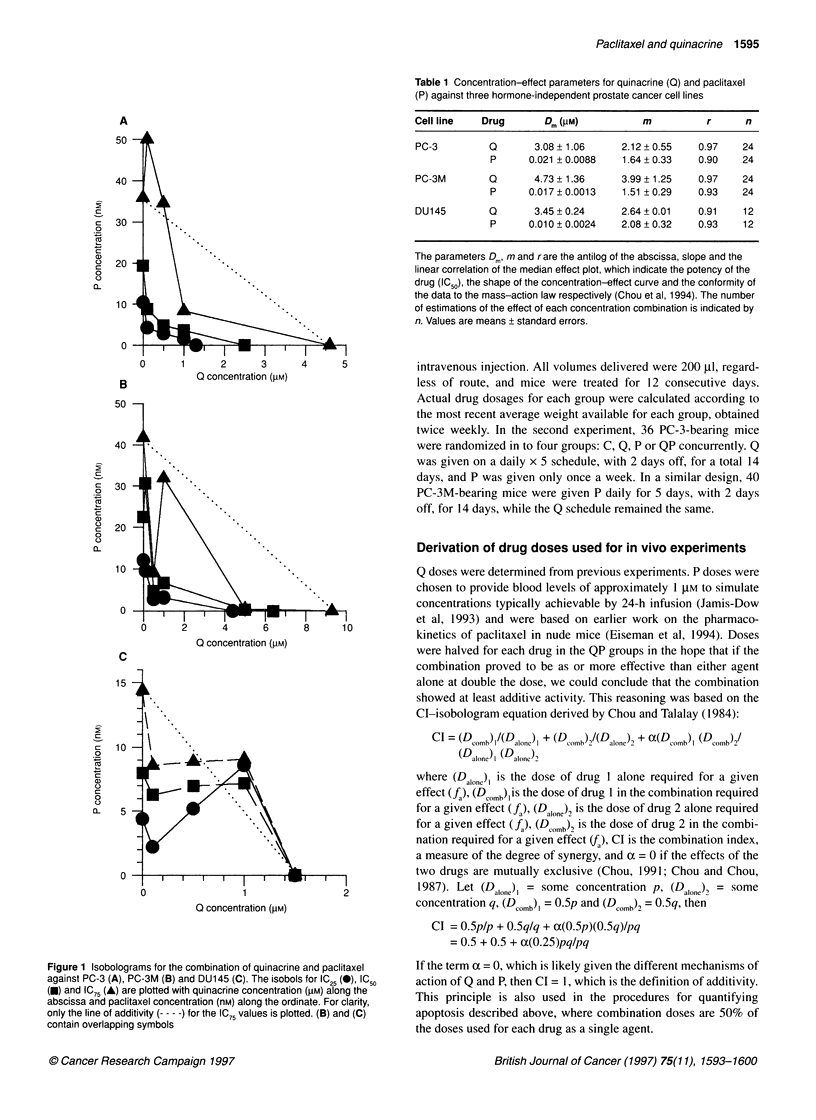
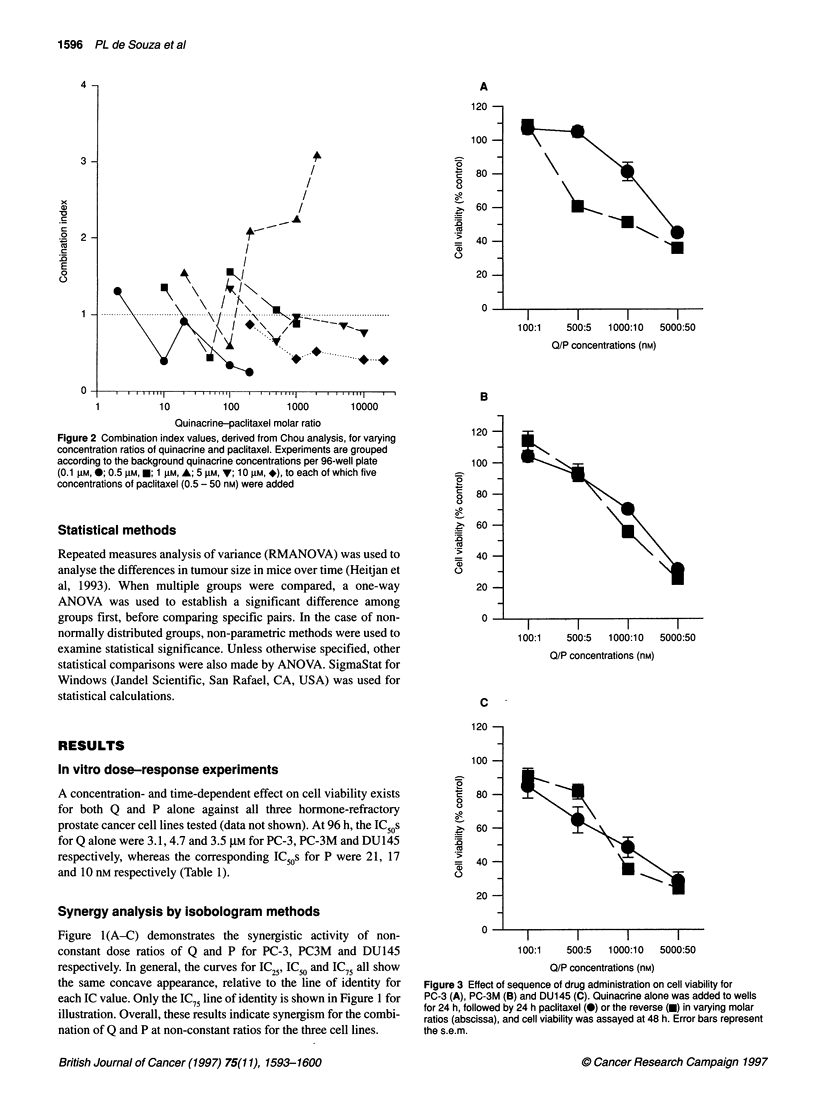
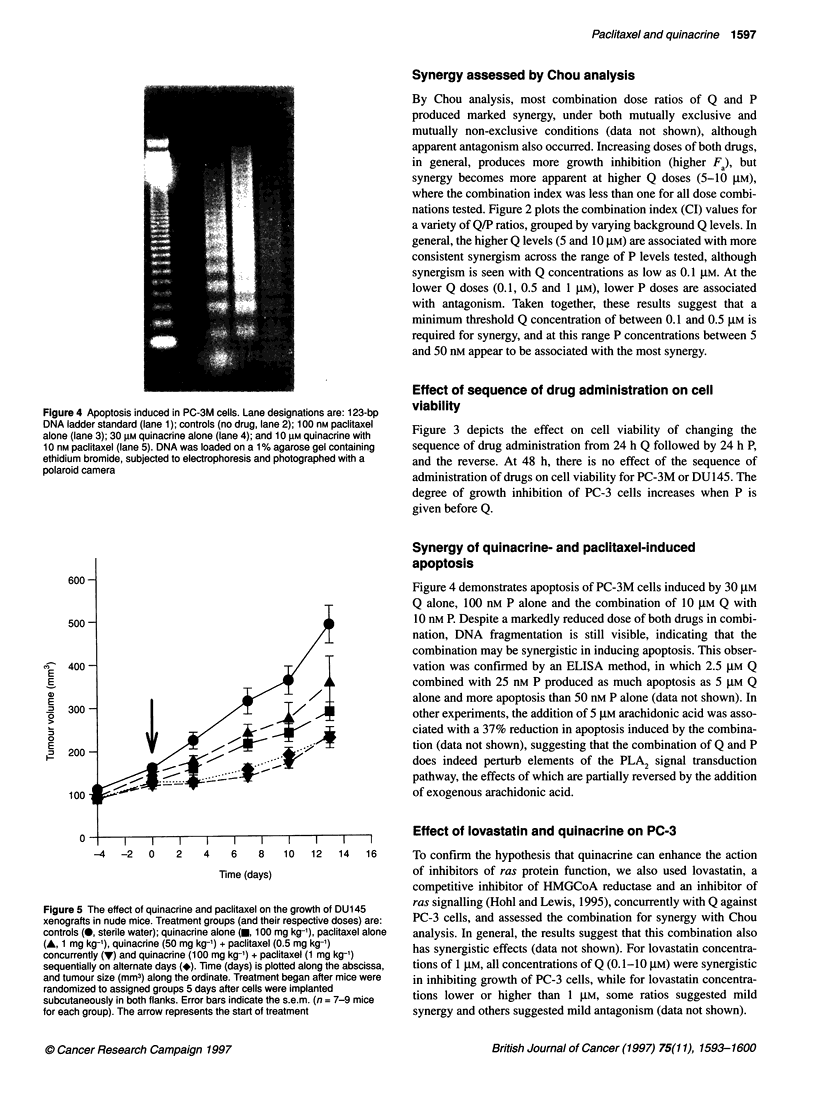
Abstract
Cytoplasmic phospholipase A2 (PLA2) is known to be phosphorylated and activated by MAP kinase (Lin et al 1993, Cell 72: 269-278), an important downstream component of signal transduction, whereas paclitaxel has been shown to inhibit isoprenylation of ras proteins (Danesi et al 1995, Mol Pharmacol 47: 1106-1111). Given that quinacrine (Q), a PLA2 inhibitor, and paclitaxel (P) might act at different sites in the cell signalling pathway, our aim was to test whether they were synergistic in combination against prostate cancer cells. Cell viability of PC-3, PC-3M and DU145 cells in 96 - well plates was assessed 96 h after drugs were added concurrently. Using Chou analysis, we demonstrated synergy for the combination against all three cell lines. Further, synergy was present under both conservative (mutually non-exclusive) and non-conservative (mutually exclusive) models. Studies in the nude mouse xenograft model support the finding of synergy in vitro. In DU145-bearing mice, Q (50 mg kg(-1)) and P (0.5 mg kg(-1)) given daily for 12 consecutive days, either concurrently or sequentially, was more effective than either drug alone, at twice the dose intensity. In an enzyme-linked immunosorbent (ELISA) apoptosis assay, arachidonic acid was able to partially reverse Q- and P-induced apoptosis, suggesting PLA2 pathway involvement. Finally, the combination of lovastatin, another inhibitor of ras isoprenylation, and quinacrine had synergistic inhibitory effects on the growth of PC-3 cells in vitro, suggesting that the combination of these two classes of compounds might serve as an attractive therapeutic approach for prostate cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babb R. R. Giardiasis. Taming this pervasive parasitic infection. Postgrad Med. 1995 Aug;98(2):155–158. [PubMed] [Google Scholar]
