Abstract
We report here the first characterization of the Sinorhizobium meliloti open reading frame SMc01113. The SMc01113 protein is a member of a highly conserved protein family, universal among bacteria. We demonstrate that the SMc01113 gene is absolutely required for S. meliloti symbiosis with alfalfa and also for the protection of the bacterium from a wide range of environmental stresses.
Under conditions of poor nitrogen availability, the alphaproteobacterium Sinorhizobium meliloti can invade and establish a chronic symbiotic infection within the host plant Medicago sativa (alfalfa) (6, 13). Numerous symbiotically deficient mutants of S. meliloti that are affected in a wide array of biochemical pathways, from intermediary metabolism to cell envelope synthesis, have been identified previously (22, 31). The characterization of these mutants has provided insights into bacterial functions necessary for each developmental stage of symbiosis. For example, mutations that disrupt exopolysaccharide production by S. meliloti strain Rm1021 result in early developmental defects characterized by aborted infection threads and the absence of bacteria in plant nodules (17, 22, 27). Other mutations, including some that alter lipopolysaccharide (LPS) synthesis, do not affect the early development of symbiosis but instead result in severe defects in later stages (7, 22). However, despite the many genetic studies that have been carried out, we still do not have a clear picture of the full genomic complement required by S. meliloti to successfully complete each developmental stage of symbiosis.
A screening strategy we developed previously for identifying symbiotically deficient mutants (11) led to the discovery that SMc01113, which encodes a protein of unknown function, is essential for symbiosis. The SMc01113 protein is highly conserved, being present in all bacteria, and its function is critically required for S. meliloti both to establish the chronic intracellular infection necessary for symbiosis and to defend against a wide range of environmental stresses.
An SMc01113::mTn5 mutant is severely defective in symbiosis with alfalfa.
We previously described a two-part screening strategy that we used to identify a number of mTn5 mutants of S. meliloti strain Rm1021 that were both sensitive to H2O2 and defective in symbiosis with alfalfa (11). The continuation of that screen identified an additional mutant disrupted in the hypothetical open reading frame SMc01113.
The SMc01113 mutant we identified is disrupted by an mTn5 transposon inserted at base 284 of the 507-bp SMc01113 open reading frame. Using the previously described methods in our screening strategy (11), we determined the H2O2 sensitivity and symbiotic defect of the original isolate and then transduced the SMc01113::mTn5 allele into the parental strain, Rm1021. We tested several transductants and confirmed that both the H2O2 sensitivity and the symbiotic defect were linked to the mTn5 insertion in SMc01113. We selected one transductant, GWBD12, for further investigation (Table 1). We will hereafter refer to strain GWBD12 as the SMc01113::mTn5 mutant.
TABLE 1.
Bacterial strains, phages, and plasmids
| Strain, phage, or plasmid | Relevant characteristics | Source (reference) |
|---|---|---|
| Strains | ||
| MT616 | E. coli MM294(pRK600); Cmr | T. Finan |
| DH5α | E. coli endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argG)U169 deoR | BRL Corp. |
| DH5α λpir | E. coli endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argG)U169 deoR oriR6K | BRL Corp. |
