Abstract
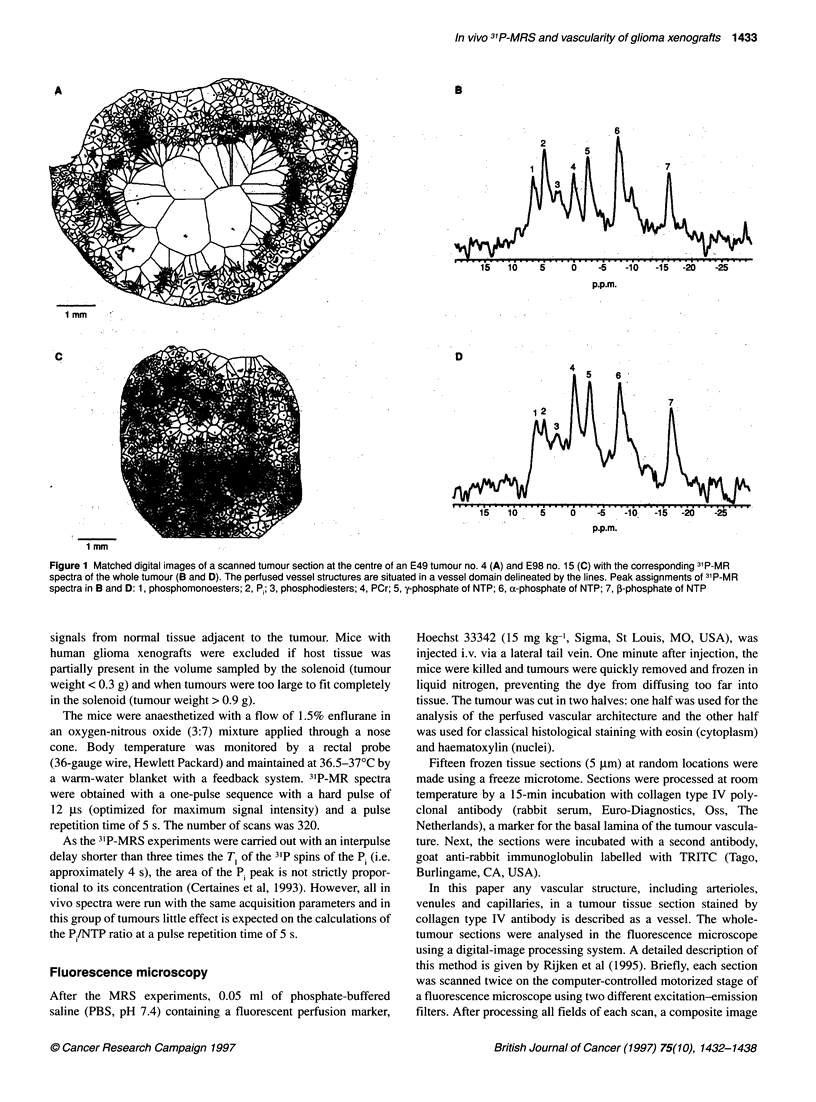
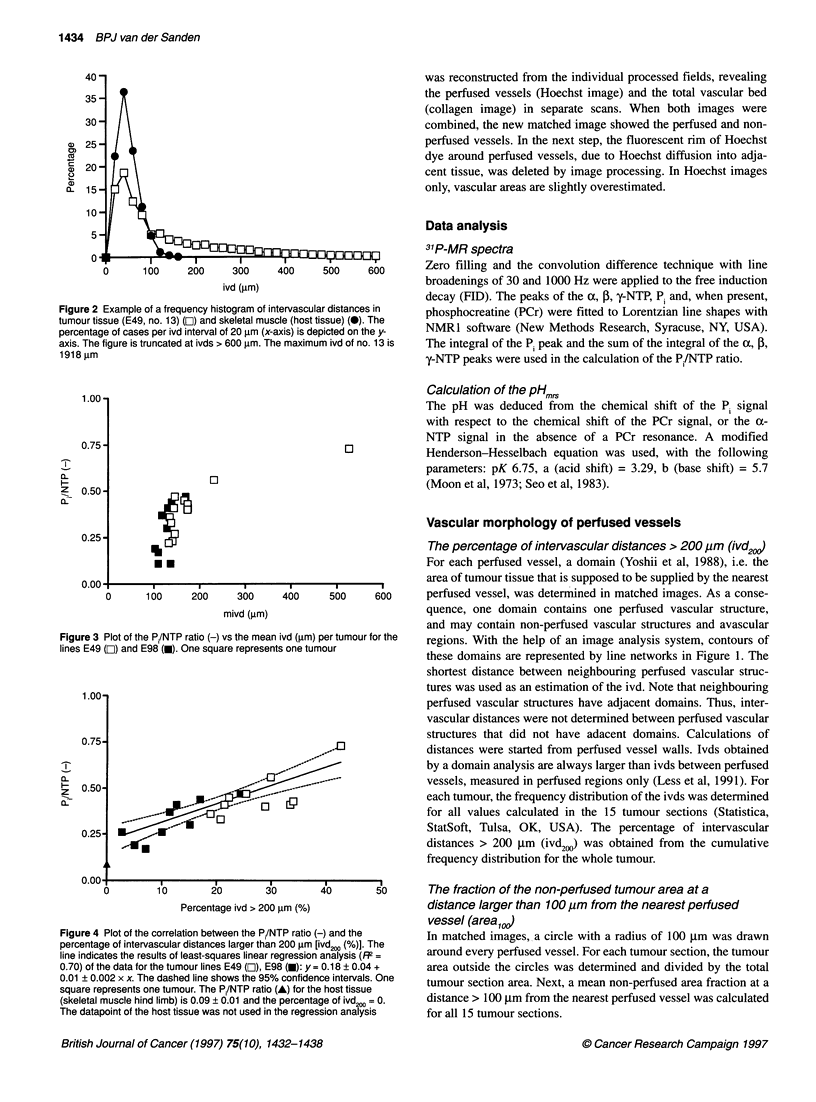
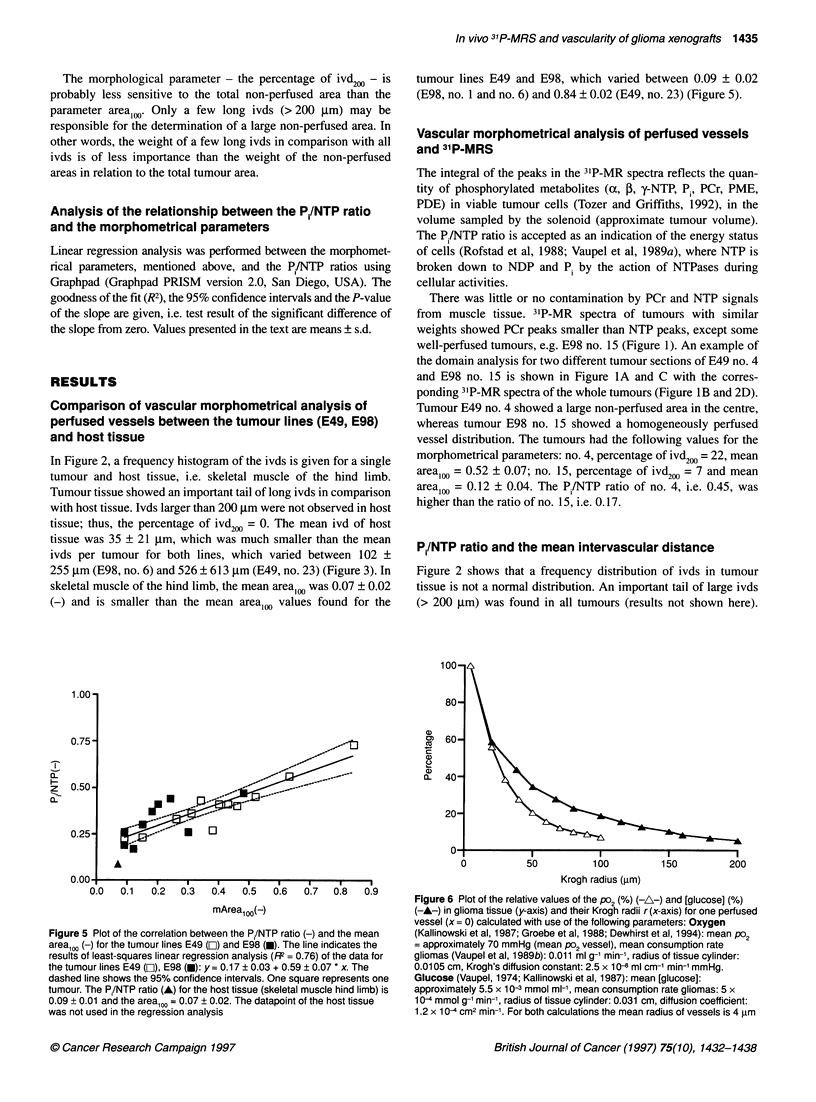
The relationship between the bioenergetic status of human glioma xenografts in nude mice and morphometric parameters of the perfused vascular architecture was studied using (31)P magnetic resonance spectroscopy (MRS), fluorescence microscopy and two-dimensional digital image analysis. Two tumour lines with a different vascular architecture were used for this study. Intervascular distances and non-perfused area fractions varied greatly between tumours of the same line and tumours of different lines. The inorganic phosphate-nucleoside triphosphate (P(i)/NTP) ratio increased rapidly as mean intervascular distances increased from 100 microm to 300 microm. Two morphometric parameters - the percentage of intervascular distances larger than 200 microm (ivd200) and the non-perfused area fraction at a distance larger than 100 microm from a nearest perfused vessel (area100), - were deduced from these experiments and related to the P(i)/NTP ratio of the whole tumour. It is assumed that an aerobic to anaerobic transition influences the bioenergetic status, i.e. the P(i)/NTP ratio increased linearly with the percentage of ivd200 and the area100.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dewhirst M. W., Secomb T. W., Ong E. T., Hsu R., Gross J. F. Determination of local oxygen consumption rates in tumors. Cancer Res. 1994 Jul 1;54(13):3333–3336. [PubMed] [Google Scholar]
- Eskey C. J., Koretsky A. P., Domach M. M., Jain R. K. Role of oxygen vs. glucose in energy metabolism in a mammary carcinoma perfused ex vivo: direct measurement by 31P NMR. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2646–2650. doi: 10.1073/pnas.90.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evelhoch J. L., Sapareto S. A., Nussbaum G. H., Ackerman J. J. Correlations between 31P NMR spectroscopy and 15O perfusion measurements in the RIF-1 murine tumor in vivo. Radiat Res. 1986 Apr;106(1):122–131. [PubMed] [Google Scholar]