- Chang J., Musser J. H., McGregor H. Phospholipase A2: function and pharmacological regulation. Biochem Pharmacol. 1987 Aug 1;36(15):2429–2436. doi: 10.1016/0006-2952(87)90512-0. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Motzer R. J., Tong Y., Bosl G. J. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst. 1994 Oct 19;86(20):1517–1524. doi: 10.1093/jnci/86.20.1517. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talaly P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J Biol Chem. 1977 Sep 25;252(18):6438–6442. [PubMed] [Google Scholar]
- Chou T. C., Tan Q. H., Sirotnak F. M. Quantitation of the synergistic interaction of edatrexate and cisplatin in vitro. Cancer Chemother Pharmacol. 1993;31(4):259–264. doi: 10.1007/BF00685668. [DOI] [PubMed] [Google Scholar]
- Danesi R., Figg W. D., Reed E., Myers C. E. Paclitaxel (taxol) inhibits protein isoprenylation and induces apoptosis in PC-3 human prostate cancer cells. Mol Pharmacol. 1995 Jun;47(6):1106–1111. [PubMed] [Google Scholar]
- Eiseman J. L., Eddington N. D., Leslie J., MacAuley C., Sentz D. L., Zuhowski M., Kujawa J. M., Young D., Egorin M. J. Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemother Pharmacol. 1994;34(6):465–471. doi: 10.1007/BF00685656. [DOI] [PubMed] [Google Scholar]
- Fenton R. G., Kung H. F., Longo D. L., Smith M. R. Regulation of intracellular actin polymerization by prenylated cellular proteins. J Cell Biol. 1992 Apr;117(2):347–356. doi: 10.1083/jcb.117.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. M., Prozialeck W. C., Hait W. N. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol Pharmacol. 1989 Jan;35(1):105–115. [PubMed] [Google Scholar]
- Hanada K., Kinoshita E., Itoh M., Hirata M., Kajiyama G., Sugiyama M. Human pancreatic phospholipase A2 stimulates the growth of human pancreatic cancer cell line. FEBS Lett. 1995 Oct 2;373(1):85–87. doi: 10.1016/0014-5793(95)01005-y. [DOI] [PubMed] [Google Scholar]