| Rm1021 | SU47 Smr | F. Ausubel |
| SMc01113 | Rm1021 SMc01113::mTn5; original isolate | This study |
| GWBD12 | Rm1021 SMc01113::mTn5; transductant | This study |
| GWBD5 | Rm1021(pMSO4) | B. Davies (10) |
| GWBD13 | SMc01113::mTn5(pMSO4) | This study |
| GWBD14 | SMc01113::mTn5(pGW2) | This study |
| Phages (Φ) | ||
| M1 | G. Campbell | |
| M5 | G. Campbell | |
| M6 | G. Campbell | |
| M7 | G. Campbell | |
| M9 | G. Campbell | |
| M10 | G. Campbell | |
| M11 | G. Campbell | |
| M12 | Generalized transducing phage | T. Finan |
| Plasmids | ||
| pCRS487 | pUT::mTn5-GNm; Apr Kms | W. Reeve (32) |
| pMSO4 | Spr | M. Barnett (2) |
| pGW2 | SMc01113 complementation plasmid | This study |
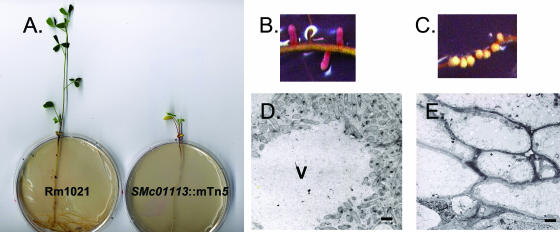
The SMc01113::mTn5 mutant exhibited a striking symbiotic defect on the plant host alfalfa. To quantify the symbiotic defect of the SMc01113::mTn5 mutant, we inoculated the SMc01113::mTn5 mutant and its Rm1021 parent onto alfalfa seedlings (24). After 4 weeks, we assessed plant height, nodule type, and the ability to fix nitrogen as measured by acetylene reduction (Table 2) (40). Rm1021-inoculated plants were on average 80% taller than the SMc01113::mTn5 mutant-inoculated plants and were a healthy green color, in contrast to the unhealthy yellow color of plants inoculated with the SMc01113::mTn5 mutant (Fig. 1A; Table 2). Nodules from Rm1021-inoculated plants were mostly pink (Table 2; Fig. 1B), a color due to leghemoglobin, which is a marker of successful symbiosis (1). In contrast, SMc01113::mTn5 mutant-inoculated plants produced only small white nodules (Table 2; Fig. 1C) indicative of failed symbiosis. Consistent with both of these observations, Rm1021-inoculated plants showed much higher levels of nitrogen fixation than those inoculated with the SMc01113::mTn5 mutant (Table 2).
TABLE 2.
Plant heights, nodule types, and nitrogenase activities for alfalfa inoculated with Rm1021 and derivative strains after 4 weeks of growtha
| Strain | Plant height (cm) | % Of pink nodules | % Of white nodules | Acetylene reduction (nmol/ nodule/h) |
|---|---|---|---|---|
| Rm1021 | 10.2 ± 1.0 | 93.6 ± 8.3 | 6.4 ± 8.3 | 19.5 ± 0.4 |
| SMc01113::mTn5 mutant | 2.2 ± 1.0 | 0 | 100 | 1.1 ± 1.1 |
| Rm1021(pMSO4) | 10.8 ± 2.0 | 93.4 ± 8.2 | 6.6 ± 7.5 | NA |
| SMc01113::mTn5(pMSO4) | 2.6 ± 0.6 | 0 | 100 | NA |
| SMc01113::mTn5(pGW2) | 11.1 ± 2.2 | 91.1 ± 9.9 | 11.1 ± 2.2 | NA |
Six plants were used for each strain. Values are means ± standard deviations. NA, not available.
FIG. 1.
Nodule morphology and ultrastructure of alfalfa inoculated with Rm1021 and the SMc01113::mTn5 mutant. (A) Plants inoculated with either Rm1021 (left) or the SMc01113::mTn5 mutant (right) after 4 weeks growth. (B) Pink nodules induced by Rm1021. (C) Small white nodules induced by the SMc01113::mTn5 strain. (D) Ultrastructure of a pink nodule induced by Rm1021 (bar, 1.0 μM). (E) Ultrastructure of a small white nodule induced by the SMc01113::mTn5 mutant. Bar = 1.0 μm Plant vacuoles (V) are indicated.
We PCR amplified SMc01113 (primers: forward, AGACTGCAGGAGGATAGGCGGCCGCGTTTATCATGAC, and reverse, CGGCTGGATCCTGAAGTCGCTCATCG; PstI and BamH1 restriction sites, respectively, are in bold) and cloned the digested product directly into the expression vector pMSO4 (2) by using standard cloning techniques (35). The resulting plasmid, pGW2, was mated into the SMc01113::mTn5 mutant as described previously (11). The symbiotic defect of the SMc01113::mTn5 mutant was fully eliminated by the ectopic expression of SMc01113 from pGW2 (Table 2).
