Abstract
The yjjQ and bglJ genes encode LuxR-type transcription factors conserved in several enterobacterial species. YjjQ is a potential virulence factor in avian pathogenic Escherichia coli. BglJ counteracts the silencing of the bgl (β-glucoside) operon by H-NS in E. coli K-12. Here we show that yjjQ and bglJ form an operon carried by E. coli K-12, whose expression is repressed by the histone-like nucleoid structuring (H-NS) protein. The LysR-type transcription factor LeuO counteracts this repression. Furthermore, the yjjP gene, encoding a membrane protein of unknown function and located upstream in divergent orientation to the yjjQ-bglJ operon, is likewise repressed by H-NS. Mapping of the promoters as well as the H-NS and LeuO binding sites within the 555-bp intergenic region revealed that H-NS binds to the center of the AT-rich regulatory region and distal to the divergent promoters. LeuO sites map to the center and to positions distal to the yjjQ promoters, while one LeuO binding site overlaps with the divergent yjjP promoter. This latter LeuO site is required for full derepression of the yjjQ promoters. The arrangement of regulatory sites suggests that LeuO restructures the nucleoprotein complex formed by H-NS. Furthermore, the data support the conclusion that LeuO, whose expression is likewise repressed by H-NS and which is a virulence factor in Salmonella enterica, is a master regulator that among other loci, also controls the yjjQ-bglJ operon and thus indirectly the presumptive targets of YjjQ and BglJ.
The enterobacterial histone-like nucleoid structuring (H-NS) protein is an abundant global repressor that affects many genes and cellular processes (16, 18, 45). H-NS controls genes related to pathogenicity and stress responses, and H-NS represses genes acquired by horizontal gene transfer, including pathogenicity islands (24, 25, 36, 46, 48). H-NS binds to AT-rich DNA with weak specificity and forms extended nucleoprotein complexes that when located close to a promoter, repress transcription (16, 51). The formation of the repressing nucleoprotein complex is initiated by H-NS dimers binding to nucleation sites, followed by their extending laterally along the DNA, a process that has been best characterized for the Escherichia coli proU operon (2, 3, 28, 37). The formation of the nucleoprotein complex can involve DNA loop formation and trapping of RNA polymerase at the promoter (9, 10, 57). DNA loop formation is likely to be based on the formation of DNA-H-NS-DNA bridges by the binding of single H-NS dimers to two DNA double helixes within the nucleoprotein complex (10, 17, 38).
Silencing by H-NS is presumably regulated gene specifically, although physiological parameters such as temperature, osmolarity, and DNA supercoiling have been postulated to pleiotropically modulate H-NS activity (45). Silencing can be relieved by the binding of specific transcription factors that disrupt or change the structure of the repressing nucleoprotein complex, by the temperature-dependent alteration of the DNA structure, and by other changes in the physiological conditions that may change DNA structure and DNA supercoiling at specific loci (16, 45, 55). Furthermore, repression by the binding of H-NS within transcription units can be affected by the promoter activity (44).
LeuO is a LysR-type regulator which is a virulence factor in Salmonella enterica and is required for biofilm formation by Vibrio cholerae (29, 42, 58). In Salmonella enterica and in E. coli, LeuO is one of the transcription factors that counteracts H-NS-mediated repression of specific loci (4, 22, 39, 60). First, the leuO gene itself is repressed by H-NS and is positively autoregulated (5, 6, 26). Relief of repression of the leuO gene, which is located upstream and in a divergent orientation to the leuABCD leucine synthesis operon, is complex. Binding of LeuO presumably delimits the spread of H-NS into the promoter and thus causes activation (4, 6, 7). In addition, the expression of leuO is coupled to the expression of the ilvIH operon located downstream of leuO. Transcriptional coupling presumably involves LeuO-induced DNA looping by LeuO binding to sites upstream of the leuO promoter and downstream of the leuO coding region (7), as well as a transcription-induced change in DNA topology (20, 21). Other systems in which LeuO activates expression include the H-NS-repressed E. coli bgl (aryl-β,d-glucoside) operon (60), the H-NS-repressed Salmonella enterica serovar Typhi ompS1 gene, and the ompS2 gene encoding outer-membrane proteins and pathogenicity determinants (12, 22, 52). Furthermore, LeuO positively regulates the Yersinia enterocolitica rovA gene, encoding a MarR-type transcriptional regulator of the inv gene, which encodes the major adherence factor invasin (30). LeuO also represses the cadC and dsrA genes in E. coli. CadC is a positive regulator of the H-NS-repressed cadBA locus, encoding an acid-inducible lysine decarboxylase required in the acid stress response (56). DsrA is a small regulatory RNA that regulates rpoS and hns translation at a low temperature (26, 31, 32, 50). Thus, LeuO, which counteracts the H-NS-mediated repression of several loci, indirectly controls H-NS and RpoS synthesis under specific conditions.
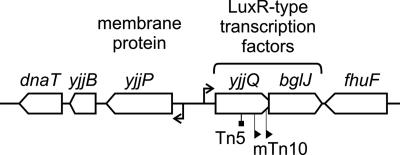
Here we analyzed the regulation of the yjjP-yjjQ-bglJ locus (Fig. 1), which is present in the enterobacterial species E. coli (including the Shigella spp.) and S. enterica. The yjjQ and bglJ genes, encoding LuxR-type transcription factors, are arranged in tandem with overlapping open reading frames and may form an operon. The yjjP gene, which encodes a membrane protein of unknown function, is mapped to a position upstream of the yjjQ gene and in a divergent orientation. (8). In avian pathogenic E. coli (APEC), the virulence of a mutant with a Tn5 insertion that disrupted yjjQ was found to be attenuated (33). Furthermore, the yjjQ mutants were negatively selected in one of two independent screens for long-term systemic infection by Salmonella enterica serovar Typhimurium in mouse (29). In E. coli K-12, mini-Tn10 insertions that cause constitutive expression of bglJ relieve H-NS-mediated repression of the bgl operon (23). Here we show that yjjQ and bglJ are expressed as an operon, which is repressed by H-NS and activated by LeuO. The divergent yjjP gene is likewise repressed by H-NS. Mapping of the divergent promoters and regulatory sites revealed that H-NS binds at the center of the extended intergenic region and distal to the divergent promoters. Similarly, LeuO binds to three regions, including a site at the center, a site distal to the yjjQ promoters, and with the highest affinity, at a site overlapping the yjjP promoter. This last site is required for the complete activation of the yjjQ promoter(s) by LeuO. The complex arrangement of divergent promoters and binding sites for H-NS and LeuO is in agreement with a mechanism of regulation where binding of LeuO and possible LeuO-induced DNA looping restructure the H-NS nucleoprotein complex and delimit spreading of H-NS to the yjjQ promoter(s).
FIG. 1.
Organization of the yjjP-yjjQ-bglJ locus mapping at 99 min of the E. coli K-12 genome in between yjjB (encoding a conserved inner membrane protein) and fhuF (encoding a ferric iron reductase protein). The yjjQ and bglJ genes encode LuxR-type transcription factors. A yjjQ::Tn5 insertion mutation attenuates the virulence of APEC (33), while mini-Tn10 insertions upstream of bglJ, causing the constitutive expression of bglJ, relieve the silencing of the bgl operon by H-NS in E. coli K-12 (23, 39). The yjjP gene encodes a membrane protein of unknown function (8).
MATERIALS AND METHODS
Strains and plasmids.
The genotypes of the E. coli strains and the relevant structures of the plasmids are given in Table 1. Transductions were performed using phage T4GT7 (61). Integration of the lacZ reporter constructs into the chromosomal phage lambda attachment site attB was performed as described previously (13, 14). The attB site was used as described previously for the integration of similar lacZ reporter fusions used in the analysis of the H-NS-mediated regulation of bgl and proU (44), and chromatin immunoprecipitation with microarray analysis (ChIP-on-chip) analysis suggested that H-NS does not bind next to attB (24). The Δhns::kanKD4 deletion mutants, in which the complete coding region of hns was deleted, were described previously (44). The Δ(yjjP-yjjQ-bglJ)::catKD3 deletion mutant was constructed by using primers S783 and S676 as described previously (11). The sequences of oligonucleotides are given in Table S2 in the supplemental material.
