Abstract
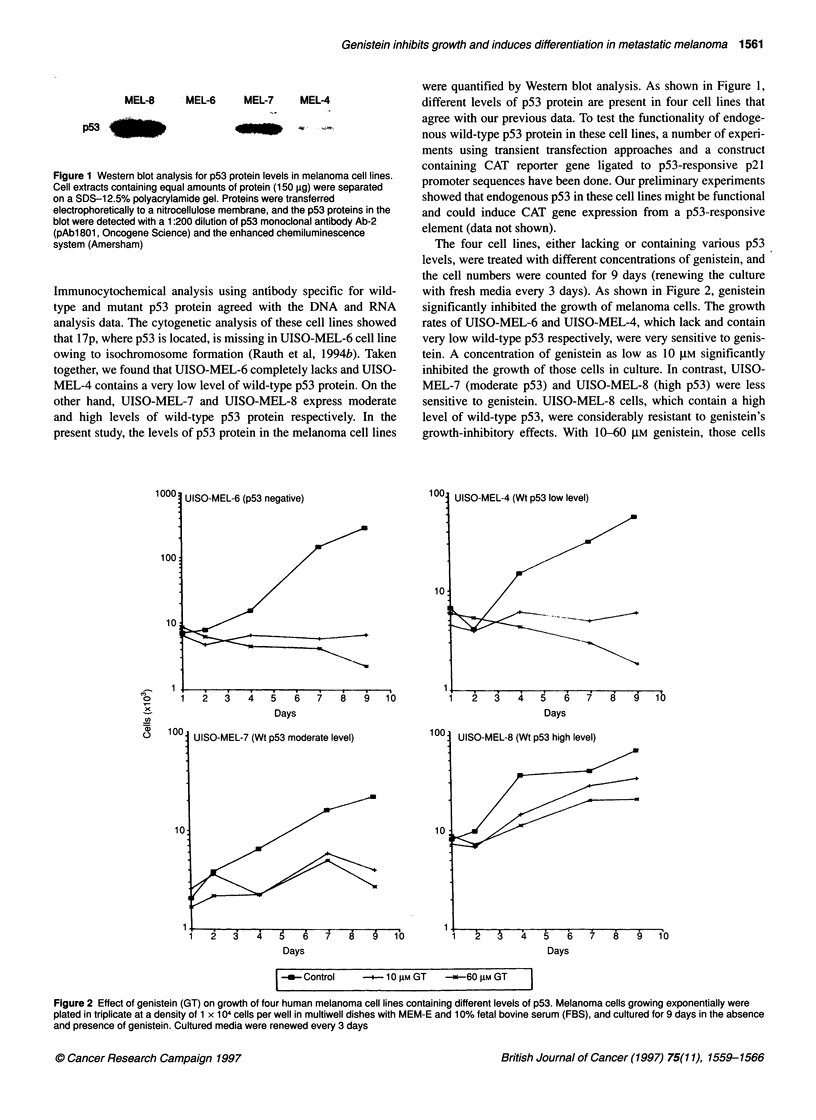
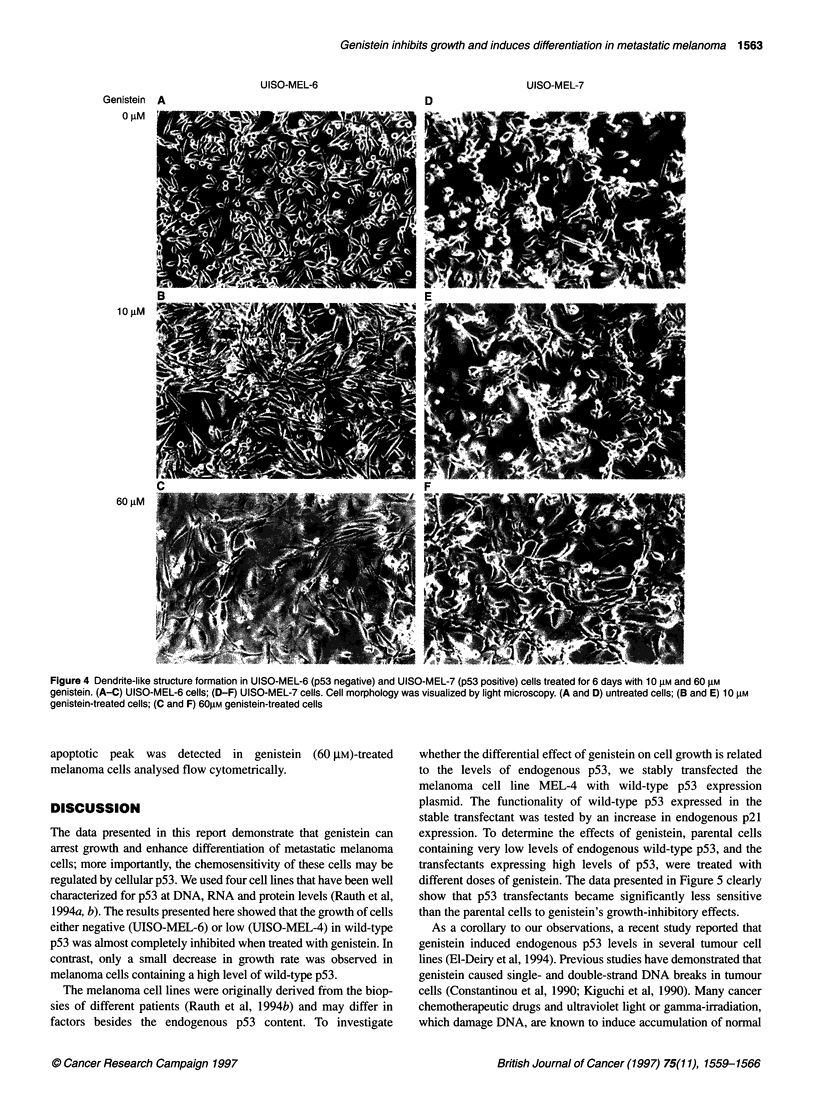
We have investigated the effect of the soybean isoflavone genistein on the growth and differentiation of human melanoma cells. Four human melanoma cell lines, either completely lacking or containing different levels of wild-type p53, were treated with genistein in vitro in culture. It has been found that genistein significantly inhibited cell growth and that the chemosensitivity might depend on cellular p53 content. Specifically, the data suggest that high levels of wild-type p53 expression make cells resistant to genistein's growth-inhibitory action. Further support for this observation came from the stable transfection studies in which p53 transfectants expressing high levels of wild-type p53 became resistant to genistein. With respect to cell differentiation, our study showed that genistein increased melanin content and tyrosinase activity and caused the cells to form dendrite-like structures. Cells lacking p53 responded more than cells with p53 to dendrite-like structure formation. We also observed that genistein-induced differentiation involved an increase in tyrosinase mRNA level; the mechanisms by which genistein increases tyrosinase transcripts remain to be elucidated. Genistein treatment of the melanoma cell lines resulted in cell cycle arrest at G2/M check point and no significant apoptosis was observed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Brozena S. J., Fenske N. A., Perez I. R. Epidemiology of malignant melanoma, worldwide incidence, and etiologic factors. Semin Surg Oncol. 1993 May-Jun;9(3):165–167. [PubMed] [Google Scholar]
- Buckley A. R., Buckley D. J., Gout P. W., Liang H., Rao Y. P., Blake M. J. Inhibition by genistein of prolactin-induced Nb2 lymphoma cell mitogenesis. Mol Cell Endocrinol. 1993 Dec;98(1):17–25. doi: 10.1016/0303-7207(93)90231-8. [DOI] [PubMed] [Google Scholar]
- Cai Q., Wei H. Effect of dietary genistein on antioxidant enzyme activities in SENCAR mice. Nutr Cancer. 1996;25(1):1–7. doi: 10.1080/01635589609514423. [DOI] [PubMed] [Google Scholar]
- Constantinou A., Kiguchi K., Huberman E. Induction of differentiation and DNA strand breakage in human HL-60 and K-562 leukemia cells by genistein. Cancer Res. 1990 May 1;50(9):2618–2624. [PubMed] [Google Scholar]
- Elledge R. M., Gray R., Mansour E., Yu Y., Clark G. M., Ravdin P., Osborne C. K., Gilchrist K., Davidson N. E., Robert N. Accumulation of p53 protein as a possible predictor of response to adjuvant combination chemotherapy with cyclophosphamide, methotrexate, fluorouracil, and prednisone for breast cancer. J Natl Cancer Inst. 1995 Aug 16;87(16):1254–1256. doi: 10.1093/jnci/87.16.1254. [DOI] [PubMed] [Google Scholar]