To gain a better understanding of the nature of the symbiotic deficiency, we examined the ultrastructure of the white nodules produced by plants inoculated with the SMc01113::mTn5 mutant by using previously described methods (19, 20). Nodule cells from plants inoculated with Rm1021 were full of bacteroids (Fig. 1D). In striking contrast, nodule cells from plants inoculated with the SMc01113::mTn5 mutant were completely devoid of bacteroids (Fig. 1E). The absence of bacteroids explains the extremely low acetylene reduction capacity of SMc01113::mTn5 mutant-inoculated plants (Table 2). In addition, plant cells of SMc01113::mTn5 mutant-induced nodules were misshapen and lacked observable vacuoles, in contrast to those of Rm1021-induced nodules (Fig. 1, compare panels D and E).
The small white nodules produced by plants inoculated with the SMc01113::mTn5 mutant were very similar to nodules induced by Rm1021 strains defective in exopolysaccharide production, both in gross morphology and in ultrastructure (23, 24). This similarity led us to test the SMc01113::mTn5 mutant for an alteration in exopolysaccharide production; however, we found no change in exopolysaccharide (succinoglycan) production by the SMc01113::mTn5 mutant as measured by calcofluor binding assays (data not shown) (23). Nonetheless, the absence of bacteroids in nodules from plants inoculated with the SMc01113::mTn5 mutant or exopolysaccharide mutants contrasted strikingly with the abundance of bacteroids in nodules induced by Rm1021 LPS mutants, which are proficient in exopolysaccharide synthesis. While LPS mutants are defective in symbiosis, nodules from plants inoculated with LPS mutants are filled with bacteroids (7). This finding suggests that the symbiotic defect of the SMc01113::mTn5 mutant resembles that of exopolysaccharide-deficient mutants in that the nodule invasion process is aborted before the release of invading bacteria into the cells of the developing nodule.
The SMc01113 protein is a member of a highly conserved protein family.
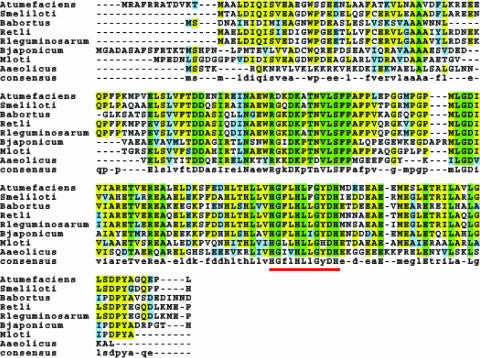
The SMc01113 gene codes for a hypothetical, highly conserved protein of unknown function (14). The protein family that includes the SMc01113 protein is designated COG0319 (37, 38). Homologs of the SMc01113 protein are found in all sequenced bacteria and are predicted to be putative metal-dependent hydrolases (37, 38). The function prediction is based on a conserved motif, H(X)3H(X)4DH (Fig. 2), that bears a resemblance to sequences in certain eukaryotic metal-dependent proteases (E. V. Koonin, personal communication). Homologs are strongly conserved throughout the alphaproteobacteria (Fig. 2). The crystal structure of the homolog from “Aquifex aeolicus” was recently resolved and revealed that the spatial arrangement of three conserved histidines may allow them to bind a metal ion (28). The same authors of the crystal structure study tested the purified protein for more than 15 different general biochemical activities but obtained only negative results in all assays (28). These findings suggest that the SMc01113 protein may have a unique or unusual substrate rather than having a generalized hydrolytic function active against a variety of substrates. The present study is the first to offer insight into the biological role(s) of this universally conserved bacterial protein.
FIG. 2.
SMc01113 protein homologs were aligned using T-Coffee (30). The red bar underlines the conserved motif used to classify this protein family. The corresponding gene names are as follows: Agrobacterium tumefaciens, Atu0358; S. meliloti, SMc01113; Brucella abortus, BruAb1_2129; Rhizobium etli, RHE_CH00374; Rhizobium leguminosarum, RL0393; Bradyrhizobium japonicum, b110793; Mesorhizobium loti, mlr5536; and “Aquifex aeolicus,” Aq1354.
The SMc01113::mTn5 mutant is sensitive to agents targeting key biological processes.