- Gerweck L. E., Fellenz M. P. The simultaneous determination of intracellular pH and cell energy status. Radiat Res. 1991 Mar;125(3):257–261. [PubMed] [Google Scholar]
- Gerweck L. E., Seneviratne T., Gerweck K. K. Energy status and radiobiological hypoxia at specified oxygen concentrations. Radiat Res. 1993 Jul;135(1):69–74. [PubMed] [Google Scholar]
- Groebe K., Vaupel P. Evaluation of oxygen diffusion distances in human breast cancer xenografts using tumor-specific in vivo data: role of various mechanisms in the development of tumor hypoxia. Int J Radiat Oncol Biol Phys. 1988 Sep;15(3):691–697. doi: 10.1016/0360-3016(88)90313-6. [DOI] [PubMed] [Google Scholar]
- Hodgkiss R. J., Jones G., Long A., Parrick J., Smith K. A., Stratford M. R., Wilson G. D. Flow cytometric evaluation of hypoxic cells in solid experimental tumours using fluorescence immunodetection. Br J Cancer. 1991 Jan;63(1):119–125. doi: 10.1038/bjc.1991.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann K. A., Linn F., Okada Y. Bioluminescence and fluoroscopic imaging of tissue pH and metabolites in experimental brain tumors of cat. NMR Biomed. 1992 Sep-Oct;5(5):259–264. doi: 10.1002/nbm.1940050511. [DOI] [PubMed] [Google Scholar]
- Ikezaki K., Black K. L., Conklin S. G., Becker D. P. Histochemical evaluation of energy metabolism in rat glioma. Neurol Res. 1992 Sep;14(4):289–293. doi: 10.1080/01616412.1992.11740072. [DOI] [PubMed] [Google Scholar]
- Kallinowski F., Runkel S., Fortmeyer H. P., Förster H., Vaupel P. L-glutamine: a major substrate for tumor cells in vivo? J Cancer Res Clin Oncol. 1987;113(3):209–215. doi: 10.1007/BF00396375. [DOI] [PubMed] [Google Scholar]
- Kreuzer F. Oxygen supply to tissues: the Krogh model and its assumptions. Experientia. 1982 Dec 15;38(12):1415–1426. doi: 10.1007/BF01955753. [DOI] [PubMed] [Google Scholar]
- Less J. R., Skalak T. C., Sevick E. M., Jain R. K. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. Cancer Res. 1991 Jan 1;51(1):265–273. [PubMed] [Google Scholar]
