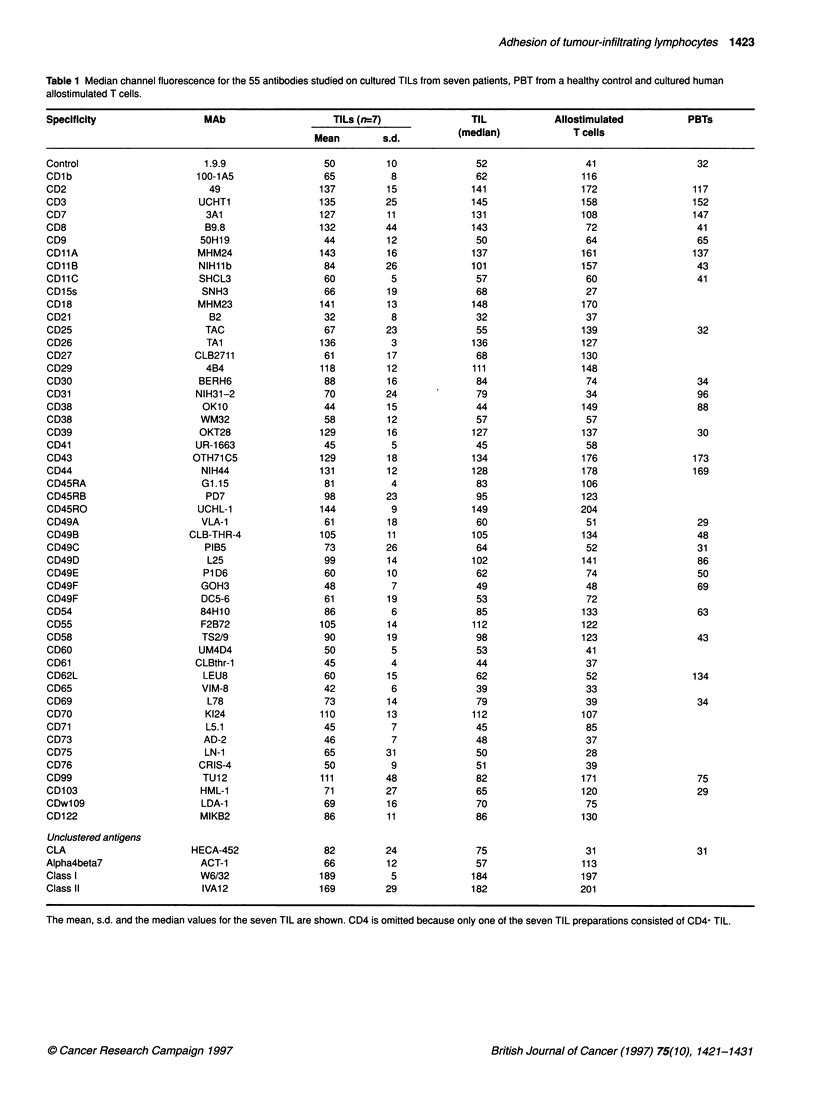
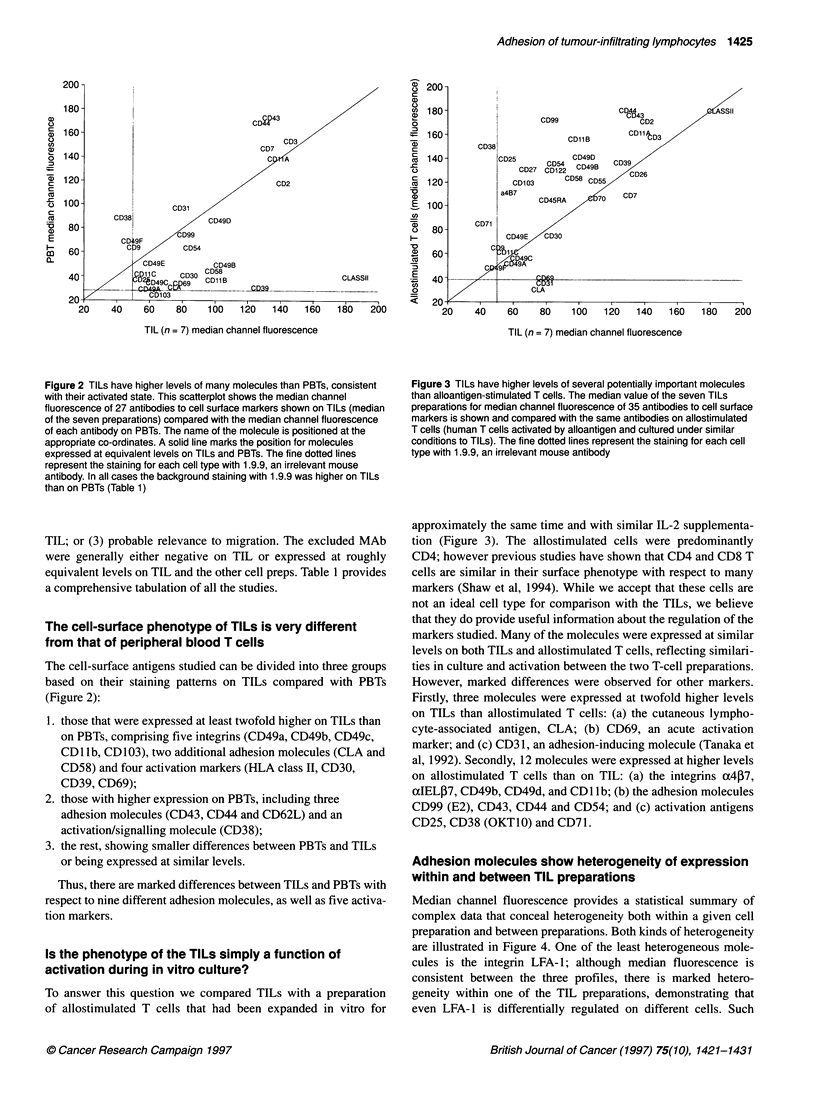
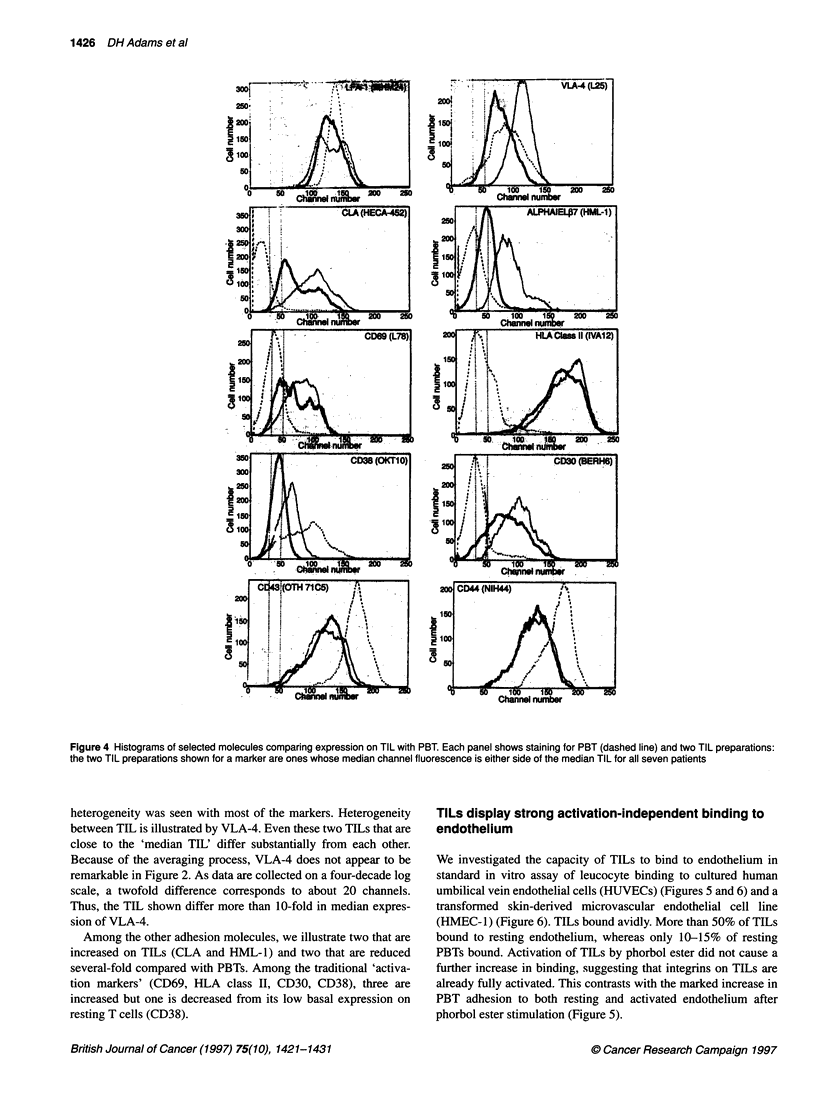
Abstract
Efficacy of cancer immunotherapy with cultured tumour-infiltrating lymphocytes (TILs) depends upon infused TILs migrating into tumour-bearing tissue, in which they mediate an anti-tumour response. For TILs to enter a tumour, they must first bind to tumour endothelium, and this process depends on TILs expressing and regulating the function of relevant cell-surface receptors. We analysed the cell-surface phenotype and endothelial binding of TILs cultured from human melanoma and compared them with peripheral blood T cells and with allostimulated T cells cultured under similar conditions. Compared with peripheral blood T cells, TILs expressed high levels of five integrins, two other adhesion molecules, including the skin homing molecule CLA, and several activation markers and showed markedly enhanced integrin-mediated adhesion to a dermal microvascular endothelial cell line in vitro. Compared with the allostimulated T cells, TILs expressed higher levels of the cutaneous lymphocyte antigen (CLA), the adhesion molecule CD31 and the activation markers CD30 and CD69, but lower levels of several other adhesion and activation molecules. These phenotypic and functional properties of TILs should have complex effects on their migration in vivo. Expression of CLA, the skin homing receptor, may increase migration to melanoma (a skin cancer), whereas integrin activation may cause non-specific binding of TILs to other endothelium. Manipulation of the culture conditions in which TILs are expanded might result in a phenotype that is more conducive to selective tumour homing in vivo.
Full text
PDF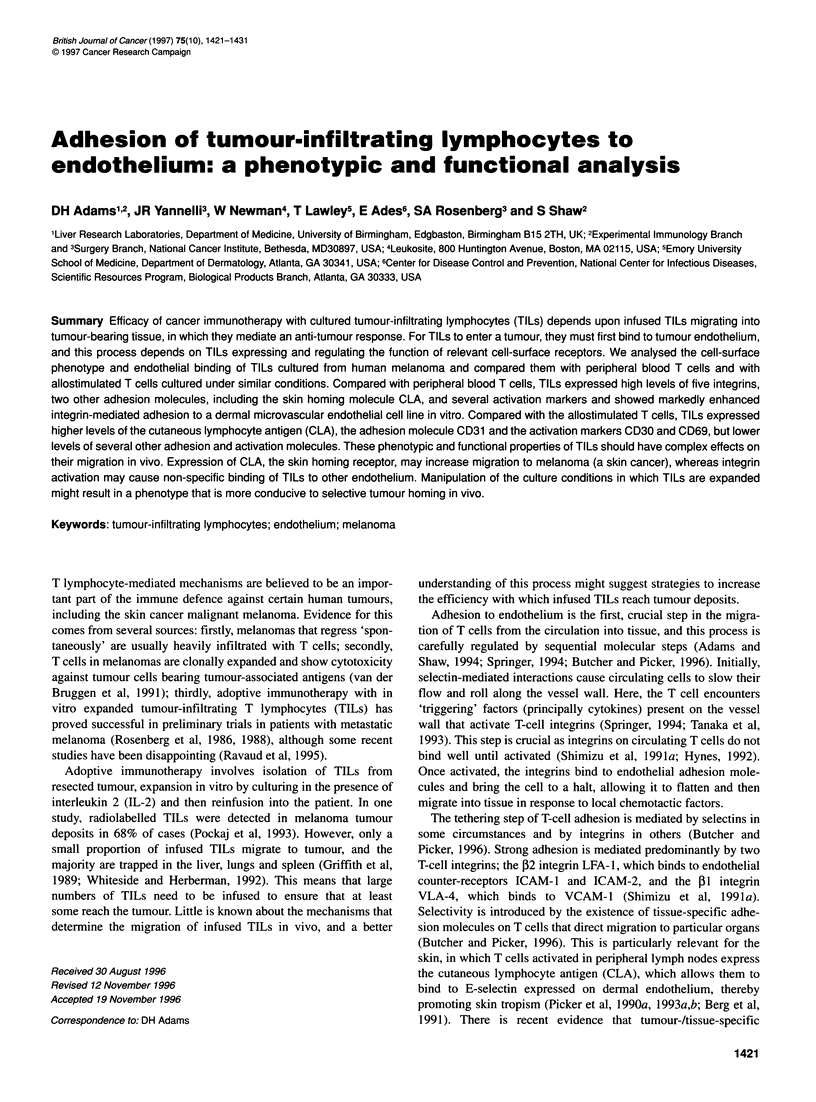






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Burra P., Hubscher S. G., Elias E., Newman W. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology. 1994 Mar;19(3):588–594. doi: 10.1002/hep.1840190308. [DOI] [PubMed] [Google Scholar]