Previous computational and biochemical analyses failed to elucidate a function for the SMc01113 protein family (28, 37). However, as described below, when we tested the SMc01113::mTn5 mutant for altered sensitivity to several different stresses, we found that the strain was sensitive to a remarkably wide spectrum of environmental stresses targeting several key cellular processes and structures. These processes included oxidative stress protection, DNA repair, cell wall synthesis, protein synthesis, and the maintenance of cell envelope stability (Tables 3 and 4; Fig. 3). We tested stress sensitivity by zone of inhibition (ZI) and efficiency of plating (EOP) assays as previously described (10, 11). All strains were grown to saturation (optical density at 600 nm of ∼6) at 30°C in Luria-Bertani broth supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2 (LB/MC) prior to assay. Rm1021 and the SMc01113::mTn5 mutant showed equivalent levels of viability in the EOP assay under nonstress conditions (data not shown). All SMc01113::mTn5 mutant sensitivities were fully eliminated by the ectopic expression of SMc01113 from pGW2 (data not shown).
TABLE 3.
ZI results for Rm1021 and SMc0113::mTn5 mutant strains screened against several environmental stresses
| Stress | ZI (cm) of:
|
|
|---|---|---|
| Rm1021 | SMc01113::mTn5 | |
| H2O2 | 3.7 ± 0.1 | 4.3 ± 0.1 |
| Menadione | 3.2 ± 0.1 | 3.2 ± 0.1 |
| MMS | 4.4 ± 0.1 | 5.0 ± 0.1 |
| Nalidixic acid | 5.4 ± 0.1 | 6.3 ± 0.1 |
| Tetracycline | 6.0 ± 0.1 | 6.6 ± 0.1 |
| Chloramphenicol | 5.5 ± 0.1 | 6.1 ± 0.1 |
| Crystal violet | 3.6 ± 0.1 | 4.2 ± 0.1 |
| Concentrated hydrochloric acid | 4.2 ± 0.1 | 4.2 ± 0.2 |
| Ampicillin | 5.1 ± 0.1 | 7.7 ± 0.1 |
| Cefotaxime | 4.1 ± 0.1 | 6.8 ± 0.1 |
TABLE 4.
Mutant phenotypes of SMc01113::mTn5 strains as determined by phenotype microarray analysis
| Testa | Difference from control strainb |
|---|---|
| Spectinomycin | −105 |
| Hygromycin B | −101 |
| Spiramycin | −103 |
| Tylosin | −125 |
Chemicals were tested in 96-well phenotype microarrays.
The OmniLog-Pm software generates time course curves for respiration (indicated by tetrazolium color formation) and calculates differences in the areas for mutant and control cells. The units are arbitrary. Negative values indicate that the control showed greater rates of respiration than the mutant. The differences are averages of values reported for two or more replicates of the mutant compared with the values for the corresponding control strain.
FIG. 3.
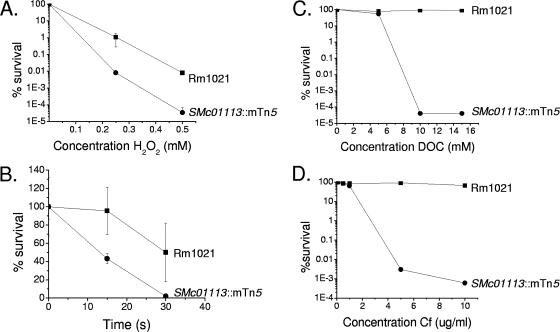
Results from EOP assays. Strains were spotted onto LB agar containing increasing amounts of H2O2 (A), DOC (C), and cefotaxime (Cf) (D). UV treatment of strains (B) was performed as described in the text. The percent survival reflects the number of CFU of the respective strain under stressed conditions relative to the number under nonstressed conditions.
(i) Oxidative stress.
We initially measured the sensitivity of the SMc01113::mTn5 mutant to H2O2 by a ZI assay, in which we observed a 16% increase of the ZI of the mutant relative to that of the parental strain Rm1021 (Table 3). To better quantify the difference in H2O2 sensitivity, we determined the EOP of each strain. We found that the SMc01113::mTn5 mutant was substantially more sensitive than Rm1021 over a range of H2O2 concentrations (Fig. 3A). We also tested the SMc01113::mTn5 mutant for sensitivity to the superoxide generator menadione (18) by a ZI assay. The SMc01113::mTn5 mutant did not show altered sensitivity to menadione (Table 3). This result suggests that the oxidative stress defect of the mutant is specific to H2O2-induced stress.