- Faber K. A., Hughes C. L., Jr The effect of neonatal exposure to diethylstilbestrol, genistein, and zearalenone on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Biol Reprod. 1991 Oct;45(4):649–653. doi: 10.1095/biolreprod45.4.649. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kichina J., Green A., Rauth S. Tumor suppressor p53 down-regulates tissue-specific expression of tyrosinase gene in human melanoma cell lines. Pigment Cell Res. 1996 Apr;9(2):85–91. doi: 10.1111/j.1600-0749.1996.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Kiguchi K., Constantinou A. I., Huberman E. Genistein-induced cell differentiation and protein-linked DNA strand breakage in human melanoma cells. Cancer Commun. 1990;2(8):271–277. doi: 10.3727/095535490820874218. [DOI] [PubMed] [Google Scholar]
- Kondo K., Tsuneizumi K., Watanabe T., Oishi M. Induction of in vitro differentiation of mouse embryonal carcinoma (F9) cells by inhibitors of topoisomerases. Cancer Res. 1991 Oct 1;51(19):5398–5404. [PubMed] [Google Scholar]
- Limtrakul P., Suttajit M., Semura R., Shimada K., Yamamoto S. Suppressive effect of soybean milk protein on experimentally induced skin tumor in mice. Life Sci. 1993;53(21):1591–1596. doi: 10.1016/0024-3205(93)90182-3. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Markovits J., Linassier C., Fossé P., Couprie J., Pierre J., Jacquemin-Sablon A., Saucier J. M., Le Pecq J. B., Larsen A. K. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989 Sep 15;49(18):5111–5117. [PubMed] [Google Scholar]
- Messina M. J., Persky V., Setchell K. D., Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- Messina M., Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. 1991 Apr 17;83(8):541–546. doi: 10.1093/jnci/83.8.541. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliacci M. C., Spinozzi F., Migliorati G., Fumi G., Smacchia M., Grignani F., Riccardi C., Nicoletti I. Genistein inhibits tumour cell growth in vitro but enhances mitochondrial reduction of tetrazolium salts: a further pitfall in the use of the MTT assay for evaluating cell growth and survival. Eur J Cancer. 1993;29A(11):1573–1577. doi: 10.1016/0959-8049(93)90297-s. [DOI] [PubMed] [Google Scholar]
- Peterson G., Barnes S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22(4):335–345. doi: 10.1002/pros.2990220408. [DOI] [PubMed] [Google Scholar]
- Peterson G., Barnes S. Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene. Biochem Biophys Res Commun. 1991 Aug 30;179(1):661–667. doi: 10.1016/0006-291x(91)91423-a. [DOI] [PubMed] [Google Scholar]