- Heitjan D. F., Manni A., Santen R. J. Statistical analysis of in vivo tumor growth experiments. Cancer Res. 1993 Dec 15;53(24):6042–6050. [PubMed] [Google Scholar]
- Hohl R. J., Lewis K. Differential effects of monoterpenes and lovastatin on RAS processing. J Biol Chem. 1995 Jul 21;270(29):17508–17512. doi: 10.1074/jbc.270.29.17508. [DOI] [PubMed] [Google Scholar]
- Hudes G. R., Nathan F. E., Khater C., Greenberg R., Gomella L., Stern C., McAleer C. Paclitaxel plus estramustine in metastatic hormone-refractory prostate cancer. Semin Oncol. 1995 Oct;22(5 Suppl 12):41–45. [PubMed] [Google Scholar]
- Isaacs W. B., Bova G. S., Morton R. A., Bussemakers M. J., Brooks J. D., Ewing C. M. Molecular biology of prostate cancer. Semin Oncol. 1994 Oct;21(5):514–521. [PubMed] [Google Scholar]
- Jamis-Dow C. A., Klecker R. W., Sarosy G., Reed E., Collins J. M. Steady-state plasma concentrations and effects of taxol for a 250 mg/m2 dose in combination with granulocyte-colony stimulating factor in patients with ovarian cancer. Cancer Chemother Pharmacol. 1993;33(1):48–52. doi: 10.1007/BF00686022. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R., Der C. J. The Ras signal transduction pathway. Cancer Metastasis Rev. 1994 Mar;13(1):67–89. doi: 10.1007/BF00690419. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993 Jan 29;72(2):269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Murata K., Egami H., Kiyohara H., Oshima S., Kurizaki T., Ogawa M. Expression of group-II phospholipase A2 in malignant and non-malignant human gastric mucosa. Br J Cancer. 1993 Jul;68(1):103–111. doi: 10.1038/bjc.1993.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso Y., Sakai N., Watari H., Yokoyama T., Kuzumaki N. Suppression of various human tumor cell lines by a dominant negative H-ras mutant. Gene Ther. 1994 Nov;1(6):403–407. [PubMed] [Google Scholar]