(ii) DNA metabolism.
Since H2O2 causes DNA damage (21), we tested the sensitivity of the SMc01113::mTn5 mutant to other agents that damage DNA: methyl methanesulfonate (MMS), UV radiation (UV), and nalidixic acid. We tested MMS and nalidixic acid sensitivities by ZI assays, while UV sensitivity was determined by serially diluting cultures on agar plates and irradiating them at 25 J/m2 for the times indicated in Fig. 3B and then determining the numbers of CFU as for EOP assays. MMS produces a variety of DNA lesions, including N3-methyladenine lesions, a lethal form that inhibits DNA synthesis and needs to be actively repaired (3, 5, 29). UV induces a variety of photoproducts that interfere with DNA replication (34). Nalidixic acid targets DNA gyrase, whose role is to overcome topological problems encountered during DNA replication (39). Strikingly, we found that the SMc01113::mTn5 mutant displayed increased sensitivity to all of these agents (Table 3; Fig. 3B). These results indicate that the SMc01113::mTn5 mutant has a general problem in dealing with DNA damage or DNA replication issues rather than a defect in a specific DNA repair process.
(iii) Protein synthesis.
Our recognition that the SMc01113::mTn5 mutant had a pleiotropic phenotype then led us to test the sensitivity of the strain to agents that inhibit protein synthesis. We found that the SMc01113::mTn5 mutant exhibited increased sensitivity to both tetracycline and chloramphenicol (Table 3). Both antibiotics inhibit translation; however, tetracycline does so by blocking the binding of the incoming aminoacylated tRNA to the A site (26, 33), while chloramphenicol inhibits peptide bond formation (9).
In addition, we used the phenotype microarray system to simultaneously test the mutant strain for further phenotypes (4). Using this technology, we found that the SMc01113::mTn5 mutant was also sensitive to the aminocyclitol spectinomycin, the aminoglycoside hygromycin B, and macrolides spiramycin and tylosin (Table 4). As each of these antibiotics affects ribosome activity in a different way, these results suggest a possible defect in ribosome structure or a general impairment of translation that increases the sensitivity of the mutant to all types of ribosome-directed antibiotics.
(iv) Cell envelope integrity.
The maintenance of cell envelope integrity is crucial for Rm1021 to develop normal symbiosis (7, 8, 12). We tested the integrity of the SMc01113::mTn5 mutant cell envelope with crystal violet and the detergent deoxycholate (DOC). Altered sensitivity to detergents is usually an indicator of a change in the bacterial cell envelope, and the hydrophobic dye crystal violet is frequently used as an indicator of alterations in the cell envelope such as those caused by changes in the LPS (12). The SMc01113::mTn5 mutant showed increased sensitivity to crystal violet when tested by a ZI assay (Table 3). The SMc01113::mTn5 mutant was also very sensitive to DOC as determined by an EOP assay (Fig. 3C). The increased sensitivity to both these agents strongly suggests that the SMc01113::mTn5 mutant has a cell envelope defect in addition to the other defects described above.
LPS constitutes the outer leaflet of the outer membrane of gram-negative bacteria. S. meliloti mutants with alterations in their LPS layer not only exhibit sensitivity to detergents but also show alteration in phage sensitivity and often have symbiotic defects (8). The striking sensitivity of the SMc01113::mTn5 mutant to DOC and crystal violet, along with its severe symbiotic defect, had us question whether the LPS layer in the mutant was drastically altered. We tested the mutant and parental strains against a panel of phage but found that both strains showed the same pattern of sensitivity and resistance (Table 5). We also found that the SMc01113::mTn5 mutant was not sensitive to low pH (Table 3), another indicator of LPS alterations (12). This result suggests that a gross LPS alteration is not the cause of the cell envelope instability of the SMc01113::mTn5 mutant.
TABLE 5.