- Adams D. H., Hubscher S. G., Fisher N. C., Williams A., Robinson M. Expression of E-selectin and E-selectin ligands in human liver inflammation. Hepatology. 1996 Sep;24(3):533–538. doi: 10.1002/hep.510240311. [DOI] [PubMed] [Google Scholar]
- Adams D. H. Lymphocyte-endothelial cell interactions in hepatic inflammation. Hepatogastroenterology. 1996 Jan-Feb;43(7):32–43. [PubMed] [Google Scholar]
- Adams D. H., Nash G. B. Disturbance of leucocyte circulation and adhesion to the endothelium as factors in circulatory pathology. Br J Anaesth. 1996 Jul;77(1):17–31. doi: 10.1093/bja/77.1.17. [DOI] [PubMed] [Google Scholar]
- Adams D. H., Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. 1994 Apr 2;343(8901):831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- Ades E. W., Candal F. J., Swerlick R. A., George V. G., Summers S., Bosse D. C., Lawley T. J. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992 Dec;99(6):683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- Aebersold P., Hyatt C., Johnson S., Hines K., Korcak L., Sanders M., Lotze M., Topalian S., Yang J., Rosenberg S. A. Lysis of autologous melanoma cells by tumor-infiltrating lymphocytes: association with clinical response. J Natl Cancer Inst. 1991 Jul 3;83(13):932–937. doi: 10.1093/jnci/83.13.932. [DOI] [PubMed] [Google Scholar]
- Ardman B., Sikorski M. A., Staunton D. E. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5001–5005. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Picker L. J. Lymphocyte homing and homeostasis. Science. 1996 Apr 5;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Clayberger C., Krensky A. M., McIntyre B. W., Koller T. D., Parham P., Brodsky F., Linn D. J., Evans E. L. Identification and characterization of two novel lymphocyte function-associated antigens, L24 and L25. J Immunol. 1987 Mar 1;138(5):1510–1514. [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Dunkley M., Pabst R., Cripps A. An important role for intestinally derived T cells in respiratory defence. Immunol Today. 1995 May;16(5):231–236. doi: 10.1016/0167-5699(95)80165-0. [DOI] [PubMed] [Google Scholar]