- Loesberg C., Van Rooij H., Nooijen W. J., Meijer A. J., Smets L. A. Impaired mitochondrial respiration and stimulated glycolysis by m-iodobenzylguanidine (MIBG). Int J Cancer. 1990 Aug 15;46(2):276–281. doi: 10.1002/ijc.2910460223. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Okunieff P. G., Koutcher J. A., Gerweck L., McFarland E., Hitzig B., Urano M., Brady T., Neuringer L., Suit H. D. Tumor size dependent changes in a murine fibrosarcoma: use of in vivo 31P NMR for non-invasive evaluation of tumor metabolic status. Int J Radiat Oncol Biol Phys. 1986 May;12(5):793–799. doi: 10.1016/0360-3016(86)90038-6. [DOI] [PubMed] [Google Scholar]
- Okunieff P., Vaupel P., Sedlacek R., Neuringer L. J. Evaluation of tumor energy metabolism and microvascular blood flow after glucose or mannitol administration using 31P nuclear magnetic resonance spectroscopy and laser Doppler flowmetry. Int J Radiat Oncol Biol Phys. 1989 Jun;16(6):1493–1500. doi: 10.1016/0360-3016(89)90954-1. [DOI] [PubMed] [Google Scholar]
- Pianet I., Merle M., Labouesse J., Canioni P. Phosphorus-31 nuclear magnetic resonance of C6 glioma cells and rat astrocytes. Evidence for a modification of the longitudinal relaxation time of ATP and Pi during glucose starvation. Eur J Biochem. 1991 Jan 1;195(1):87–95. doi: 10.1111/j.1432-1033.1991.tb15679.x. [DOI] [PubMed] [Google Scholar]
- Rhodes C. G., Wise R. J., Gibbs J. M., Frackowiak R. S., Hatazawa J., Palmer A. J., Thomas D. G., Jones T. In vivo disturbance of the oxidative metabolism of glucose in human cerebral gliomas. Ann Neurol. 1983 Dec;14(6):614–626. doi: 10.1002/ana.410140604. [DOI] [PubMed] [Google Scholar]
- Rijken P. F., Bernsen H. J., van der Kogel A. J. Application of an image analysis system to the quantitation of tumor perfusion and vascularity in human glioma xenografts. Microvasc Res. 1995 Sep;50(2):141–153. doi: 10.1006/mvre.1995.1048. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., DeMuth P., Fenton B. M., Sutherland R. M. 31P nuclear magnetic resonance spectroscopy studies of tumor energy metabolism and its relationship to intracapillary oxyhemoglobin saturation status and tumor hypoxia. Cancer Res. 1988 Oct 1;48(19):5440–5446. [PubMed] [Google Scholar]