Phage sensitivities of Rm1021 and SMc01113::mTn5 strains
| Strain | Sensitivitya to phage:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ΦM1 | ΦM5 | ΦM6 | ΦM7 | ΦM9 | ΦM10 | ΦM11 | ΦM12 | |
| Rm1021 | S | S | S | S | S | R | R | S |
| SMc01113:: mTn5 mutant | S | S | S | S | S | R | R | S |
R, resistant; S, sensitive.
(v) Peptidoglycan synthesis.
The severe sensitivity of the SMc01113::mTn5 mutant to agents that affect outer cell envelope integrity led us to question whether additional components of the cell envelope may also be compromised. We tested the SMc01113::mTn5 mutant against two inhibitors of peptidoglycan synthesis, ampicillin and cefotaxime. Both ampicillin and cefotaxime inhibit the transpeptidase reaction required to cross-link glycan-linked peptide chains to form the mature peptidoglycan layer (25). The SMc01113::mTn5 mutant was severely sensitive to both cell wall inhibitors (Table 3). To better quantify this sensitivity, we performed an EOP assay to measure cefotaxime sensitivity and found a 105-fold increase in sensitivity at high doses compared to that of the parental strain (Fig. 3D). Since the peptidoglycan layer connects the inner and outer membranes, a weakened cell wall may perturb the outer membrane, leading to increased sensitivity to other cell envelope-destabilizing agents, such as DOC and crystal violet.
In addition to the wide range of sensitivities, we found that the SMc01113::mTn5 mutant had a modest growth defect in LB/MC (doubling time of Rm1021 [mean ± standard deviation], 3.0 ± 0.1 h, versus the doubling time of the SMc01113::mTn5 mutant, 4.4 ± 0.3 h). We do not feel that this growth rate defect was a major cause of the increased sensitivity of the SMc01113::mTn5 mutant to the wide range of stresses. For each stress tested by the ZI assay, we also tested at least one similar stress by the EOP assay and showed increased sensitivity of the SMc01113::mTn5 mutant in both assays. For example, the SMc01113::mTn5 mutant displayed increased cefotaxime sensitivity in both ZI and EOP assays (Table 2; Fig. 3D).
The S. meliloti SMc01113 gene is a member of a highly conserved family that is found in every bacterium whose genome has been sequenced and is said to be one of the 206 genes that are required for definition as a bacterium (15). This physiological study offers the first insights into the biological function of this gene family in any bacterium. We were struck by the wide spectrum of stresses to which the SMc01113::mTn5 mutant was sensitive. These results would seem to imply that the SMc01113 protein either plays numerous independent biological roles or instead affects a fundamental cellular function that involves a wide range of processes. Considering the striking diversity of the chemical structures and modes of action of agents to which the SMc01113::mTn5 mutant was sensitive, we consider it more likely that the SMc01113 protein affects one central biological function rather than playing an active role in many different stress responses.
In addition to identifying phenotypes of the SMc01113::mTn5 mutant, we used computational analysis to identify gene neighbors of SMc01113 and its homologs throughout the bacterial domain (36, 41). The repeated occurrence of genes in the same neighborhood in genomes has been shown to indicate a functional association between the proteins these genes encode (36). Our analyses showed that the SMc01113 gene and its homologs were always present near genes that function in RNA and phospholipid metabolism. A defect in either RNA or phospholipid metabolism may explain why the SMc01113::mTn5 mutant is sensitive to so many agents or treatments. It is also interesting that the neighboring gene SMc01111 (lnt) is required for acid tolerance and the neighboring gene SMc01110 (phrR) is a transcriptional regulator that is inducible by low pH (16). While we did not observe increased sensitivity to acid in the SMc01113::mTn5 mutant (Table 3), it is still possible that SMc01113 is involved in pH tolerance in S. meliloti, perhaps in planta if not in the free-living state.
While its biochemical function remains elusive, our results clearly show that SMc01113 is absolutely required for Rm1021 to establish an intracellular infection of alfalfa (Fig. 1). Since homologs of SMc01113 are found in every sequenced bacterium, this gene cannot have evolved solely for function in rhizobial symbiosis. Nevertheless, in the many nodulation assays we have carried out with the SMc01113::mTn5 mutant, we have never observed the formation of a pink nodule (data not shown). This result suggests that the function of the SMc01113 gene cannot be compensated for by any other gene or pathway in S. meliloti during symbiosis. Serious effort will be required to identify the functional defect of such a pleiotropic mutant. Future work to identify the specific pathway in which the SMc01113 protein functions will not only increase our understanding of symbiosis but will also expand our knowledge of the role this novel protein plays in all bacteria.