- Faustman D., Lacy P., Davie J., Hauptfeld V. Prevention of allograft rejection by immunization with donor blood depleted of Ia-bearing cells. Science. 1982 Jul 9;217(4555):157–158. doi: 10.1126/science.6806903. [DOI] [PubMed] [Google Scholar]
- Fisher L. L., Ottaway C. A. The kinetics of migration of murine CD4 and CD8 lymphocytes in vivo. Reg Immunol. 1990;3(3):156–162. [PubMed] [Google Scholar]
- Graber N., Gopal T. V., Wilson D., Beall L. D., Polte T., Newman W. T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol. 1990 Aug 1;145(3):819–830. [PubMed] [Google Scholar]
- Griffith K. D., Read E. J., Carrasquillo J. A., Carter C. S., Yang J. C., Fisher B., Aebersold P., Packard B. S., Yu M. Y., Rosenberg S. A. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J Natl Cancer Inst. 1989 Nov 15;81(22):1709–1717. doi: 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Horgan K. J., Van Seventer G. A., Shimizu Y., Shaw S. Hyporesponsiveness of "naive" (CD45RA+) human T cells to multiple receptor-mediated stimuli but augmentation of responses by co-stimuli. Eur J Immunol. 1990 May;20(5):1111–1118. doi: 10.1002/eji.1830200525. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Balch C. M. Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating into human metastatic melanomas. Cancer Res. 1986 Jun;46(6):3011–3017. [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits A. I., Moscicki R. A., Kurnick J. T., Camerini D., Bhan A. K., Baird L. G., Erikson M., Colvin R. B. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol. 1984 Oct;133(4):1857–1862. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Manjunath N., Johnson R. S., Staunton D. E., Pasqualini R., Ardman B. Targeted disruption of CD43 gene enhances T lymphocyte adhesion. J Immunol. 1993 Aug 1;151(3):1528–1534. [PubMed] [Google Scholar]
