Abstract
Receptors for granulocyte colony-stimulating factor (G-CSFRs) have been confirmed on the cell surfaces of several non-haematopoietic cell types, including bladder cancer cells. This observation has naturally led to the hypothesis that the expression of G-CSFR on these cells may enhance their growth by G-CSF. In this study, the expression of G-CSFR was determined in both established human bladder cancer cell lines and primary bladder cancers. We studied five different human bladder cancer cell lines (KU-1, KU-7, T-24, NBT-2 and KK) and 26 newly diagnosed bladder tumours. G-CSFR mRNA expressions on cultured cell lines were determined using the reverse transcriptase polymerase chain reaction (RT-PCR) method. Furthermore, the G-CSFR binding experiments on the cultured cell lines were conducted using the Na(125)I-labelled G-CSF ligand-binding assay method. Moreover, the G-CSFR mRNA expressions on primary bladder tumour specimens were assessed using the in situ RT-PCR method. Three out of the five cultured cell lines (KU-1, NBT-2 and KK) exhibited G-CSFR mRNA signals when the RT-PCR method was used. The G-CSFR binding experiments showed an equilibrium dissociation constant (K[d]) of 490 pM for KU-1, 340 pM for NBT-2 and 103 pM for KK cells. With in situ RT-PCR, the tumour cells of 6 out of 26 primary bladder tumour specimens (23.1%) presented positive G-CSFR mRNA signals. Thus, in this study, G-CSFR expression was frequently observed on bladder cancer cells. Therefore, the clinical use of G-CSF for patients with bladder cancer should be selected with great care.
Full text
PDF

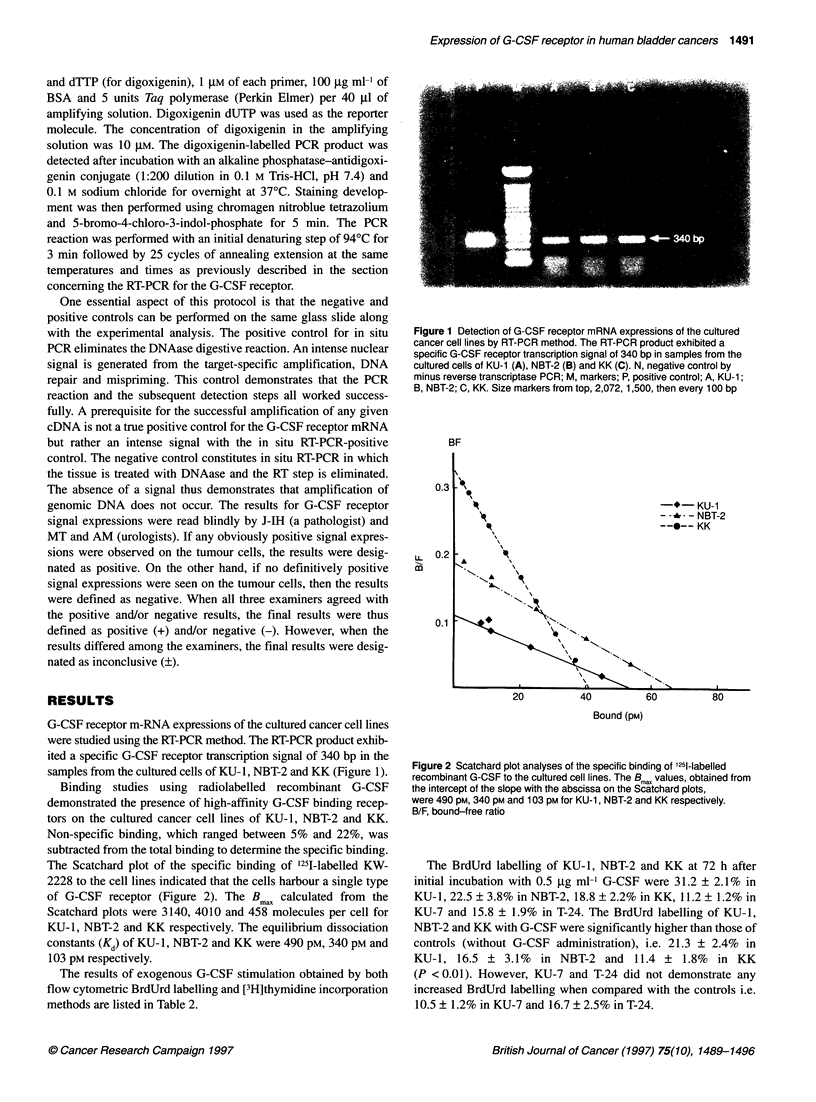
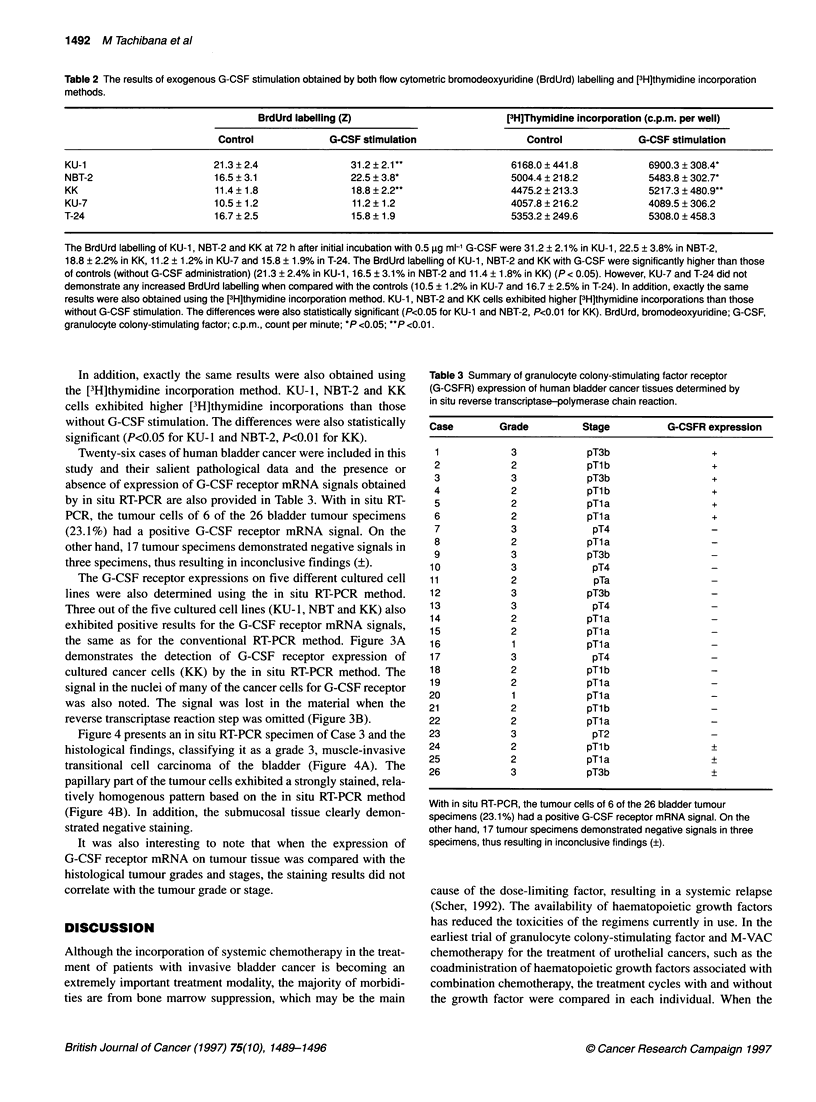
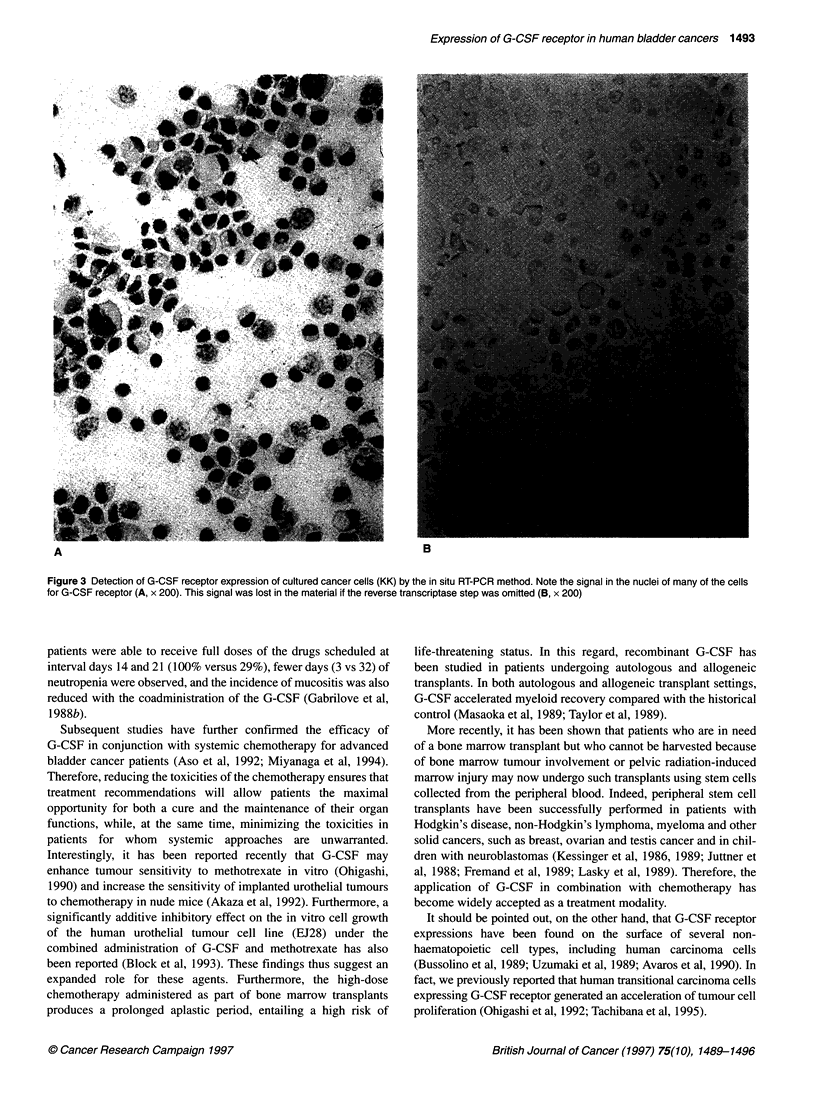
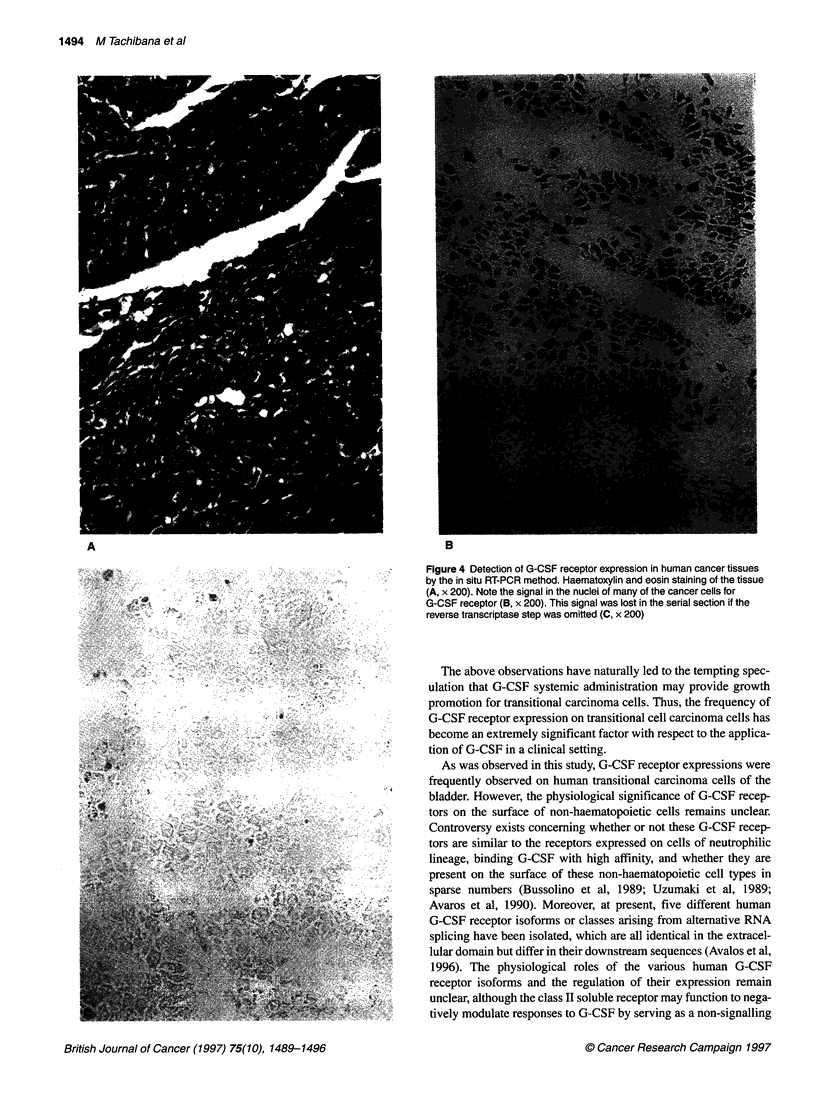


