Abstract
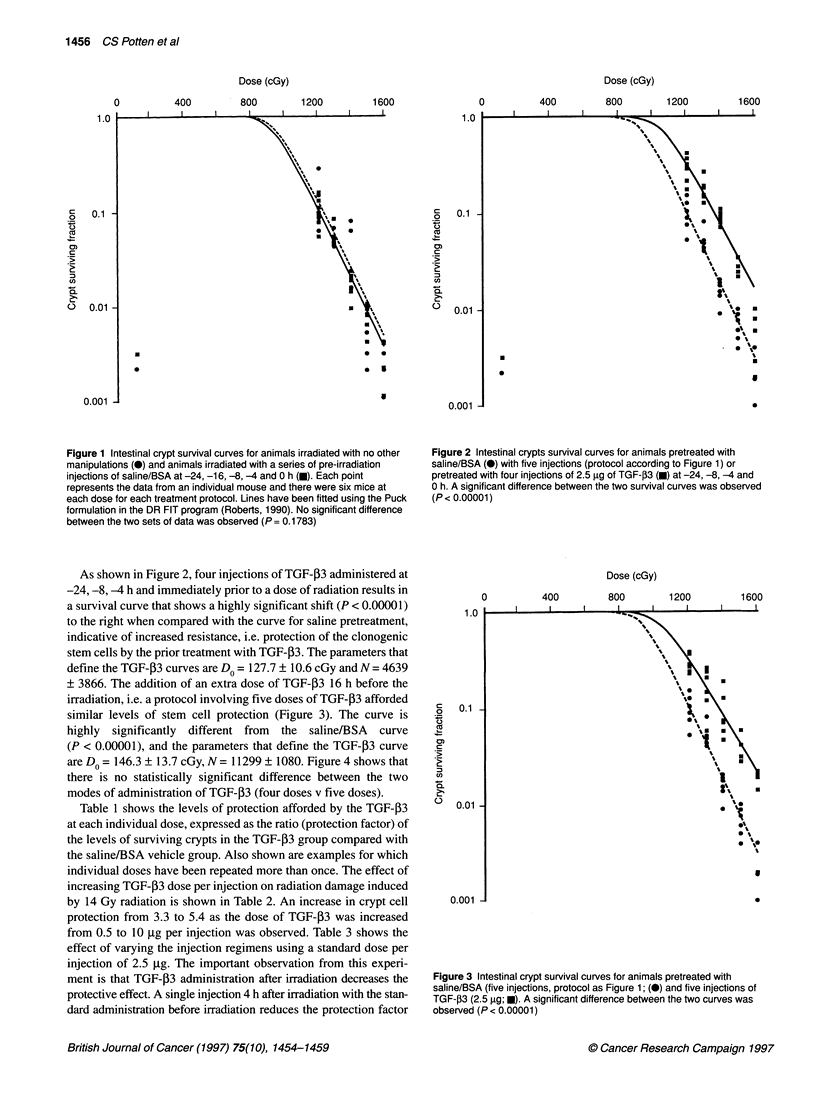
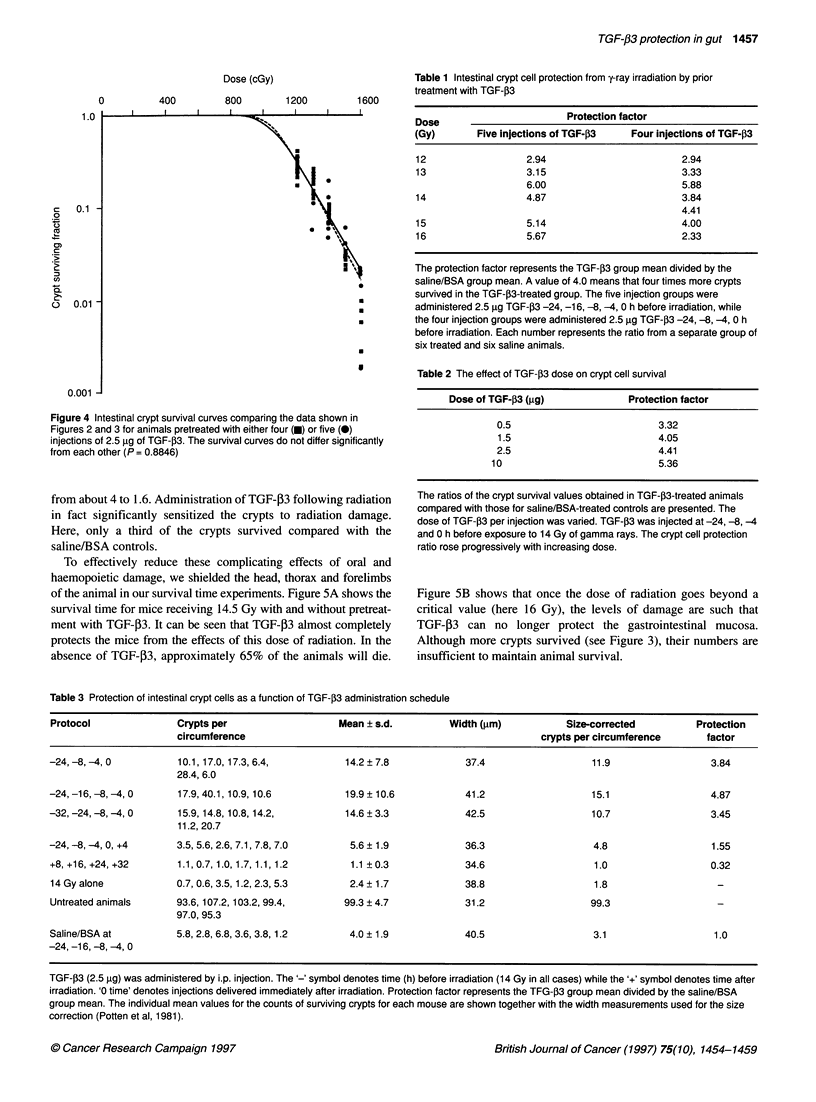
The gastrointestinal tract, with its rapid cell replacement, is sensitive to cytotoxic damage and can be a site of dose-limiting toxicity in cancer therapy. Here, we have investigated the use of one growth modulator to manipulate the cell cycle status of gastrointestinal stem cells before cytotoxic exposure to minimize damage to this normal tissue. Transforming growth factor beta-3 (TGF-beta3), a known inhibitor of cell cycle progression through G1, was used to alter intestinal crypt stem cell sensitivity before 12-16 Gy of gamma irradiation, which was used as a model cytotoxic agent. Using a crypt microcolony assay as a measure of functional competence of gastrointestinal stem cells, it was shown that the administration of TGF-beta3 over a 24-h period before irradiation increased the number of surviving crypts by four- to six-fold. To test whether changes in crypt survival are reflected in the well-being of the animal, survival time analyses were performed. After 14.5 Gy of radiation, only 35% of the animals survived within a period of about 12 days, while prior treatment with TGF-beta3 provided significant protection against this early gastrointestinal animal death, with 95% of the treated animals surviving for greater than 30 days.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Geng Y., Weinberg R. A. Transforming growth factor beta effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10315–10319. doi: 10.1073/pnas.90.21.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorzewski K., Ruscetti F. W., Usui N., Damia G., Longo D. L., Carlino J. A., Keller J. R., Wiltrout R. H. Recombinant transforming growth factor beta 1 and beta 2 protect mice from acutely lethal doses of 5-fluorouracil and doxorubicin. J Exp Med. 1994 Sep 1;180(3):1047–1057. doi: 10.1084/jem.180.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry J. H., Potten C. S., Roberts N. P. The gastrointestinal syndrome and mucosal clonogenic cells: relationships between target cell sensitivities, LD50 and cell survival, and their modification by antibiotics. Radiat Res. 1983 Oct;96(1):100–112. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Iwata K. K., Fryling C. M., Knott W. B., Todaro G. J. Isolation of tumor cell growth-inhibiting factors from a human rhabdomyosarcoma cell line. Cancer Res. 1985 Jun;45(6):2689–2694. [PubMed] [Google Scholar]