- Matsuyama T., Yamada A., Kay J., Yamada K. M., Akiyama S. K., Schlossman S. F., Morimoto C. Activation of CD4 cells by fibronectin and anti-CD3 antibody. A synergistic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989 Oct 1;170(4):1133–1148. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Poggi A., Pende D., Tripodi G., Orengo A. M., Pella N., Augugliaro R., Bottino C., Ciccone E., Moretta L. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med. 1991 Dec 1;174(6):1393–1398. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Sung S. S., Bjorndahl J. M., Fu S. M. Human T cell activation. IV. T cell activation and proliferation via the early activation antigen EA 1. J Exp Med. 1989 Mar 1;169(3):677–689. doi: 10.1084/jem.169.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Michie S. A., Rott L. S., Butcher E. C. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990 May;136(5):1053–1068. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Terstappen L. W., Rott L. S., Streeter P. R., Stein H., Butcher E. C. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990 Nov 15;145(10):3247–3255. [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Bergstresser P. R., Terstappen L. W. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993 Feb 1;150(3):1122–1136. [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Buck D., Terstappen L. W. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J Immunol. 1993 Feb 1;150(3):1105–1121. [PubMed] [Google Scholar]
- Pockaj B. A., Sherry R. M., Wei J. P., Yannelli J. R., Carter C. S., Leitman S. F., Carasquillo J. A., Steinberg S. M., Rosenberg S. A., Yang J. C. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclophosphamide and correlation with response. Cancer. 