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaza H., Fukushima H., Koiso K., Aso Y. Enhancement of chemotherapeutic effects by recombinant human granulocyte colony-stimulating factor on implanted mouse bladder cancer cells (MBT-2). Cancer. 1992 Feb 15;69(4):997–1002. doi: 10.1002/1097-0142(19920215)69:4<997::aid-cncr2820690428>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Aso Y., Akaza H. Effect of recombinant human granulocyte colony-stimulating factor in patients receiving chemotherapy for urogenital cancer. Urological rhG-CSF Study Group. J Urol. 1992 Apr;147(4):1060–1064. doi: 10.1016/s0022-5347(17)37468-2. [DOI] [PubMed] [Google Scholar]
- Avalos B. R. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996 Aug 1;88(3):761–777. [PubMed] [Google Scholar]
- Bailly J. D., Pourquier P., Jaffrézou J. P., Duchayne E., Cassar G., Bordier C., Laurent G. Effect of 5637-conditioned medium and recombinant cytokines on P-glycoprotein expression in a human GM-CSF-dependent leukemic myeloid cell line. Leukemia. 1995 Oct;9(10):1718–1725. [PubMed] [Google Scholar]
- Basu A., Raghunath M., Bishayee S., Das M. Inhibition of tyrosine kinase activity of the epidermal growth factor (EGF) receptor by a truncated receptor form that binds to EGF: role for interreceptor interaction in kinase regulation. Mol Cell Biol. 1989 Feb;9(2):671–677. doi: 10.1128/mcb.9.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block T., Treiber U., Geffken B., Vogel M., Hanauske A. R., Schmid F., Hartung R., Busch R. Inhibitory effects on in vitro cell growth of human urothelial tumor cell lines under the combined administration of hematopoietic growth factors and clinically relevant antineoplastic agents. Urol Res. 1993 May;21(3):217–221. doi: 10.1007/BF00590039. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Defilippi P., Turrini F., Sanavio F., Edgell C. J., Aglietta M., Arese P., Mantovani A. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989 Feb 2;337(6206):471–473. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crawford J., Ozer H., Stoller R., Johnson D., Lyman G., Tabbara I., Kris M., Grous J., Picozzi V., Rausch G. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991 Jul 18;325(3):164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- Demetri G. D., Griffin J. D. Granulocyte colony-stimulating factor and its receptor. Blood. 1991 Dec 1;78(11):2791–2808. [PubMed] [Google Scholar]
- Fermand J. P., Levy Y., Gerota J., Benbunan M., Cosset J. M., Castaigne S., Seligmann M., Brouet J. C. Treatment of aggressive multiple myeloma by high-dose chemotherapy and total body irradiation followed by blood stem cells autologous graft. Blood. 1989 Jan;73(1):20–23. [PubMed] [Google Scholar]
- Gabrilove J. L., Jakubowski A., Fain K., Grous J., Scher H., Sternberg C., Yagoda A., Clarkson B., Bonilla M. A., Oettgen H. F. Phase I study of granulocyte colony-stimulating factor in patients with transitional cell carcinoma of the urothelium. J Clin Invest. 1988 Oct;82(4):1454–1461. doi: 10.1172/JCI113751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juttner C. A., To L. B., Ho J. Q., Bardy P. G., Dyson P. G., Haylock D. N., Kimber R. J. Early lympho-hemopoietic recovery after autografting using peripheral blood stem cells in acute non-lymphoblastic leukemia. Transplant Proc. 1988 Feb;20(1):40–42. [PubMed] [Google Scholar]