TABLE 1.
Strains and plasmids
| Strain | Relevant genotype or structurea | Reference or construction sourceb |
|---|---|---|
| EK25 | MC4100 leuO::cat (stored as S2706) | 26 |
| MG1655 | CGSC6300 E. coli K-12 wild type (stored as S527) | Coli Genetic Stock Center |
| S219 | CSH50 Δbgl-AC11 stpA::tet | 15 |
| S541 | CSH50 Δbgl-AC11 ΔlacZ | 14 |
| S3010 | S541 Δhns::kanKD4 | 44 |
| S3346 | S541 Δhns::FRT | 44 |
| S3108 | S541 attB::[SpecryjjP(+182 to −450) lacZ] | S541 × pKES141 |
| S3322 | S541 attB::[SpecryjjP(+182 to −450) lacZ] Δ(yjjP-bglJ)::catKD3 | S3108/pKD46 × pKD3 PCR S676/S783c |
| S3375 | S541 Δ(yjjP-bglJ)::catKD3 | S541/pKD46 × pKD3 PCR S676/S783c |
| S3469 | S541 Δ(yjjP-bglJ)::FRT | S3375 × pCP20 flp |
| S3470 | S541 attB::[SpecryjjP(+182 to −450) lacZ] Δ(yjjP-bglJ)::FRT | S3322 × pCP20 flp |
| S3472 | S3469 attB::[SpecryjjQ(−347 to +166) lacZ] | S3469 × pKES111 |
| S3474 | S3469 attB::[SpecryjjQ(−147 to +166) lacZ] | S3469 × pKES119 |
| S3476 | S3469 attB::[SpecryjjQ(−347 to +26) lacZ] | S3469 × pKES157 |
| S3478 | S3469 attB::[SpecryjjQ(−147 to +26) lacZ] | S3469 × pKES158 |
| S3480 | S3469 attB::[SpecryjjQ(−87 to +26) lacZ] | S3469 × pKES159 |
| S3482 | S3469 attB::[SpecryjjQ(−47 to +26) lacZ] | S3469 × pKES160 |
| S3484 | S3469 attB::[SpecryjjP(+25 to −450) lacZ] | S3469 × pKES172 |
| S3486 | S3469 attB::[SpecryjjP(+25 to −250) lacZ] | S3469 × pKES173 |
| S3488 | S3469 attB::[SpecryjjP(+25 to −50) lacZ] | S3469 × pKES174 |
| S3494 | S3470 leuO::cat | S3470 × T4GT7 (EK25) |
| S3496 | S3470 Δhns::kanKD4 | S3470 × T4GT7 (S3010) |
| S3587 | S3470 Δhns::kanKD4leuO::cat | S3494 × T4GT7 (S3010) |
| S3588 | S3472 leuO::cat | S3472 × T4GT7 (EK25) |
| S3590 | S3472 Δhns::kanKD4 | S3472 × T4GT7 (S3010) |
| S3592 | S3474 leuO::cat | S3474 × T4GT7 (EK25) |
| S3594 | S3474 Δhns::kanKD4 | S3474 × T4GT7 (S3010) |
| S3596 | S3476 leuO::cat | S3476 × T4GT7 (EK25) |
| S3598 | S3476 Δhns::kanKD4 | S3476 × T4GT7 (S3010) |
| S3600 | S3478 leuO::cat | S3478 × T4GT7 (EK25) |
| S3602 | S3478 Δhns::kanKD4 | S3478 × T4GT7 (S3010) |
| S3604 | S3480 leuO::cat | S3480 × T4GT7 (EK25) |
| S3606 | S3480 Δhns::kanKD4 | S3480 × T4GT7 (S3010) |
| S3608 | S3488 leuO::cat | S3488 × T4GT7 (EK25) |
| S3610 | S3482 Δhns::kanKD4 | S3482 × T4GT7 (S3010) |
| S3612 | S3484 leuO::cat | S3484 × T4GT7 (EK25) |
| S3614 | S3484 Δhns::kanKD4 | S3484 × T4GT7 (S3010) |
| S3616 | S3486 leuO::cat | S3486 × T4GT7 (EK25) |
| S3618 | S3486 Δhns::kanKD4 | S3486 × T4GT7 (S3010) |
| S3620 | S3482 leuO::cat | S3488 × T4GT7 (EK25) |
| S3622 | S3488 Δhns::kanKD4 | S3488 × T4GT7 (S3010) |
| S3631 | S3472 Δhns::kanKD4leuO::cat | S3588 × T4GT7 (S3010) |
| S3633 | S3474 Δhns::kanKD4leuO::cat | S3592 × T4GT7 (S3010) |
| S3635 | S3476 Δhns::kanKD4leuO::cat | S3596 × T4GT7 (S3010) |
| S3637 | S3478 Δhns::kanKD4leuO::cat | S3600 × T4GT7 (S3010) |
| S3638 | S3480 Δhns::kanKD4leuO::cat | S3604 × T4GT7 (S3010) |
| S3641 | S3488 Δhns::kanKD4leuO::cat | S3609 × T4GT7 (S3010) |
| S3642 | S3484 Δhns::kanKD4leuO::cat | S3612 × T4GT7 (S3010) |
| S3645 | S3486 Δhns::kanKD4leuO::cat | S3616 × T4GT7 (S3010) |
| S3646 | S3482 Δhns::kanKD4leuO::cat | S3620 × T4GT7 (S3010) |
| S3663 | S3470 Δhns::FRT leuO::cat | S3587 × pCP20 flp |
| S3665 | S3472 Δhns::FRT leuO::cat | S3631 × pCP20 flp |
| S3667 | S3474 Δhns::FRT leuO::cat | S3633 × pCP20 flp |
| S3669 | S3476 Δhns::FRT leuO::cat | S3635 × pCP20 flp |
| S3671 | S3478 Δhns::FRT leuO::cat | S3637 × pCP20 flp |
| S3673 | S3488 Δhns::FRT leuO::cat | S3641 × pCP20 flp |
| S3675 | S3484 Δhns::FRT leuO::cat | S3642 × pCP20 flp |
| S3677 | S3486 Δhns::FRT leuO::cat | S3645 × pCP20 flp |
| S3689 | S3480 Δhns::FRT leuO::cat | S3638 × pCP20 flp |
| S3691 | S3482 Δhns::FRT leuO::cat | S3646 × pCP20 flp |
All experiments were performed using isogenic E. coli K-12 CSH50 (40) derivatives. Integrations into attB carry the spectinomycin resistance (Specr) gene, fragments encompassing the yjjQ or yjjP promoter with positions given relative to that of the transcription start and the lacZ gene.
The construction of strains by transduction with phage T4GT7 by replacement of genes with resistance cassettes and by deletion of the resistance gene cassettes present in the Δhns and Δ(yjjP-bglJ) mutants using plasmid pCP20 were performed as described previously (11, 14).
Strain transformed with a PCR fragment generated with primers S676 and S783 using pKD3 as the template, as described elsewhere (11).
Plasmids (shown in Table S1 in the supplemental material) were constructed according to standard techniques (1, 53). All lacZ reporter fusions are descendants of the plasmid pKES15, which carries the pACYC177 origin, the neo gene, the attP gene, and an omegon-spectinomycin resistance cassette (14). Site-specific mutations and fusions to lacZ were constructed using restriction fragments or PCR fragments. All regions of plasmids that were derived from PCR fragments were sequenced. The relevant structures of the plasmids are shown schematically in the figures in which they are used and are given in Table S1 in the supplemental material. Details for the constructions and the compiled sequences of the plasmids are available upon request. Media and plates were used as described previously (14, 41). The final concentrations of the antibiotics added were 25 μg/ml kanamycin, 50 μg/ml ampicillin, 15 μg/ml chloramphenicol, 50 μg/ml spectinomycin, and 12.5 μg/ml tetracycline, where necessary.
Determination of β-galactosidase activities.