Acknowledgments
This work was supported by National Institutes of Health grant GM31010 to G.C.W. and a National Sciences and Engineering Research Council of Canada graduate scholarship to B.W.D. G.C.W. is an American Cancer Society Research Professor.
Footnotes
Published ahead of print on 30 November 2007.
REFERENCES
- 1.Appleby, C. A. 1974. Biological nitrogen fixation. North Holland Publishing Co., Amsterdam, The Netherlands.
- 2.Barnett, M. J., V. Oke, and S. R. Long. 2000. New genetic tools for use in the Rhizobiaceae and other bacteria. BioTechniques 29240-242, 244-245. [DOI] [PubMed] [Google Scholar]
- 3.Beranek, D. T. 1990. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. 23111-30. [DOI] [PubMed] [Google Scholar]
- 4.Bochner, B. R., P. Gadzinski, and E. Panomitros. 2001. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 111246-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boiteux, S., O. Huisman, and J. Laval. 1984. 3-Methyladenine residues in DNA induce the SOS function sfiA in Escherichia coli. EMBO J. 32569-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewin, N. J. 1991. Development of the legume root nodule. Annu. Rev. Cell Biol. 7191-226. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, G. R., B. L. Reuhs, and G. C. Walker. 2002. Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core. Proc. Natl. Acad. Sci. USA 993938-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, G. R., L. A. Sharypova, H. Scheidle, K. M. Jones, K. Niehaus, A. Becker, and G. C. Walker. 2003. Striking complexity of lipopolysaccharide defects in a collection of Sinorhizobium meliloti mutants. J. Bacteriol. 1853853-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutsogeorgopoulos, C. 1966. On the mechanism of action of chloramphenicol in protein synthesis. Biochim. Biophys. Acta 129214-217. [DOI] [PubMed] [Google Scholar]
- 10.Davies, B. W., and G. C. Walker. 2007. Disruption of sitA compromises Sinorhizobium meliloti for manganese uptake required for protection against oxidative stress. J. Bacteriol. 1892101-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, B. W., and G. C. Walker. 2007. Identification of novel Sinorhizobium meliloti mutants compromised for oxidative stress protection and symbiosis. J. Bacteriol. 1892110-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson, G. P., R. M. Roop II, and G. C. Walker. 2002. Deficiency of a Sinorhizobium meliloti BacA mutant in alfalfa symbiosis correlates with alteration of the cell envelope. J. Bacteriol. 1845625-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, R. F., and S. R. Long. 1992. Rhizobium-plant signal exchange. Nature 357655-660. [DOI] [PubMed] [Google Scholar]
- 14.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293668-672. [DOI] [PubMed] [Google Scholar]
- 15.Gil, R., F. J. Silva, J. Pereto, and A. Moya. 2004. Determination of the core of a minimal bacterial gene set. Microbiol. Mol. Biol. Rev. 68518-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glenn, A. R., W. G. Reeve, R. P. Tiwari, and M. J. Dilworth. 1999. Acid tolerance in the root nodule bacteria. Novartis Found. Symp. 221112-126. [PubMed] [Google Scholar]
- 17.Gonzalez, J. E., G. M. York, and G. C. Walker. 1996. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene 179141-146. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell, B., and J. M. C. Gutteridge. 1999. Free radicals in biology and medicine, 3rd ed. Oxford University Press, Oxford, United Kingdom.