- Kessinger A., Armitage J. O., Landmark J. D., Weisenburger D. D. Reconstitution of human hematopoietic function with autologous cryopreserved circulating stem cells. Exp Hematol. 1986 Mar;14(3):192–196. [PubMed] [Google Scholar]
- Kessinger A., Armitage J. O., Smith D. M., Landmark J. D., Bierman P. J., Weisenburger D. D. High-dose therapy and autologous peripheral blood stem cell transplantation for patients with lymphoma. Blood. 1989 Sep;74(4):1260–1265. [PubMed] [Google Scholar]
- Lasky L. C., Bostrom B., Smith J., Moss T. J., Ramsay N. K. Clinical collection and use of peripheral blood stem cells in pediatric patients. Transplantation. 1989 Apr;47(4):613–616. doi: 10.1097/00007890-198904000-00010. [DOI] [PubMed] [Google Scholar]
- Masaoka T., Takaku F., Kato S., Moriyama Y., Kodera Y., Kanamaru A., Shimosaka A., Shibata H., Nakamura H. Recombinant human granulocyte colony-stimulating factor in allogeneic bone marrow transplantation. Exp Hematol. 1989 Nov;17(10):1047–1050. [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Mizutani Y., Okada Y., Terachi T., Kakehi Y., Yoshida O. Serum granulocyte colony-stimulating factor levels in patients with urinary bladder tumour and various urological malignancies. Br J Urol. 1995 Nov;76(5):580–586. doi: 10.1111/j.1464-410x.1995.tb07782.x. [DOI] [PubMed] [Google Scholar]
- Morstyn G., Campbell L., Souza L. M., Alton N. K., Keech J., Green M., Sheridan W., Metcalf D., Fox R. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988 Mar 26;1(8587):667–672. doi: 10.1016/s0140-6736(88)91475-4. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of 125I-labeled granulocyte colony-stimulating factor to normal murine hemopoietic cells. J Cell Physiol. 1985 Aug;124(2):313–321. doi: 10.1002/jcp.1041240222. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Lidonnici K., MacConnell P., Lane B. Intracellular localization of polymerase chain reaction (PCR)-amplified hepatitis C cDNA. Am J Surg Pathol. 1993 Jul;17(7):683–690. doi: 10.1097/00000478-199307000-00005. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., MacConnell P. B., Simsir A., Valea F., French D. L. Correlation of the in situ detection of polymerase chain reaction-amplified metalloproteinase complementary DNAs and their inhibitors with prognosis in cervical carcinoma. Cancer Res. 1995 Jan 15;55(2):267–275. [PubMed] [Google Scholar]
- Ohigashi T. Granulocyte-colony stimulating factor enhances the cytotoxic effects of methotrexate to bladder cancer cells in vitro. Keio J Med. 1990 Dec;39(4):254–260. doi: 10.2302/kjm.39.254. [DOI] [PubMed] [Google Scholar]
- Ohigashi T., Tachibana M., Tazaki H., Nakamura K. Bladder cancer cells express functional receptors for granulocyte-colony stimulating factor. J Urol. 1992 Jan;147(1):283–286. doi: 10.1016/s0022-5347(17)37214-2. [DOI] [PubMed] [Google Scholar]
- Ohno R., Tomonaga M., Kobayashi T., Kanamaru A., Shirakawa S., Masaoka T., Omine M., Oh H., Nomura T., Sakai Y. Effect of granulocyte colony-stimulating factor after intensive induction therapy in relapsed or refractory acute leukemia. N Engl J Med. 1990 Sep 27;323(13):871–877. doi: 10.1056/NEJM199009273231304. [DOI] [PubMed] [Google Scholar]
- Park L. S., Waldron P. E., Friend D., Sassenfeld H. M., Price V., Anderson D., Cosman D., Andrews R. G., Bernstein I. D., Urdal D. L. Interleukin-3, GM-CSF, and G-CSF receptor expression on cell lines and primary leukemia cells: receptor heterogeneity and relationship to growth factor responsiveness. Blood. 1989 Jul;74(1):56–65. [PubMed] [Google Scholar]