The β-galactosidase assays were performed as described previously (41). Cultures in LB medium were inoculated from fresh overnight cultures to an optical density at 600 nm (OD600) of 0.1 and harvested after growth at 37°C to an OD600 of 0.5. Isopropyl-β,d-thiogalactoside (IPTG) was added to the overnight and the exponential cultures to a concentration of 1 mM for the induction of leuO expression (carried on the plasmid pKEDR13). The enzyme activities were determined from at least three independent cultures, and standard deviations were less than 15%.
Northern blotting and primer extension.
RNA was isolated from cultures grown in LB medium to an OD600 of 0.5, using an SV total RNA isolation system (Promega). For Northern blotting analyses, 10 μg of RNA was separated by using denaturing acrylamide gels (6% acrylamide/bisacrylamide, 19:1; 7 M urea; 0.9× Tris-borate-EDTA [TBE]) next to an RNA marker (high-range RNA ruler ladder; Fermentas), and blotted onto a Hybond N+ nylon membrane (GE-Healthcare). The membranes were baked for 2 h at 80°C (1) and then stained with methylene blue (0.03% methylene blue in 0.3 M Na-acetate [pH 5.2]) to identify the marker bands and to verify uniform loading and transfer of the RNA. To generate the yjjQ riboprobe, a PCR fragment that carries a terminal T7 promoter sequence was amplified using primers S529 and S843 (see Table S2 in the supplemental material). Then, the riboprobe was synthesized using 0.2 pmol of the PCR fragment in 20 μl of 0.5 mM ATP, 0.5 mM CTP, 0.5 mM GTP, 5 μM UTP, [α-32P]UTP (50 μCi, 800 Ci/mmol), T7 RNA polymerase transcription buffer, and 10 units of T7 RNA polymerase according to the instructions of the supplier (Fermentas). Unincorporated nucleotides were removed by passing the samples over Sephadex-G50 nick columns (GE-Healthcare), and the labeling efficiency was determined by counting 1 μl of the final eluate of 400 μl. The membranes were incubated for 3 h at 65°C with hybridization solution (5× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA {pH 7.7}], 5× Denhardt solution, 50% [wt/vol] formamide, 0.5% [wt/vol]), sodium dodecyl sulfate, and 72 μg of denatured herring sperm DNA, as described previously (1), and then hybridized for 16 h at 65°C with the probes added to hybridization solution and washed as described previously (1). The membranes were exposed to X-ray film or to a phosphorimager plate and then scanned, using a Typhoon imager (GE-Healthcare).
For primer extensions, 5 pmol of the oligonucleotides S700 and S699 (see Table S2 in the supplemental material) were end labeled with [γ-32P]ATP (50 μCi/6,000 Ci/mmol), using 20 units of T4 polynucleotide kinase (Fermentas). Unincorporated nucleotides were removed by passing the sample through a Sephadex-G50 nick column (GE-Healthcare). Five micrograms of total RNA was incubated with 4 μl of the 32P-labeled oligonucleotide (approximately 50 fmol) in a total volume of 10 μl for 5 min at 65°C and cooled on ice. Then, samples of all deoxynucleoside triphosphates (dNTPs) (2 μl of a 10 mM dNTP mixture), 4 μl 5× cDNA buffer, 1 μl 0.1 M dithiothreitol (DTT), 2 μl of H2O, and 1 μl (15 units) of Thermoscript reverse transcriptase (Invitrogen) were added, and the samples were incubated at 50°C for 45 min. The reaction was stopped by heating the solution to 85°C for 5 min, the sample was phenol and chloroform extracted, and then the DNA was ethanol precipitated and resuspended in 5 μl of H2O. A stop solution (T7 sequencing kit; USB) was added, and the samples were separated next to a sequencing ladder on a denaturing sequencing gel (6% acrylamide/bisacrylamide, 19:1; 7 M urea, 0.9× TBE). The sequencing ladders were generated using the same labeled primers and the T7 sequencing kit (USB), as described above, with the following modifications. Briefly, 16 μl of the end-labeled primer with 2 μg of plasmid DNA were ethanol precipitated and resuspended in 12 μl of H2O. Then, 2 μl of annealing buffer, 4 μl labeling mixture (1.375 μM of all dNTPs, 333.5 mM NaCl), and 2 μl of diluted T7-DNA polymerase were added. After the solution was incubated for 5 min at room temperature, 4.5-μl aliquots were transferred to tubes containing 2.5 μl of the A, C, G, and T termination mixtures, respectively, and the reaction was stopped 5 min later by adding 5 μl of stop solution.
Purification of LeuO and H-NS.
For the purification of C-terminally histidine-tagged LeuO (LeuO-His6), strain S541 was transformed with the pKEAP21 plasmid (lacIq tac leuO-His6, Ampr). One-liter cultures of LB medium plus ampicillin were inoculated to an OD600 of 0.1, using a fresh overnight culture of S541/pKEAP21. The cultures were grown to an OD600 of 0.3, at which point IPTG was added to a final concentration of 1 mM. Then, the cultures were grown for an additional 1 h and harvested on ice, having reached an OD600 of 0.8. The cells were spun down and washed twice with Mg-saline (10 mM MgSO4, 0.85% NaCl), and the pellets were stored in aliquots at −80°C. For lysate preparation, the pellets derived from 5 liter of culture were resuspended in 16 ml lysis buffer (20 mM Tris-HCl [pH 7.5], at 4°C, 100 mM KCl, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride [PMSF], 5% glycerol, 50 mM imidazole), and the cells were lysed by sonication. The lysates were cleared by high-speed centrifugation and by filtration through a 0.2-μm filter unit. Then, 10 ml of the lysate was loaded onto a 1-ml HisTrap HP column equilibrated with the same buffer, using an Äkta fast-performance liquid chromatography system (GE-Healthcare). The column was washed with the same buffer containing 100 mM imidazole. LeuO-His6 was eluted by increasing the imidazole concentration stepwise to 200 mM and 500 mM. A fraction of the eluate with 500 mM imidazole contained the highest LeuO-His6 concentration of 120 μg/ml (or 3.4 μM) LeuO-His6 (∼37 kDa) and was stored in aliquots at −80°C.
H-NS was purified essentially as described previously (15). Transformants of strain S219 (stpA::tet) with plasmids pFDY400 (a high-copy pBR derivative plasmid carrying the hns gene under control of the tac promoter) and pFDX500 (a pACYC derivative carrying the lacIq gene) were grown in LB medium with ampicillin and kanamycin to an OD600 of 0.8. The expression of hns was induced by adding 1 mM IPTG, and cells were harvested after 90 min of further growth. The cell pellets were resuspended in 10 ml of H-NS lysis buffer (100 mM NH4Cl, 30 mM Tris-HCl [pH 7.5], 1 mM EDTA, 10% glycerol, 1 mM PMSF, 7 mM β-mercaptoethanol) per liter of culture, and the cells were lysed by sonication. The lysates were cleared by two subsequent high-speed centrifugations. In total, 30 ml of lysate was loaded onto a 50-ml phosphocellulose (P11; Whatman) column preequilibrated with the same buffer. Proteins were eluted with an NH4Cl gradient, with H-NS eluting at approximately 300 mM NH4Cl. The pooled fractions containing H-NS were diluted to a final buffer concentration of 100 mM NH4Cl, 30 mM Tris-HCl (pH 7.5), 1 mM EDTA, 10% glycerol, 1 mM PMSF, and 7 mM β-mercaptoethanol and loaded onto a heparin fast-flow column (GE-Healthcare). Again, an NH4Cl gradient was used, with H-NS eluting at approximately 700 mM NH4Cl. The pooled fractions were diluted to adjust the buffer to 100 mM NH4Cl and then loaded onto a 1-ml Q-Sepharose column (GE-Healthcare) equilibrated with 100 mM KCl, 30 mM Tris-HCl (pH 7.5), 1 mM EDTA, 10% glycerol, 1 mM PMSF, 7 mM β-mercaptoethanol. From this column, H-NS eluted at approximately 300 mM KCl. Fractions containing H-NS were pooled and rebuffered using a Centricon YM-3 centrifugal filter unit (Millipore). H-NS (∼15.5 kDa) was stored at a concentration of 60 ng/μl (or 4 μM) in 75 mM KCl, 20 mM Tris-HCl (pH 7.5), 1.5 mM β-mercaptoethanol, and 1 mM DTT.