- Seo Y., Murakami M., Watari H., Imai Y., Yoshizaki K., Nishikawa H., Morimoto T. Intracellular pH determination by a 31P-NMR technique. The second dissociation constant of phosphoric acid in a biological system. J Biochem. 1983 Sep;94(3):729–734. doi: 10.1093/oxfordjournals.jbchem.a134413. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Bhujwalla Z. M., Tozer G. M., Rodrigues L. M., Maxwell R. J., Morgan R., Howe F. A., Griffiths J. R. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992 Nov-Dec;5(6):351–359. doi: 10.1002/nbm.1940050606. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Griffiths J. R. The contribution made by cell death and oxygenation to 31P MRS observations of tumour energy metabolism. NMR Biomed. 1992 Sep-Oct;5(5):279–289. doi: 10.1002/nbm.1940050515. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Vaupel P., Kelleher D. K., Engel T. Stable bioenergetic status despite substantial changes in blood flow and tissue oxygenation in a rat tumour. Br J Cancer. 1994 Jan;69(1):46–49. doi: 10.1038/bjc.1994.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P., Okunieff P., Kallinowski F., Neuringer L. J. Correlations between 31P-NMR spectroscopy and tissue O2 tension measurements in a murine fibrosarcoma. Radiat Res. 1989 Dec;120(3):477–493. [PubMed] [Google Scholar]
- Wendland M. F., Iyer S. B., Fu K. K., Lam K. N., James T. L. Correlations between in vivo 31P MRS measurements, tumor size, cell survival, and hypoxic fraction in the murine EMT6 tumor. Magn Reson Med. 1992 Jun;25(2):217–232. doi: 10.1002/mrm.1910250202. [DOI] [PubMed] [Google Scholar]
- Yoshii Y., Sugiyama K. Intercapillary distance in the proliferating area of human glioma. Cancer Res. 1988 May 15;48(10):2938–2941. [PubMed] [Google Scholar]
- de Certaines J. D., Larsen V. A., Podo F., Carpinelli G., Briot O., Henriksen O. In vivo 31P MRS of experimental tumours. NMR Biomed. 1993 Nov-Dec;6(6):345–365. doi: 10.1002/nbm.1940060602. [DOI] [PubMed] [Google Scholar]