- Piao Y. F., Okabe T. Receptor binding of human granulocyte colony-stimulating factor to the blast cells of myeloid leukemia. Cancer Res. 1990 Mar 15;50(6):1671–1674. [PubMed] [Google Scholar]
- Scher H. I. Systemic chemotherapy in regionally advanced bladder cancer. Theoretical considerations and results. Urol Clin North Am. 1992 Nov;19(4):747–759. [PubMed] [Google Scholar]
- Tachibana M., Miyakawa A., Tazaki H., Nakamura K., Kubo A., Hata J., Nishi T., Amano Y. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995 Aug 1;55(15):3438–3443. [PubMed] [Google Scholar]
- Tachibana M. Studies on cellular adhesiveness in five different culture cell lines derived from carcinoma of the urinary bladder. Keio J Med. 1982 Oct;31(3):127–148. doi: 10.2302/kjm.31.127. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Jagannath S., Spitzer G., Spinolo J. A., Tucker S. L., Fogel B., Cabanillas F. F., Hagemeister F. B., Souza L. M. Recombinant human granulocyte colony-stimulating factor hastens granulocyte recovery after high-dose chemotherapy and autologous bone marrow transplantation in Hodgkin's disease. J Clin Oncol. 1989 Dec;7(12):1791–1799. doi: 10.1200/JCO.1989.7.12.1791. [DOI] [PubMed] [Google Scholar]
- Uzumaki H., Okabe T., Sasaki N., Hagiwara K., Takaku F., Tobita M., Yasukawa K., Ito S., Umezawa Y. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9323–9326. doi: 10.1073/pnas.86.23.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis J. D., Johnson M. L., Faunt L. M., Mercado M., Baumann G. Influence of the high-affinity growth hormone (GH)-binding protein on plasma profiles of free and bound GH and on the apparent half-life of GH. Modeling analysis and clinical applications. J Clin Invest. 1993 Feb;91(2):629–641. doi: 10.1172/JCI116243. [DOI] [PMC free article] [PubMed] [Google Scholar]