Electrophoretic mobility shift assays (EMSA).
DNA fragments were amplified by PCR (see Table S2 in the supplemental material), and their approximate concentrations were determined by the comparison of band intensities in agarose gels. Binding of H-NS and LeuO was carried out in 10-μl samples containing 20 to 25 ng of DNA per fragment in the case of the longer fragments or with 10 ng of DNA in the case of 75-bp fragments. The binding buffer was 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 2 mM DTT, and 10% glycerol, and the amounts of protein added are indicated in Fig. 3. The binding reaction was incubated at 30°C for 20 min, and then 6 μl of each sample was separated next to a GeneRuler size ladder (Fermentas) on 8% nondenaturing polyacrylamide gels (acrylamide/bisacrylamide, 29:1; 0.5× TBE) that were run under cold conditions (4°C). The gels were stained with ethidium bromide for visualization of the DNA.
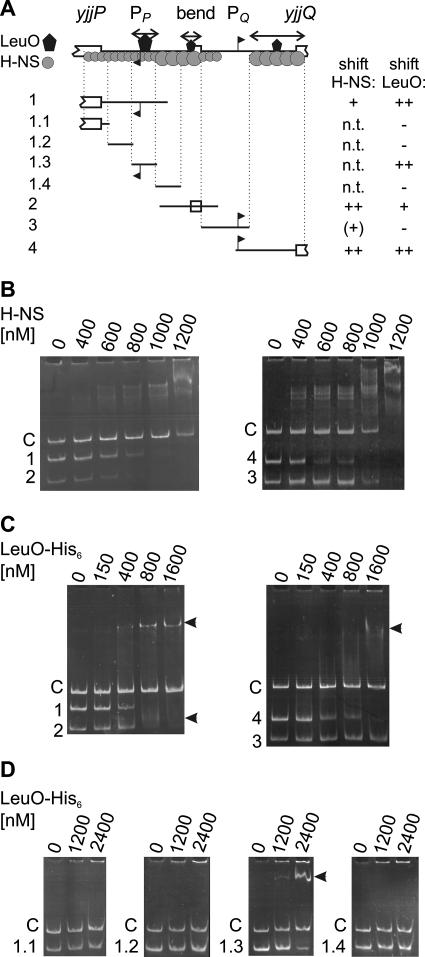
FIG. 3.
Sequence of the intergenic region of the yjjP and yjjQ divergent genes. The putative transcription starts mapped by primer extension are indicated and the possible −10 and −35 boxes are marked. The endpoints of fragments used for the construction of the yjjQ and yjjP promoter-lacZ fusions are marked with arrows with closed and open arrowheads, respectively. The endpoints of fragments used in the gel shift assays are marked by brackets and are labeled with “f” and the number of the fragment. A predicted bend sequence is underlined. Putative H-NS nucleation sites with seven or more matches to the 10-bp H-NS consensus (28) are indicated in boldface letters.
RESULTS
H-NS represses and LeuO activates the yjjQ-bglJ operon.
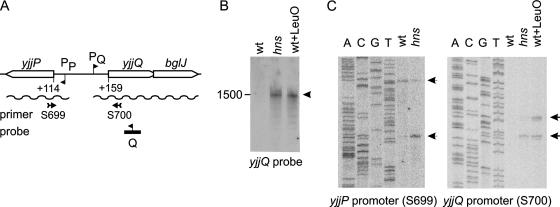
To determine whether the overlapping yjjQ and bglJ genes form an operon, Northern blotting was performed, using a riboprobe complementary to yjjQ (Fig. 2). With RNA isolated from the wild-type strain S541 carrying the native chromosomal yjjP-yjjQ-bglJ locus, no signal was detected (Fig. 2B). However, with RNA isolated from the isogenic Δhns mutant, a weak signal was detected (Fig. 2B). The signal corresponded to an RNA of approximately 1,500 bases, which is the size expected for the transcription of yjjQ and bglJ as a bicistronic mRNA, suggesting that yjjQ and bglJ form an operon. The results show, furthermore, that H-NS represses the expression of this yjjQ-bglJ operon. In agreement with findings for repression by H-NS, the intergenic region between the yjjQ and the yjjP genes located upstream and in a divergent orientation shows features reminiscent of H-NS-repressed loci (16). The sequence is very AT rich (Fig. 3), and a strong bend at the center of this region is predicted by bend.it software (http://hydra.icgeb.trieste.it/∼kristian/dna/bend_it.html) (43).
FIG. 2.
Mapping of the transcription units and the yjjP and yjjQ promoters. (A) Schematic map of the yjjP-yjjQ-bglJ locus. Shown are positions of a riboprobe used in Northern blotting (B) and oligonucleotides S699 and S700 (see Table S2 in the supplemental material) used in the primer extension analyses (C) are indicated. (B) Northern blotting analysis of RNA isolated from the wild-type strain S541 (wt), the Δhns mutant S3346 (hns), and the S541 strain transformed with plasmid pKEDR13 for the expression of LeuO (wt+LeuO) and separated with a denaturing gel, and the blot was hybridized to a riboprobe complementary to the yjjQ RNA. (C) Primer extension mapping of the yjjP promoter and the yjjQ promoter of RNA isolated from the wild-type, the hns mutant, and the wild type expressing LeuO (as in panel B) using the end-labeled oligonucleotides S699 and S700, respectively. The reaction products were separated next to a sequencing ladder generated with the same end-labeled primer. The 5′ end of the yjjP mRNA maps to 113 bases upstream of the yjjP translation start codon, with a second faint signal at 155 bases upstream of yjjP. The 5′ end of the yjjQ mRNA maps to 158 upstream of the yjjQ translation start codon, with a second signal mapping to 172 upstream of the yjjQ promoter codon AUG (Fig. 3).
Furthermore, we determined whether the transcription factor LeuO regulates expression of the yjjQ-bglJ operon (Fig. 2, and see discussion below). The rationale for this experiment was that the constitutive expression of BglJ as well as that of LeuO relieves H-NS-mediated repression of the bgl operon (23, 39, 60). Since, the leuO gene is also repressed by H-NS (7, 26), LeuO was provided in trans by the induction of plasmid-encoded leuO under the control of a lacIq tac promoter cassette with IPTG (using the pKEDR13 plasmid) (39). Northern analysis of RNA isolated from the wild-type strain expressing LeuO revealed signals obtained with the yjjQ probe that were similar to the signals obtained with RNA isolated from the Δhns mutant (Fig. 2B), which suggests that LeuO activates the yjjQ-bglJ operon.
Mapping of the yjjQ and yjjP transcription start sites.
To map the transcription start sites of the yjjQ-bglJ operon and of the divergent yjjP gene, primer extension analyses were performed using end-labeled oligonucleotides complementary to the 5′ end of yjjP and yjjQ, respectively (Fig. 2A and C). For the yjjQ promoter, no signals were obtained with RNA isolated from the wild-type strain. However, with RNA isolated from the Δhns mutant, as well as with RNA isolated after the induction of LeuO, expression of the wild-type signals was obtained (Fig. 2C). One signal was mapped to the 5′ end of the yjjQ-bglJ operon-specific RNA to a position 158 bp upstream of the yjjQ coding region (Fig. 3). A second signal, which was more pronounced when LeuO was provided in trans than that of the Δhns mutant, mapped to a position 172 bp upstream of the yjjQ coding region (Fig. 2C). For both of the putative transcription start sites, −10 boxes can be identified in the sequence, and a modest −35 may be present at the proper distance to the putative transcription start that was mapped to a position 158 bp upstream of the yjjQ coding region (Fig. 3). For the yjjP promoter, two signals were obtained. One of the signals was detected only for RNA isolated from the Δhns mutant (Fig. 2C). The position of this signal suggests that the 5′ end of the yjjP gene-specific RNA maps to a position 113 bp upstream of the yjjP open reading frame (Fig. 2C). The second signal was mapped to a position 155 bp upstream of the yjjP gene coding region. Putative −10 boxes are located at the proper distance to both, while no −35 boxes are evident in the sequence (Fig. 3). Taken together, the data confirm the regulation of the yjjQ-bglJ operon by H-NS and LeuO, and they demonstrate the repression of yjjP by H-NS. The primer extension analyses further reveal that both of the divergent transcription units, yjjQ-bglJ as well as yjjP, carry long untranslated leader sequences of at least 158 and 113 bases, respectively.