- Pretlow T. G., Delmoro C. M., Dilley G. G., Spadafora C. G., Pretlow T. P. Transplantation of human prostatic carcinoma into nude mice in Matrigel. Cancer Res. 1991 Jul 15;51(14):3814–3817. [PubMed] [Google Scholar]
- Ruch R. J., Madhukar B. V., Trosko J. E., Klaunig J. E. Reversal of ras-induced inhibition of gap-junctional intercellular communication, transformation, and tumorigenesis by lovastatin. Mol Carcinog. 1993;7(1):50–59. doi: 10.1002/mc.2940070109. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegsmund M. J., Cardarelli C., Aksentijevich I., Sugimoto Y., Pastan I., Gottesman M. M. Ketoconazole effectively reverses multidrug resistance in highly resistant KB cells. J Urol. 1994 Feb;151(2):485–491. doi: 10.1016/s0022-5347(17)34999-6. [DOI] [PubMed] [Google Scholar]
- Tokumoto H., Croxtall J. D., Choudhury Q., Flower R. J. Phospholipase A2-induced stimulation of A549 lung adenocarcinoma cell line proliferation. Biochim Biophys Acta. 1993 Sep 8;1169(3):236–242. doi: 10.1016/0005-2760(93)90246-6. [DOI] [PubMed] [Google Scholar]
- Voeller H. J., Wilding G., Gelmann E. P. v-rasH expression confers hormone-independent in vitro growth to LNCaP prostate carcinoma cells. Mol Endocrinol. 1991 Feb;5(2):209–216. doi: 10.1210/mend-5-2-209. [DOI] [PubMed] [Google Scholar]
- Wallace D. J. Antimalarial agents and lupus. Rheum Dis Clin North Am. 1994 Feb;20(1):243–263. [PubMed] [Google Scholar]
- Yamashita S., Yamashita J., Ogawa M. Overexpression of group II phospholipase A2 in human breast cancer tissues is closely associated with their malignant potency. Br J Cancer. 1994 Jun;69(6):1166–1170. doi: 10.1038/bjc.1994.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R., Sherman I. W., Maguire P. A., De Boeck H. A nuclear magnetic resonance study of the interactions of the antimalarials chloroquine, quinacrine, quinine and mefloquine with lipids extracted from normal human erythrocytes. Mol Biochem Parasitol. 1990 Jan 1;38(1):33–39. doi: 10.1016/0166-6851(90)90202-w. [DOI] [PubMed] [Google Scholar]