- Morstyn G., Foote M., Perkins D., Vincent M. The clinical utility of granulocyte colony-stimulating factor: early achievements and future promise. Stem Cells. 1994;12 (Suppl 1):213–228. doi: 10.1002/stem.5530120718. [DOI] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Al-Barwari S. E., Hume W. J., Searle J. Circadian rhythms of presumptive stem cells in three different epithelia of the mouse. Cell Tissue Kinet. 1977 Nov;10(6):557–568. doi: 10.1111/j.1365-2184.1977.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Owen G., Hewitt D., Chadwick C. A., Hendry H., Lord B. I., Woolford L. B. Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut. 1995 Jun;36(6):864–873. doi: 10.1136/gut.36.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Rezvani M., Hendry J. H., Moore J. V., Major D. The correction of intestinal microcolony counts for variation in size. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Sep;40(3):321–326. doi: 10.1080/09553008114551251. [DOI] [PubMed] [Google Scholar]
- Roberts S. A. DRFIT: a program for fitting radiation survival models. Int J Radiat Biol. 1990 Jun;57(6):1243–1246. doi: 10.1080/09553009014551321. [DOI] [PubMed] [Google Scholar]
- Schlunegger M. P., Cerletti N., Cox D. A., McMaster G. K., Schmitz A., Grütter M. G. Crystallization and preliminary X-ray analysis of recombinant human transforming growth factor beta 2. FEBS Lett. 1992 May 25;303(1):91–93. doi: 10.1016/0014-5793(92)80484-x. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995 May 15;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Sonis S. T., Lindquist L., Van Vugt A., Stewart A. A., Stam K., Qu G. Y., Iwata K. K., Haley J. D. Prevention of chemotherapy-induced ulcerative mucositis by transforming growth factor beta 3. Cancer Res. 1994 Mar 1;54(5):1135–1138. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers H. R., Elkind M. M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]