Binding of H-NS and LeuO to the intergenic region of the yjjP-yjjQ-bglJ locus.
The expression directed by the divergent yjjQ and yjjP promoters is repressed by H-NS. In addition, LeuO activates the yjjQ promoter. To analyze the binding of H-NS and LeuO to the intergenic region, EMSAs were performed by using fragments ranging in size from 140 to 243 bp (Fig. 3 and Fig. 4 and see Table S2 in the supplemental material). In addition, a 360-bp fragment derived from the lacZ gene was used as a negative control. Mixtures of fragments 1 and 2 and of fragments 3 and 4 with the lacZ control fragment were incubated with increasing amounts of H-NS and then separated on native acrylamide gels (Fig. 4B). As shown in Fig. 4B, fragments 2 and 4 were shifted very efficiently by H-NS; fragment 2 encompasses the predicted bend region located between the divergent yjjP and yjjQ promoters, while fragment 4 was mapped to a position downstream of the yjjQ promoter (from positions −7 to +193 relative to that of the yjjQ transcription start site, which was mapped to 158 bp upstream of yjjQ); fragment 1, which encompasses the yjjP promoter and sequences located downstream to it (from position −80 to + 163 relative to that of the yjjP transcription start at 113 bp upstream of yjjP) was also shifted specifically (Fig. 4B); fragment 3, which encompasses the yjjQ promoter was not shifted compared to that of the lacZ control fragment, which suggests that in this region no sites or only weak H-NS sites are present. This lack of binding between H-NS and fragment 3 is not due to its small size (140 bp), since a 140-bp fragment mapped to within fragment 2 was efficiently shifted by H-NS (data not shown). Taken together, these results demonstrate that H-NS binds to the center region between the divergent promoters and to sites located distal to the two promoters (Fig. 4A). Furthermore, scanning of the DNA sequence for putative H-NS nucleation sites, using the H-NS consensus (28), revealed several putative matches which cluster in the predicted bend region and downstream of the yjjQ promoter (Fig. 3). The locations of these putative sites are in agreement with specific binding of H-NS to fragments 2 and 4, encompassing these two regions.
FIG. 4.
Binding of H-NS and LeuO to the regulatory region of the yjjP-yjjQ-bglJ locus. (A) Schematic representation of the intergenic region. Regions bound by H-NS and LeuO, as deduced from the results shown in panels B to D, are indicated by a black polygon (LeuO) and gray circles forming an extended complex (H-NS). The positions of the fragments used in the EMSA are schematically indicated (with the specific positions given in Fig. 3). n.t., not tested. (B) Binding of H-NS. Fragments 1 (243 bp) and 2 (170 bp) and the 360-bp lacZ control fragment “C” as well as fragments 3 (140 bp) and 4 (200 bp) and the 360-bp lacZ control were mixed and incubated with the indicated amounts of H-NS. Subsequently, the samples were separated on native acrylamide gels stained with ethidium bromide. (C) Binding of LeuO-His6 to fragments 1 to 4 in comparison to the binding of the 360-bp control fragment “C.” (D) Binding of LeuO to fragments 1.1 to 1.4 (75 bp each) in comparison to that of the 140-bp lacZ control fragment “C.”
Binding of LeuO was likewise analyzed by EMSAs, using C-terminal LeuO-His6 (Fig. 4C). These assays demonstrated binding of LeuO to fragments 1, 2, and 4 (Fig. 4B), while no retardation of fragment 3 encompassing the yjjQ promoter was detected (Fig. 4C). Binding of LeuO to fragment 1 resulted in a stable shift, while binding of LeuO to fragments 2 and 4 appeared as a smear, suggesting that the LeuO binding site with the highest affinity was mapped to fragment 1. The mapping of the LeuO binding sites is summarized in Fig. 4A. No LeuO consensus binding sequence is available to date.
LeuO counteracts the repression of yjjQ by H-NS.
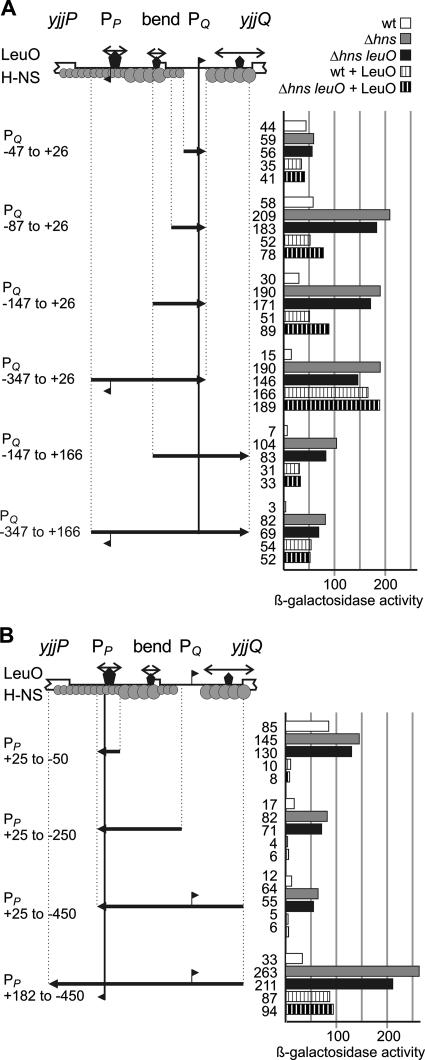
To further analyze the regulation of yjjQ by H-NS and LeuO, a series of fragments encompassing the putative yjjQ promoters was fused to a promoterless lacZ gene as the reporter for expression (Fig. 5A). The yjjQ promoter-lacZ fusions were integrated into the chromosome at the attB site of strain S541 (ΔlacZ), and the expression directed by these fusions was analyzed in the wild-type strain, in the Δhns mutant, and in the Δhns leuO::cat double mutant. All strains carry a deletion of the chromosomal yjjP-yjjQ-bglJ locus. In addition, the role of LeuO in the regulation of the yjjQ promoter-lacZ fusions was analyzed with the wild-type strain as well as with the Δhns leuO double mutant by using the pKEDR13 plasmid carrying leuO under the control of the lacIq tac promoter cassette (Fig. 5A).
FIG. 5.
Regulation of the yjjQ and the yjjP promoters by H-NS and LeuO. (A) The expression directed by the chromosomal yjjQ promoter-lacZ fusions was determined in the wild-type strain, the Δhns mutant, and the Δhns leuO::cat double mutant, as well as in transformants of the wild-type strain and the Δhns leuO::cat double with plasmid pKEDR13 for the expression of LeuO. The expression of LeuO was induced with 1 mM IPTG in the overnight and exponential cultures. The positions of the fragments are given relative to that of the transcription start of the yjjQ promoter mapping to 158 bp upstream of the yjjQ coding region. Binding of H-NS and LeuO is schematically indicated as in Fig. 4. (B) Expression analyses using chromosomal yjjP promoter-lacZ fusions. Data are represented as in panel A, where positions are given relative to the yjjP promoter mapping to 113 bp upstream of the translation start.
