S. C., J. R. Lindsey, J. Gibbs-Erwin, G. H. Cassell, and J. W. Simecka. 1998. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect. Immun. 663485-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassell, G. H., and B. C. Cole. 1981. Mycoplasmas as agents of human disease. N. Engl. J. Med. 30480-89. [DOI] [PubMed] [Google Scholar]
- 8.Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36465-478. [DOI] [PubMed] [Google Scholar]
- 9.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. van Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 2801435-1438. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, T. M., and A. Sher. 1998. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J. Immunol. 1605428-5435. [PubMed] [Google Scholar]
- 11.Duckett, N. S., S. Olmos, D. M. Durrant, and D. W. Metzger. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 732306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca-Aten, M., A. M. Rios, A. Mejias, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., and R. D. Hardy. 2005. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am. J. Respir. Cell Mol. Biol. 32201-210. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca-Aten, M., C. M. Salvatore, A. Mejias, A. M. Rios, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., and R. D. Hardy. 2005. Evaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 494128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalo, J. A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. Wells, A. Proudfoot, A. C. Martinez, M. Dorf, T. Bjerke, A. J. Coyle, and J. C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberger, M. J., S. L. Kunkel, R. M. Strieter, N. W. Lukacs, J. Bramson, J. Gauldie, F. L. Graham, M. Hitt, J. M. Danforth, and T. J. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 1573006-3012. [PubMed] [Google Scholar]
- 16.Gubler, U., A. O. Chua, D. S. Schoenhaut, C. M. Dwyer, W. McComas, R. Motyka, N. Nabavi, A. G. Wolitzky, P. M. Quinn, P. C. Familletti, et al. 1991. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl. Acad. Sci. USA 884143-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156766-775. [DOI] [PubMed] [Google Scholar]
- 18.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 693869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy, R. D., A. M. Rios, S. Chavez-Bueno, H. S. Jafri, J. Hatfield, B. B. Rogers, G. H. McCracken, and O. Ramilo. 2003. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob. Agents Chemother. 471614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy, R. D., T. Prescot Atkinson, and G. H. Cassell. 2005. Mucosal immunology, 3rd ed., vol. 2. Elsevier Academic Press, Philadelphia, PA.
- 21.Hayakawa, M., H. Taguchi, S. Kamiya, Y. Fujioka, H. Watanabe, S. Kawai, and H. Kobayashi. 2002. Animal model of Mycoplasma pneumoniae infection using germfree mice. Clin. Diagn. Lab. Immunol. 9669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickman-Davis, J. M. 2002. Role of innate immunity in respiratory mycoplasma infection. Front. Biosci. 7d1347-d1355. [DOI] [PubMed] [Google Scholar]
- 23.Kannan, T. R., and J. B. Baseman. 2006. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc. Natl. Acad. Sci. USA 1036724-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraft, M. 2000. The role of bacterial infections in asthma. Clin. Chest Med. 21301-313. [DOI] [PubMed] [Google Scholar]
- 26.Kraft, M., G. H. Cassell, J. E. Henson, H. Watson, J. Williamson, B. P. Marmion, C. A. Gaydos, and R. J. Martin. 1998. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am. J. Respir. Crit. Care Med. 158998-1001. [DOI] [PubMed] [Google Scholar]
- 27.Kraft, M., and Q. Hamid. 2006. Mycoplasma in severe asthma. J. Allergy Clin. Immunol. 1171197-1198. [DOI] [PubMed] [Google Scholar]
- 28.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4471-481. [DOI] [PubMed] [Google Scholar]
- 29.Michelow, I. C., K. Olsen, J. Lozano, N. K. Rollins, L. B. Duffy, T. Ziegler, J. Kauppila, M. Leinonen, and G. H. McCracken, Jr. 2004. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113701-707. [DOI] [PubMed] [Google Scholar]
- 30.Monack, D., J. J. Munoz, M. G. Peacock, W. J. Black, and S. Falkow. 1989. Expression of pertussis toxin correlates with pathogenesis in Bordetella species. J. Infect. Dis. 159205-210. [DOI] [PubMed] [Google Scholar]
- 31.Nastala, C. L., H. D. Edington, T. G. McKinney, H. Tahara, M. A. Nalesnik, M. J. Brunda, M. K. Gately, S. F. Wolf, R. D. Schreiber, W. J. Storkus, et al. 1994. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J. Immunol. 1531697-1706. [PubMed] [Google Scholar]
- 32.Nolt, D., and J. L. Flynn. 2004. Interleukin-12 therapy reduces the number of immune cells and pathology in lungs of mice infected with Mycobacterium tuberculosis. Infect. Immun. 722976-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pammit, M. A., V. N. Budhavarapu, E. K. Raulie, K. E. Klose, J. M. Teale, and B. P. Arulanandam. 2004. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob. Agents Chemother. 484513-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potvin, D. M., D. W. Metzger, W. T. Lee, D. N. Collins, and A. I. Ramsingh. 2003. Exogenous interleukin-12 protects against lethal infection with coxsackievirus B4. J. Virol. 778272-8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raupach, B., and S. H. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13417-428. [DOI] [PubMed] [Google Scholar]
- 36.Salvatore, C. M., M. Fonseca-Aten, K. Katz-Gaynor, A. M. Gomez, A. Mejias, C. Somers, S. Chavez-Bueno, G. H. McCracken, and R. D. Hardy. 2007. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infect. Immun. 75236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sathiyaseelan, J., R. Goenka, M. Parent, R. M. Benson, E. A. Murphy, D. M. Fernandes, A. S. Foulkes, and C. L. Baldwin. 2006. Treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity. Cell. Immunol. 2431-9. [DOI] [PubMed] [Google Scholar]
- 38.Schwarze, J., E. Hamelmann, K. L. Bradley, K. Takeda, and E. W. Gelfand. 1997. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 100226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stern, A. S., F. J. Podlaski, J. D. Hulmes, Y. C. Pan, P. M. Quinn, A. G. Wolitzky, P. C. Familletti, D. L. Stremlo, T. Truitt, R. Chizzonite, et al. 1990. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 876808-6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 844008-4027. [PubMed] [Google Scholar]
- 41.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13251-276. [DOI] [PubMed] [Google Scholar]
- 42.van Schaik, S. M., G. Enhorning, I. Vargas, and R. C. Welliver. 1998. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J. Infect. Dis. 177269-276. [DOI] [PubMed] [Google Scholar]
- 43.Woolard, M. D., R. D. Hardy, and J. W. Simecka. 2004. IL-4-independent pathways exacerbate methacholine-induced airway hyperreactivity during mycoplasma respiratory disease. J. Allergy Clin. Immunol. 114645-649. [DOI] [PubMed] [Google Scholar]
- 44.Woolard, M. D., D. Hudig, L. Tabor, J. A. Ivey, and J. W. Simecka. 2005. NK cells in gamma-interferon-deficient mice suppress lung innate immunity against Mycoplasma spp. Infect. Immun. 736742-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, C., J. Ferrante, M. K. Gately, and J. Magram. 1997. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J. Immunol. 1591658-1665. [PubMed] [Google Scholar]
- 46.Wu, C., X. Wang, M. Gadina, J. J. O'Shea, D. H. Presky, and J. Magram. 2000. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J. Immunol. 1656221-6228. [DOI] [PubMed] [Google Scholar]
- 47.Wubbel, L., L. Muniz, A. Ahmed, M. Trujillo, C. Carubelli, C. McCoig, T. Abramo, M. Leinonen, and G. H. McCracken, Jr. 1999. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr. Infect. Dis. J. 1898-104. [DOI] [PubMed] [Google Scholar]