1994 Mar 15;73(6):1731–1737. doi: 10.1002/1097-0142(19940315)73:6<1731::aid-cncr2820730630>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Poppema S., Bröcker E. B., de Leij L., Terbrack D., Visscher T., Ter Haar A., Macher E., Thé T. H., Sorg C. In situ analysis of the mononuclear cell infiltrate in primary malignant melanoma of the skin. Clin Exp Immunol. 1983 Jan;51(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Ravaud A., Legrand E., Delaunay M. M., Bussières E., Coulon V., Cany L., Huet S., Verdier D., Kind M., Chomy F. A phase I trial of repeated tumour-infiltrating lymphocyte (TIL) infusion in metastatic melanoma. Br J Cancer. 1995 Feb;71(2):331–336. doi: 10.1038/bjc.1995.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Corless C., Bevilacqua M. P. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol. 1991 Feb;138(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Rohde D., Schlüter-Wigger W., Mielke V., von den Driesch P., von Gaudecker B., Sterry W. Infiltration of both T cells and neutrophils in the skin is accompanied by the expression of endothelial leukocyte adhesion molecule-1 (ELAM-1): an immunohistochemical and ultrastructural study. J Invest Dermatol. 1992 May;98(5):794–799. doi: 10.1111/1523-1747.ep12499959. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Salmi M., Granfors K., Leirisalo-Repo M., Hämäläinen M., MacDermott R., Leino R., Havia T., Jalkanen S. Selective endothelial binding of interleukin-2-dependent human T-cell lines derived from different tissues. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11436–11440. doi: 10.1073/pnas.89.23.11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M., Grenman R., Grenman S., Nordman E., Jalkanen S. Tumor endothelium selectively supports binding of IL-2-propagated tumor-infiltrating lymphocytes. J Immunol. 1995 Jun 1;154(11):6002–6012. [PubMed] [Google Scholar]
- Schieferdecker H. L., Ullrich R., Weiss-Breckwoldt A. N., Schwarting R., Stein H., Riecken E. O., Zeitz M. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990 Apr 1;144(7):2541–2549. [PubMed] [Google Scholar]