The lacZ fusion of the smallest yjjQ promoter fragment, which encompasses sequences from position −47 to +26 (relative to the transcription start site mapping 158 bp upstream of yjjQ), directed 44 units of β-galactosidase activity in the wild-type strain, 59 units in the Δhns mutant, and 56 units in the Δhns leuO double mutant (Fig. 5A, PQ −47 to +26). When LeuO was provided in trans (by the induction of the plasmid-encoded leuO gene), the expression level decreased slightly to 35 units in the wild-type strain and to 41 units in the Δhns leuO double mutant (Fig. 5A, PQ −47 to +26). These data confirm the primer extension result that promoters map within the fragment. The results further show that the fragment lacks binding sites for regulation by H-NS and LeuO. When the promoter fragment was extended to include sequences from positions −87 to +26 and −147 to +26, the expression was repressed by H-NS, suggesting that H-NS binds in between −147 and −47 (Fig. 5A, PQ −87 to +26 and PQ −147 to +26). Furthermore, the unrepressed expression level directed by these fusions in the hns mutant was fourfold increased compared to that in the PQ (−47 to +26) fusion, which may indicate that upstream DNA contacts by RNA polymerase are important for efficient transcription initiation. Providing LeuO in trans had no effect on the expression of these yjjQ promoter-lacZ fusions in the wild-type strain but caused repression in the Δhns leuO double mutant (Fig. 5A, PQ −87 to +26 and PQ −147 to +26), suggesting the presence of a weak LeuO binding site between positions −147 and −47. In contrast, the expression of the yjjQ promoter-lacZ fusion encompassing position −347 to +26 was activated by LeuO and was repressed more efficiently by H-NS than the shorter constructs were (Fig. 5A, PQ −347 to +26). This suggests the presence of a LeuO binding site in between positions −347 and −147 and also binding of H-NS within that region. The presence of regulatory sites for LeuO and H-NS between positions −347 and −147 is further supported by the result obtained with the yjjQ promoter −147-to-+166 and −347-to-+166 fusions. Again, LeuO had little effect on the expression of the −147-to-+166 promoter fusion but efficiently activated the −347-to-+166 promoter fusion in the wild-type strain. Furthermore, these two fusions, which include sequences downstream of the promoter (up to position +166) are more specifically repressed by H-NS (up to ∼27-fold) than the corresponding fusions that lack the downstream region. The expression level directed by the −347-to-+166 fusion had a 27-fold increase (from 3 units in the wild-type strain to 82 units in the hns mutants), while the expression of the corresponding −347-to-+26 fusion had only a 13-fold increase, from 15 units in the wild-type strain to 190 units in the hns mutant (Fig. 5A). Similarly, the expression level directed by the −147-to-+166 fusion had a ∼15-fold increase (compare the wild-type strain with the hns mutant), while the expression of the −147-to-+26 fusion had a ∼6-fold increase (Fig. 5A). These data suggest binding of H-NS to the leader DNA fragment. The finding that the absolute expression level was higher for the +26 than for the +166 fusions (Fig. 5A, compare, e.g., 190 units for the −347-to-+26 fusion in the hns mutant with 104 units for the −347-to-+166 fusion) may indicate that the leader contains signals that decrease expression, possibly at the posttranscriptional level. Taken together, the data are in agreement with the binding of H-NS over the complete intergenic region, with the exception of the yjjQ promoter, as determined by the EMSA (Fig. 4). H-NS sites appear to map to positions downstream of the yjjQ promoter, to the center region, and further upstream to between positions −47 and −347 (Fig. 5A). Furthermore, the data suggest that a LeuO binding site important for derepression of the yjjQ promoter maps to between positions −347 and −147.
Regulation of the yjjP promoter by H-NS and LeuO.
Similar to the analysis performed for the yjjQ promoter region, four yjjP promoter-lacZ fusions were constructed and integrated into the chromosomal attB site (Fig. 5B). The yjjP promoter-lacZ fusion with the smallest fragment, encompassing sequences from position −50 to +25 (relative to that of the yjjP transcription start site that was mapped to a position 113 bp upstream of yjjP) directed the expression of 85 units of β-galactosidase activity in the wild-type strain, 145 units in the Δhns mutant, and 130 units in the Δhns leuO double mutant, confirming that a promoter maps on this fragment. Interestingly, when LeuO was provided in trans, expression dropped to 10 units in the wild-type strain and to 8 units in the Δhns leuO double mutant (Fig. 5B), suggesting that LeuO binds next to this yjjP promoter and represses it. Extending the yjjP promoter fragment to include the central region (Fig. 5B, PP −250 to +25) or the complete intergenic region (Fig. 5B, PP −450 to +25) resulted in more effective repression by H-NS, in agreement with results described above that suggest that H-NS binds to the central region and to sequences distal to the divergent yjjQ promoter region (Fig. 5). These extended yjjP promoter-lacZ fusions were also repressed by LeuO (Fig. 5B, PP −250 to +25 and PP −450 to +25). Furthermore, the expression level directed by a yjjP promoter-lacZ fusion that encompasses the complete intergenic region (from position −450 to +182 relative to that of the transcription start site mapping to a position 113 bp upstream of yjjP) increased from 33 units in the wild-type strain to 263 units and 211 units in the Δhns and the Δhns leuO double mutants, respectively (Fig. 5B), demonstrating that in the complete context, H-NS represses the yjjP promoter ∼eightfold and suggesting that H-NS also binds in between the yjjP promoter and the yjjP gene. Interestingly, this full construct was activated by LeuO in the wild-type strain and repressed only 2.2-fold by LeuO in the Δhns leuO double mutant (Fig. 5B), indicative of a more complex pattern of regulation. Possibly, LeuO has a dual role in the regulation of the yjjP promoter; it may repress the promoter directly and also counteract its repression by H-NS when the binding site downstream of the yjjP promoter is present. The location of binding sites for LeuO and H-NS deduced from the yjjP promoter-lacZ fusions are summarized schematically in Fig. 5B.
To further address the regulation of the yjjP promoter by LeuO, gel retardation experiments were performed using a series of 75-bp fragments that cover the yjjP promoter and the leader region (Fig. 4D, fragments 1.1 to 1.4). The fragments were mixed with a 140-bp control fragment derived from lacZ to exclude unspecific binding. In this shift assay, LeuO bound only to the 75-bp fragment that encompassed the yjjP promoter but not to the other fragments. This fragment encompasses positions −50 to +25 relative to that of the transcription start and is, thus, identical to the fragment used in the yjjP promoter-lacZ fusion carrying the smallest yjjP promoter fragment (Fig. 5B). Binding of LeuO to this fragment is in agreement with repression of the yjjP promoter by LeuO.
DISCUSSION
We have shown that the yjjP-yjjQ-bglJ locus is regulated by H-NS and the LysR-type regulator LeuO. The yjjQ and bglJ genes encoding LuxR-type transcription factors form an operon whose repression by H-NS is counteracted by LeuO. The yjjP gene located upstream of the yjjQ-bglJ operon and in a divergent orientation is likewise repressed by H-NS but is not activated by LeuO. The H-NS binding sites are presumably spread throughout the extended 555-bp intergenic region, except for the yjjQ core promoter. For LeuO, three sites were mapped. One of these LeuO sites that overlapped the divergent yjjP promoter was required for the complete derepression of the promoter of the yjjQ-bglJ operon. The arrangement of binding sites suggests that H-NS forms an extended repressing nucleoprotein complex that prevents transcription initiation at both divergent promoters. Binding of LeuO and putative concomitant DNA looping could prevent spreading of the H-NS nucleoprotein complex into the yjjQ promoter and thus relieve its repression by H-NS. Repression of yjjP, yjjQ, and bglJ by H-NS has also been detected by genome scale analyses with E. coli and S. enterica (36, 46, 48).