- Pisha E., Chai H., Lee I. S., Chagwedera T. E., Farnsworth N. R., Cordell G. A., Beecher C. W., Fong H. H., Kinghorn A. D., Brown D. M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995 Oct;1(10):1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- Powers T. P., Shows T. B., Davidson R. L. Pigment-cell-specific genes from fibroblasts are transactivated after chromosomal transfer into melanoma cells. Mol Cell Biol. 1994 Feb;14(2):1179–1190. doi: 10.1128/mcb.14.2.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth S., Davidson R. L. Suppression of tyrosinase gene expression by bromodeoxyuridine in Syrian hamster melanoma cells is not due to its incorporation into upstream or coding sequences of the tyrosinase gene. Somat Cell Mol Genet. 1993 May;19(3):285–293. doi: 10.1007/BF01233076. [DOI] [PubMed] [Google Scholar]
- Rauth S., Green A., Bratescu L., Das Gupta T. K. Chromosome abnormalities in metastatic melanoma. In Vitro Cell Dev Biol Anim. 1994 Feb;30A(2):79–84. doi: 10.1007/BF02631396. [DOI] [PubMed] [Google Scholar]
- Rauth S., Kichina J., Green A., Bratescu L., Das Gupta T. K. Establishment of a human melanoma cell line lacking p53 expression and spontaneously metastasizing in nude mice. Anticancer Res. 1994 Nov-Dec;14(6B):2457–2463. [PubMed] [Google Scholar]
- Schweigerer L., Christeleit K., Fleischmann G., Adlercreutz H., Wähälä K., Hase T., Schwab M., Ludwig R., Fotsis T. Identification in human urine of a natural growth inhibitor for cells derived from solid paediatric tumours. Eur J Clin Invest. 1992 Apr;22(4):260–264. doi: 10.1111/j.1365-2362.1992.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Spinozzi F., Pagliacci M. C., Migliorati G., Moraca R., Grignani F., Riccardi C., Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk Res. 1994 Jun;18(6):431–439. doi: 10.1016/0145-2126(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Steele V. E., Pereira M. A., Sigman C. C., Kelloff G. J. Cancer chemoprevention agent development strategies for genistein. J Nutr. 1995 Mar;125(3 Suppl):713S–716S. doi: 10.1093/jn/125.3_Suppl.713S. [DOI] [PubMed] [Google Scholar]
- Traganos F., Ardelt B., Halko N., Bruno S., Darzynkiewicz Z. Effects of genistein on the growth and cell cycle progression of normal human lymphocytes and human leukemic MOLT-4 and HL-60 cells. Cancer Res. 1992 Nov 15;52(22):6200–6208. [PubMed] [Google Scholar]
- Watanabe T., Kondo K., Oishi M. Induction of in vitro differentiation of mouse erythroleukemia cells by genistein, an inhibitor of tyrosine protein kinases. Cancer Res. 1991 Feb 1;51(3):764–768. [PubMed] [Google Scholar]
- Wei H., Bowen R., Cai Q., Barnes S., Wang Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc Soc Exp Biol Med. 1995 Jan;208(1):124–130. doi: 10.3181/00379727-208-43844. [DOI] [PubMed] [Google Scholar]
- Wei H., Wei L., Frenkel K., Bowen R., Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993;20(1):1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]