- Schweighoffer T., Tanaka Y., Tidswell M., Erle D. J., Horgan K. J., Luce G. E., Lazarovits A. I., Buck D., Shaw S. Selective expression of integrin alpha 4 beta 7 on a subset of human CD4+ memory T cells with Hallmarks of gut-trophism. J Immunol. 1993 Jul 15;151(2):717–729. [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990 Jul 1;145(1):59–67. [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Kansas G. S., Munro J. M., Griffin J. D., Gimbrone M. A., Jr, Tedder T. F. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991 Oct 15;147(8):2565–2573. [PubMed] [Google Scholar]
- Spiess P. J., Yang J. C., Rosenberg S. A. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J Natl Cancer Inst. 1987 Nov;79(5):1067–1075. [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Swerlick R. A., Lee K. H., Li L. J., Sepp N. T., Caughman S. W., Lawley T. J. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992 Jul 15;149(2):698–705. [PubMed] [Google Scholar]
- Takada Y., Elices M. J., Crouse C., Hemler M. E. The primary structure of the alpha 4 subunit of VLA-4: homology to other integrins and a possible cell-cell adhesion function. EMBO J. 1989 May;8(5):1361–1368. doi: 10.1002/j.1460-2075.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Albelda S. M., Horgan K. J., van Seventer G. A., Shimizu Y., Newman W., Hallam J., Newman P. J., Buck C. A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992 Jul 1;176(1):245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugores A., Alonso M. A., Sánchez-Madrid F., de Landázuri M. O. Human T cell activation through the activation-inducer molecule/CD69 enhances the activity of transcription factor AP-1. J Immunol. 1992 Apr 1;148(7):2300–2306. [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- Whiteside T. L., Herberman R. B. Extravasation of antitumor effector cells. Invasion Metastasis. 1992;12(2):128–146. [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]