For the activation of H-NS-repressed loci, several mechanisms have been reported (reviewed in references 16, 45, 51, and 55). For example, in the case of the virF promoter in Shigella flexneri, DNA bending is temperature dependent, and repression is relieved at 37°C (49). Repression of the hdeAB promoter in E. coli depends on the trapping of σ70-associated RNA polymerase, which induces a bend, while transcription initiation of σS-associated RNA polymerase is not repressed (57). Furthermore, binding of a transcription factor next to an essential H-NS nucleation site can prevent the binding of H-NS, as shown for the relief of the H-NS-mediated repression of the icsB promoter in Shigella flexneri by VirB (59). Alternatively, a bound transcription factor may form a barrier that prevents spreading of the H-NS nucleoprotein complex into the promoter, as proposed for autoregulation of the H-NS-repressed leuO gene and the divergent leuABCD operon encoding enzymes of the leucine synthesis pathway (7). In the case of the antagonistic regulation of the yjjQ-bglJ operon, analyzed here, the arrangement of the binding sites indicates that LeuO may form a DNA loop by binding to the yjjP promoter and to the center of the intergenic region. LeuO-mediated DNA looping may prevent spreading of the H-NS complex into the promoter of the yjjQ-bglJ operon. It is also possible that H-NS traps RNA polymerase at the yjjQ promoter by binding upstream and downstream and that LeuO prevents the formation of that repressing DNA loop. In a genome scale analysis, RNA polymerase and H-NS were both found to be associated with the yjjQ locus (48). An alternative mechanism in which LeuO prevents the binding of H-NS to a nucleation site seems less likely. Such a mechanism was shown for LeuO-mediated antirepression of the H-NS and StpA-repressed Salmonella enterica ompS1 gene (12). However, in the case of the yjjP-yjjQ-bglJ locus, H-NS binds to the center of the intergenic region with the highest affinity, while LeuO binds with highest affinity to the yjjP promoter fragment. DNA looping by LeuO is further supported by the result that the yjjQ promoter is moderately repressed by LeuO in the hns mutant when the distal LeuO site overlapping the yjjP promoter is missing (Fig. 4A).
The divergent arrangement of transcription units at the yjjP-yjjQ-bglJ locus raises the possibility that transcription-induced supercoiling (34) plays a role in its antagonistic regulation by H-NS and LeuO, which again would relate to autoregulation of the leuO gene (20, 21, 62). A similar divergent arrangement and topological coupling has also been shown to be important for the regulation of the ilvYC locus by the LysR-type transcription factor IlvY (47). In addition, divergent transcription units are a feature common to many loci that are regulated by LysR-type transcription factors (54).
Regulation of the yjjQ-bglJ operon by H-NS and LeuO adds an additional aspect to the role of LeuO in the emerging regulatory network important for in vivo induction of genes and for pathogenicity. LeuO is involved in the control of biofilm formation and pathogenicity in several bacteria (29, 42, 58). YjjQ encodes a putative pathogenicity determinant in APEC and in S. enterica (see the introduction) (29, 33). BglJ counteracts repression of the bgl operon by H-NS, as does LeuO (23, 39, 60). Although the importance of LeuO has been clearly defined in in vivo models for several bacterial pathogens, the regulatory effect of LeuO under in vitro growth conditions has so far been observed only when LeuO was constitutively overexpressed. In addition, no coinducer (or effector) of LeuO has been defined so far, although the activity of other transcription factors of the LysR-family is controlled by the binding of small molecules (see for example references 19, 27, 35, and 54). Furthermore, it is a common feature of LysR-type transcription factors that the effector is not required for the binding of the LysR-type regulator to DNA but alters its interaction with the DNA (27, 35). For example, the LysR-type transcription factor ArgP acts either as a repressor (when associated with lysine) or as an activator (when associated with arginine), stimulating promoter clearing at argO (27).
Supplementary Material
Acknowledgments
We thank Kathleen Plamper for excellent technical assistance, Andreas Paukner for the pKEAP21 plasmid and the S3375 strain, Nagarajavel V. for purified H-NS, and Lorna Moll for the S3322 strain and for conducting the initial experiments.
The work was funded by Deutsche Forschungsgemeinschaft Grant Schn 371/8.
Footnotes
Published ahead of print on 30 November 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2005. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, NY.
- 2.Badaut, C., R. Williams, V. Arluison, E. Bouffartigues, B. Robert, H. Buc, and S. Rimsky. 2002. The degree of oligomerization of the H-NS nucleoid structuring protein is related to specific binding to DNA. J. Biol. Chem. 27741657-41666. [DOI] [PubMed] [Google Scholar]
- 3.Bouffartigues, E., M. Buckle, C. Badaut, A. Travers, and S. Rimsky. 2007. H-NS cooperative binding to high-affinity sites in a regulatory element results in transcriptional silencing. Nat. Struct. Mol. Biol. 14441-448. [DOI] [PubMed] [Google Scholar]
- 4.Chen, C. C., M. Y. Chou, C. H. Huang, A. Majumder, and H. Y. Wu. 2005. A cis-spreading nucleoprotein filament is responsible for the gene silencing activity found in the promoter relay mechanism. J. Biol. Chem. 2805101-5112. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. C., M. Fang, A. Majumder, and H. Y. Wu. 2001. A 72-base pair AT-rich DNA sequence element functions as a bacterial gene silencer. J. Biol. Chem. 2769478-9485. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. C., M. Ghole, A. Majumder, Z. Wang, S. Chandana, and H. Y. Wu. 2003. LeuO-mediated transcriptional derepression. J. Biol. Chem. 27838094-38103. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C. C., and H. Y. Wu. 2005. LeuO protein delimits the transcriptionally active and repressive domains on the bacterial chromosome. J. Biol. Chem. 28015111-15121. [DOI] [PubMed] [Google Scholar]
- 8.Daley, D. O., M. Rapp, E. Granseth, K. Melen, D. Drew, and G. von Heijne. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 3081321-1323. [DOI] [PubMed] [Google Scholar]
- 9.Dame, R. T., C. Wyman, R. Wurm, R. Wagner, and N. Goosen. 2002. Structural basis for H-NS-mediated trapping of RNA polymerase in the open initiation complex at the rrnB P1. J. Biol. Chem. 2772146-2150. [DOI] [PubMed] [Google Scholar]
- 10.Dame, R. T., M. C. Noom, and G. J. L. Wuite. 2006. Bacterial chromatin organization by H-NS protein unravelled using dual DNA manipulation. Nature 444387-390. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De la Cruz, M. A., M. Fernandez-Mora, C. Guadarrama, M. A. Flores-Valdez, V. H. Bustamante, A. Vazquez, and E. Calva. 2007. LeuO antagonizes H-NS and StpA-dependent repression in Salmonella enterica ompS1. Mol. Microbiol. 66727-743. [DOI] [PubMed] [Google Scholar]
- 13.Diederich, L., L. J. Rasmussen, and W. Messer. 1992. New cloning vectors for integration into the lambda attachment site attB of the Escherichia coli chromosome. Plasmid 2814-24. [DOI] [PubMed] [Google Scholar]
- 14.Dole, S., S. Kühn, and K. Schnetz. 2002. Post-transcriptional enhancement of Escherichia coli bgl operon silencing by limitation of BglG-mediated antitermination at low transcription rates. Mol. Microbiol. 43217-226. [DOI] [PubMed] [Google Scholar]
- 15.Dole, S., V. Nagarajavel, and K. Schnetz. 2004. The histone-like nucleoid structuring protein H-NS represses the Escherichia coli bgl operon downstream of the promoter. Mol. Microbiol. 52589-600. [DOI] [PubMed] [Google Scholar]
- 16.Dorman, C. J. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2391-400. [DOI] [PubMed] [Google Scholar]
- 17.Dorman, C. J. 2007. Probing bacterial nucleoid structure with optical tweezers. BioEssays 29212-216. [DOI] [PubMed] [Google Scholar]
- 18.Dorman, C. J. 2007. H-NS, the genome sentinel. Nat. Rev. Microbiol. 5157-161. [DOI] [PubMed] [Google Scholar]
- 19.Ezezika, O. C., S. Haddad, T. J. Clark, E. L. Neidle, and C. Momany. 2007. Distinct effector-binding sites enable synergistic transcriptional activation by BenM, a LysR-type regulator. J. Mol. Biol. 367616-629. [DOI] [PubMed] [Google Scholar]