- 19.Hirsch, A. M., M. Bang, and F. M. Ausubel. 1983. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J. Bacteriol. 155367-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch, A. M., S. R. Long, M. Bang, N. Haskins, and F. M. Ausubel. 1982. Structural studies of alfalfa roots infected with nodulation mutants of Rhizobium meliloti. J. Bacteriol. 151411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imlay, J. A., and S. Linn. 1987. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J. Bacteriol. 1692967-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, K. M., H. Kobayashi, B. W. Davies, M. E. Taga, and G. C. Walker. 2007. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat. Rev. Microbiol. 5619-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leigh, J. A., J. W. Reed, J. F. Hanks, A. M. Hirsch, and G. C. Walker. 1987. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 51579-587. [DOI] [PubMed] [Google Scholar]
- 24.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 826231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madigan, M. T., J. M. Martinko, and J. P. Parker. 1997. Biology of microorganisms, 8th ed. Prentice Hall, Upper Saddle River, NJ.
- 26.Maxwell, I. H. 1967. Partial removal of bound transfer RNA from polysomes engaged in protein synthesis in vitro after addition of tetracycline. Biochim. Biophys. Acta 138337-346. [DOI] [PubMed] [Google Scholar]
- 27.Niehaus, K., and A. Becker. 1998. The role of microbial surface polysaccharides in the Rhizobium-legume interaction. Subcell. Biochem. 2973-116. [DOI] [PubMed] [Google Scholar]
- 28.Oganesyan, V., D. Busso, J. Brandsen, S. Chen, J. Jancarik, R. Kim, and S. H. Kim. 2003. Structure of the hypothetical protein AQ_1354 from Aquifex aeolicus. Acta Crystallogr. D 591219-1223. [DOI] [PubMed] [Google Scholar]
- 29.Pegg, A. E. 1984. Methylation of the O6 position of guanine in DNA is the most likely initiating event in carcinogenesis by methylating agents. Cancer Investig. 2223-231. [DOI] [PubMed] [Google Scholar]
- 30.Poirot, O., E. O'Toole, and C. Notredame. 2003. Tcoffee@igs: a web server for computing, evaluating and combining multiple sequence alignments. Nucleic Acids Res. 313503-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prell, J., and P. Poole. 2006. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 14161-168. [DOI] [PubMed] [Google Scholar]
- 32.Reeve, W. G., R. P. Tiwari, P. S. Worsley, M. J. Dilworth, A. R. Glenn, and J. G. Howieson. 1999. Constructs for insertional mutagenesis, transcriptional signal localization and gene regulation studies in root nodule and other bacteria. Microbiology 1451307-1316. [DOI] [PubMed] [Google Scholar]
- 33.Ross, J. I., E. A. Eady, J. H. Cove, and W. J. Cunliffe. 1998. 16S rRNA mutation associated with tetracycline resistance in a gram-positive bacterium. Antimicrob. Agents Chemother. 421702-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rupp, W. D., and P. Howard-Flanders. 1968. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J. Mol. Biol. 31291-304. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Snel, B., G. Lehmann, P. Bork, and M. A. Huynen. 2000. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 283442-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatusov, R. L., N. D. Fedorova, J. D. Jackson, A. R. Jacobs, B. Kiryutin, E. V. Koonin, D. M. Krylov, R. Mazumder, S. L. Mekhedov, A. N. Nikolskaya, B. S. Rao, S. Smirnov, A. V. Sverdlov, S. Vasudevan, Y. I. Wolf, J. J. Yin, and D. A. Natale. 2003. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatusov, R. L., D. A. Natale, I. V. Garkavtsev, T. A. Tatusova, U. T. Shankavaram, B. S. Rao, B. Kiryutin, M. Y. Galperin, N. D. Fedorova, and E. V. Koonin. 2001. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2922-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tse-Dinh, Y. C. 2007. Exploring DNA topoisomerases as targets of novel therapeutic agents in the treatment of infectious diseases. Infect. Disord. Drug Targets 73-9. [DOI] [PubMed] [Google Scholar]
- 40.Turner, G. L., and A. H. Gibson. 1980. Measurement of nitrogen fixation by indirect means. John Wiley and Sons, Chichester, United Kingdom.
- 41.von Mering, C., L. J. Jensen, M. Kuhn, S. Chaffron, T. Doerks, B. Kruger, B. Snel, and P. Bork. 2007. STRING 7: recent developments in the integration and prediction of protein interactions. Nucleic Acids Res. 35D358-D362. [DOI] [PMC free article] [PubMed] [Google Scholar]