Abstract
Although breast carcinomas are considered to originate from glandular epithelial cells, some exhibit 'squamoid features', comprising stratification with a gradient in the nuclear-cytoplasmic ratio within individual cancer cell nests on microscopy. In parallel with a histological review of squamoid features, we immunohistochemically investigated the expression of involucrin, a marker of terminal squamous differentiation, in 223 breast carcinomas with one to three regional nodal metastases but no distant metastases and analysed their association with other clinicopathological parameters to explore their clinical and biological implications. Squamoid features and involucrin expression, detected in 22% and 27% of cases respectively, correlated with each other and were associated with high-grade atypia, a solid-nest pattern, cancer cell necrosis on histology and negative oestrogen receptor status. The incidence of regional recurrences was higher in patients with involucrin expression, whereas bone metastases were less frequent in groups with squamoid features or with diffuse (> or = 10%) involucrin expression. Both squamoid features and involucrin expression, which were considered to be derived either from differentiation into keratinocytes or from some kind of cellular degeneration caused by high turnover rate, are suggested to influence the biological behaviour of breast cancer cells in vivo, and they may be effective in predicting the most likely recurrence sites.
Full text
PDF

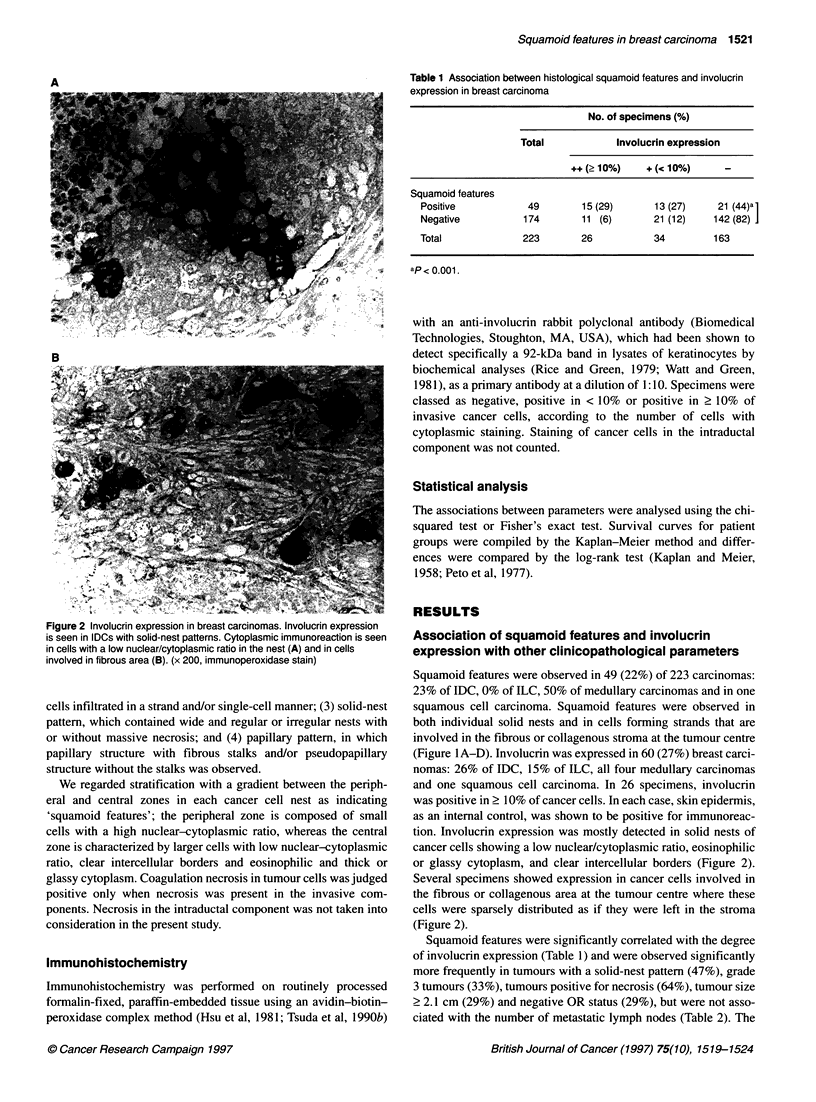



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouizar Z., Spyratos F., Deytieux S., de Vernejoul M. C., Jullienne A. Polymerase chain reaction analysis of parathyroid hormone-related protein gene expression in breast cancer patients and occurrence of bone metastases. Cancer Res. 1993 Nov 1;53(21):5076–5078. [PubMed] [Google Scholar]
- Fisher E. R., Gregorio R. M., Fisher B., Redmond C., Vellios F., Sommers S. C. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975 Jul;36(1):1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kamby C., Andersen J., Ejlertsen B., Birkler N. E., Rytter L., Zedeler K., Thorpe S. M., Nørgaard T., Rose C. Histological grade and steroid receptor content of primary breast cancer--impact on prognosis and possible modes of action. Br J Cancer. 1988 Oct;58(4):480–486. doi: 10.1038/bjc.1988.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen P., Aaltomaa S., Kosma V. M., Syrjänen K. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer. 1994;30A(14):2068–2073. doi: 10.1016/0959-8049(94)00342-3. [DOI] [PubMed] [Google Scholar]
- Lynley A. M., Dale B. A. The characterization of human epidermal filaggrin. A histidine-rich, keratin filament-aggregating protein. Biochim Biophys Acta. 1983 Apr 14;744(1):28–35. doi: 10.1016/0167-4838(83)90336-9. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Mehrel T., Hohl D., Rothnagel J. A., Longley M. A., Bundman D., Cheng C., Lichti U., Bisher M. E., Steven A. C., Steinert P. M. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990 Jun 15;61(6):1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- Murphy G. F., Flynn T. C., Rice R. H., Pinkus G. S. Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J Invest Dermatol. 1984 May;82(5):453–457. doi: 10.1111/1523-1747.ep12260945. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Said J. W., Nash G., Sassoon A. F., Shintaku I. P., Banks-Schlegel S. Involucrin in lung tumors. A specific marker for squamous differentiation. Lab Invest. 1983 Nov;49(5):563–568. [PubMed] [Google Scholar]
- Schmid C., Zatloukal K., Beham A., Denk H. Involucrin expression in breast carcinomas: an immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1993;423(3):161–167. doi: 10.1007/BF01614766. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Hirohashi S., Shimosato Y., Hirota T., Tsugane S., Watanabe S., Terada M., Yamamoto H. Correlation between histologic grade of malignancy and copy number of c-erbB-2 gene in breast carcinoma. A retrospective analysis of 176 cases. Cancer. 1990 Apr 15;65(8):1794–1800. doi: 10.1002/1097-0142(19900415)65:8<1794::aid-cncr2820650821>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Hirohashi S., Shimosato Y., Tanaka Y., Hirota T., Tsugane S., Shiraishi M., Toyoshima K., Yamamoto T., Terada M. Immunohistochemical study on overexpression of c-erbB-2 protein in human breast cancer: its correlation with gene amplification and long-term survival of patients. Jpn J Cancer Res. 1990 Apr;81(4):327–332. doi: 10.1111/j.1349-7006.1990.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhol M. J., Antonioli D. A., Pinkus G. S., Burke L., Rice R. H. Immunoperoxidase staining for involucrin: a potential diagnostic aid in cervicovaginal pathology. Hum Pathol. 1982 Dec;13(12):1095–1099. doi: 10.1016/s0046-8177(82)80245-1. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Green H. Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol. 1981 Sep;90(3):738–742. doi: 10.1083/jcb.90.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]