- 20.Fang, M., and H. Y. Wu. 1998. Suppression of leu-500 mutation in topA+ Salmonella typhimurium strains. The promoter relay at work. J. Biol. Chem. 27329929-29934. [DOI] [PubMed] [Google Scholar]
- 21.Fang, M., and H.-Y. Wu. 1998. A promoter relay mechanism for sequential gene activation. J. Bacteriol. 180626-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Mora, M., J. L. Puente, and E. Calva. 2004. OmpR and LeuO positively regulate the Salmonella enterica serovar Typhi ompS2 porin gene. J. Bacteriol. 1862909-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giel, M., M. Desnoyer, and J. Lopilato. 1996. A mutation in a new gene, bglJ, activates the bgl operon in Escherichia coli K-12. Genetics 143627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grainger, D. C., D. Hurd, M. D. Goldberg, and S. J. Busby. 2006. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 344642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 4020-36. [DOI] [PubMed] [Google Scholar]
- 26.Klauck, E., J. Bohringer, and R. Hengge-Aronis. 1997. The LysR-like regulator LeuO in Escherichia coli is involved in the translational regulation of rpoS by affecting the expression of the small regulatory DsrA-RNA. Mol. Microbiol. 25559-569. [DOI] [PubMed] [Google Scholar]
- 27.Laishram, R. S., and J. Gowrishankar. 2007. Environmental regulation operating at the promoter clearance step of bacterial transcription. Genes Dev. 211258-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang, B., N. Blot, E. Bouffartigues, M. Buckle, M. Geertz, C. O. Gualerzi, R. Mavathur, G. Muskhelishvili, C. L. Pon, S. Rimsky, S. Stella, M. M. Babu, and A. Travers. 2007. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 356330-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawley, T. D., K. Chan, L. J. Thompson, C. C. Kim, G. R. Govoni, and D. M. Monack. 2006. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrenz, M. B., and V. L. Miller. 2007. Comparative analysis of the regulation of rovA from the pathogenic Yersiniae. J. Bacteriol. 1895963-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lease, R. A., M. E. Cusick, and M. Belfort. 1998. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc. Natl. Acad. Sci. USA 9512456-12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lease, R. A., and S. A. Woodson. 2004. Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J. Mol. Biol. 3441211-1223. [DOI] [PubMed] [Google Scholar]
- 33.Li, G., C. Laturnus, C. Ewers, and L. H. Wieler. 2005. Identification of genes required for avian Escherichia coli septicemia by signature-tagged mutagenesis. Infect. Immun. 732818-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, L. F., and J. C. Wang. 1987. Supercoiling of the DNA template during transcription. Proc. Natl. Acad. Sci. USA 847024-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorca, G. L., A. Ezersky, V. V. Lunin, J. R. Walker, S. Altamentova, E. Evdokimova, M. Vedadi, A. Bochkarev, and A. Savchenko. 2007. Glyoxylate and pyruvate are antagonistic effectors of the Escherichia coli IclR transcriptional regulator. J. Biol. Chem. 28216476-16491. [DOI] [PubMed] [Google Scholar]
- 36.Lucchini, S., G. Rowley, M. D. Goldberg, D. Hurd, M. Harrison, and J. C. D. Hinton. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucht, J. M., P. Dersch, B. Kempf, and E. Bremer. 1994. Interactions of the nucleoid-associated DNA-binding protein H-NS with the regulatory region of the osmotically controlled proU operon of Escherichia coli. J. Biol. Chem. 2696578-6586. [PubMed] [Google Scholar]
- 38.Luijsterburg, M. S., M. C. Noom, G. J. Wuite, and R. T. Dame. 2006. The architectural role of nucleoid-associated proteins in the organization of bacterial chromatin: a molecular perspective. J. Struct. Biol. 156262-272. [DOI] [PubMed] [Google Scholar]
- 39.Madhusudan, S., A. Paukner, Y. Klingen, and K. Schnetz. 2005. Independent regulation of H-NS mediated silencing of the bgl operon at two levels: upstream by BglJ and LeuO and downstream by DnaKJ. Microbiology 1513349-3359. [DOI] [PubMed] [Google Scholar]
- 40.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 41.Miller, J. H. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Moorthy, S., and P. I. Watnick. 2005. Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol. Microbiol. 571623-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munteanu, M. G., K. Vlahovicek, S. Parthasarathy, I. Simon, and S. Pongor. 1998. Rod models of DNA: sequence-dependent anisotropic elastic modelling of local bending phenomena. Trends Biochem. Sci. 23341-347. [DOI] [PubMed] [Google Scholar]
- 44.Nagarajavel, V., S. Madhusudan, S. Dole, A. R. Rahmouni, and K. Schnetz. 2007. Repression by binding of H-NS within the transcription unit. J. Biol. Chem. 28223622-23630. [DOI] [PubMed] [Google Scholar]
- 45.Navarre, W. W., M. McClelland, S. J. Libby, and F. C. Fang. 2007. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 211456-1471. [DOI] [PubMed] [Google Scholar]
- 46.Navarre, W. W., S. Porwollik, Y. Wang, M. McClelland, H. Rosen, S. J. Libby, and F. C. Fang. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313236-238. [DOI] [PubMed] [Google Scholar]
- 47.Opel, M. L., S. M. Arfin, and G. W. Hatfield. 2001. The effects of DNA supercoiling on the expression of operons of the ilv regulon of Escherichia coli suggest a physiological rationale for divergently transcribed operons. Mol. Microbiol. 391109-1115. [DOI] [PubMed] [Google Scholar]
- 48.Oshima, T., S. Ishikawa, K. Kurokawa, H. Aiba, and N. Ogasawara. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA Polymerase. DNA Res. 13141-153. [DOI] [PubMed] [Google Scholar]
- 49.Prosseda, G., M. Falconi, M. Giangrossi, C. O. Gualerzi, G. Micheli, and B. Colonna. 2004. The virF promoter in Shigella: more than just a curved DNA stretch. Mol. Microbiol. 51523-537. [DOI] [PubMed] [Google Scholar]
- 50.Repoila, F., and S. Gottesman. 2001. Signal transduction cascade for regulation of RpoS: temperature regulation of DsrA. J. Bacteriol. 1834012-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rimsky, S. 2004. Structure of the histone-like protein H-NS and its role in regulation and genome superstructure. Curr. Opin. Microbiol. 7109-114. [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-Morales, O., M. Fernández-Mora, I. Hernández-Lucas, A. Vázquez, J. L. Puente, and E. Calva. 2006. Salmonella enterica serovar Typhimurium ompS1 and ompS2 mutants are attenuated for virulence in mice. Infect. Immun. 741398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 54.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47597-626. [DOI] [PubMed] [Google Scholar]
- 55.Schröder, O., and R. Wagner. 2002. The bacterial regulatory protein H-NS—a versatile modulator of nucleic acid structures. Biol. Chem. 383945-960. [DOI] [PubMed] [Google Scholar]
- 56.Shi, X., and G. N. Bennett. 1995. Effects of multicopy LeuO in the expression of the acid-inducible lysine decarboxylase gene in Escherichia coli. J. Bacteriol. 177810-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin, M., M. Song, J. H. Rhee, Y. Hong, Y. J. Kim, Y. J. Seok, K. S. Ha, S. H. Jung, and H. E. Choy. 2005. DNA looping-mediated repression by histone-like protein H-NS: specific requirement of Eσ70 as a cofactor for looping. Genes Dev. 192388-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tenor, J. L., B. A. McCormick, F. M. Ausubel, and A. Aballay. 2004. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr. Biol. 141018-1024. [DOI] [PubMed] [Google Scholar]
- 59.Turner, E. C., and C. J. Dorman. 2007. H-NS antagonism in Shigella flexneri by VirB, a virulence gene transcription regulator that is closely related to plasmid partition factors. J. Bacteriol. 1893403-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueguchi, C., T. Ohta, C. Seto, T. Suzuki, and T. Mizuno. 1998. The leuO gene-product has a latent ability to relieve the bgl silencing in Escherichia coli. J. Bacteriol. 180190-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson, G. G., K. Y. K. Young, G. J. Edlin, and W. Konigsberg. 1979. High-frequency generalised transduction by bacteriophage T4. Nature 28080-82. [DOI] [PubMed] [Google Scholar]
- 62.Wu, H.-Y., J. Tan, and M. Fang. 1995. Long-range interaction between two promoters: activation of the leu-500 promoter by a distant upstream promoter. Cell 82445-451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.