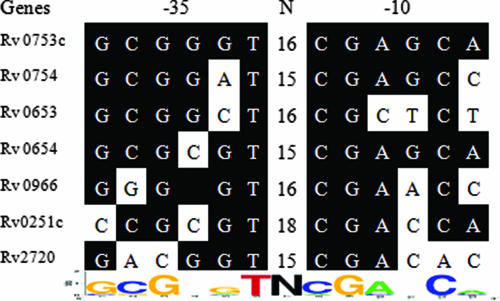Abstract
The sigG gene of Mycobacterium tuberculosis was disrupted by homologous recombination, and the genes regulated by SigG were examined by real-time reverse-transcription PCR and microarray studies. The SigG consensus promoter recognition sequence was identified as GCGNGT-N15-18-CGANCA. A ΔsigG mutant was found to be more resistant to mitomycin C treatment than the wild-type strain, indicating that it may be involved in the SOS response in M. tuberculosis.
Mycobacterium tuberculosis causes disease in humans and has been one of the most important human pathogens since it was first identified 125 years ago. It is estimated that approximately 9 million new cases of tuberculosis and 2 million deaths due to this disease occur each year (6). A unique feature of M. tuberculosis is its ability to persist as a latent infection, where it lies dormant and escapes detection by the host immune system. The environmental stress response in bacteria is controlled by the extracytoplasmic function (ECF) sigma factors, which contribute to the resistance to stress conditions, such as high and low temperature, oxidative stress, carbon starvation, and low pH. The number of ECF sigma factors varies in different bacterial species. In this study, we focused on the ECF sigma factor gene, sigG. In a previous study it was found that sigG expression is 10- to 100-fold lower than the expression of the other M. tuberculosis sigma factor genes under normal in vitro growth conditions (9). However, a previous report showed that sigG was one of the most highly induced genes during macrophage infection and that its expression was increased 9.7-fold (2). The promoter region of sigG is similar to that of the recA gene, which has a key role in the SOS response (5). These data suggested that SigG is unlikely to play a significant role in bacterial physiology during in vitro growth in rich medium but that it may be conditionally up-regulated in response to certain environmental signals. In this study, we constructed a sigG deletion mutant and characterized its expression patterns and phenotypes compared to wild-type M. tuberculosis.
Construction of ΔsigG mutant.
sigG mutant construction was carried out as described previously (21) using the suicide vector pCK0686 (7). The left flanking region of sigG was amplified with primers sigGP1 and sigGP2, and the right flanking region was amplified with primers sigGP3 and sigGP4 (see Table S1 in the supplemental material). After amplification by PCR, the two flanks were ligated into pCK0686. To construct the complemented strain, approximately 5.5 kb of the region containing the sigG gene was amplified using primers gcomp1 and gcomp2 and then cloned into the pMH94 vector (8) at the XbaI sites. Putative mutant candidates were screened by PCR with the RT-sigG primer set (Fig. 1B) and by Southern blotting (Fig. 1C and D). The ΔsigG mutant strain was confirmed by the absence of a 17.5-kb band using the sigG gene probe (Fig. 1C) and the appearance of a 12-kb band using the hygromycin gene probe (Fig. 1D). Complementation was achieved by cloning a 5.5-kb sigG-containing fragment into pMH94 and introducing this plasmid into the mutant strain (Fig. 1C).
FIG. 1.
Confirmation of the M. tuberculosis sigG deletion mutant. The ΔsigG mutant was confirmed by PCR and Southern blotting. (A) Physical map of the genes around sigG and the restriction endonuclease sites used for Southern blotting. Chromosomal DNA was purified from the parental wild-type, ΔsigG mutant, and complemented strains. PCR was performed with primers RT-sigG1 and RT-sigG2, which were designed to bind to the deleted region, and with primer RT-sigA for the control. (B) Identification of mutant strain by PCR. Lane 1, molecular marker (1-kb Plus ladder; Invitrogen); lane 2, wild type with RT-sigG; lane 3, ΔsigG mutant with RT-sigG; lane 4, wild type with RT-sigA; lane 5, ΔsigG mutant with RT-sigA. (C) Left panel, ethidium bromide stain of gel prior to transfer. Right panel, Southern blot in which chromosomal DNA from wild-type, ΔsigG mutant, and complemented strains were digested with restriction enzyme XbaI and hybridized with the sigG gene probe generated by PCR with primers RT-sigG1 and RT-sigG2. Lane 1, marker (Dig marker III; Roche); lane 2, wild-type strain; lane 3, ΔsigG mutant strain; lane 4, complemented strain. (D) Southern blot in which chromosomal DNA was digested with XhoI and hybridized with the hygromycin gene-specific probe. Lane 1, marker (Dig marker III; Roche); lane 2, wild type; lane 3, ΔsigG mutant strain.
Recently, a sequence similar to the recAp1 promoter of the recA gene was identified in the upstream region of sigG (5), which suggested that sigG may be involved in the SOS response mechanism. However, using the TubercuList database (http://genolist.pasteur.fr/TubercuList/), this promoter region was located in the open reading frame of the sigG gene. Therefore, we determined the exact transcription start point of the sigG gene by the random amplification of cDNA ends technique (see Fig. S1 in the supplemental material). The transcription start point of the sigG gene was identified as an A residue 5 bp from the putative start site, GTG (see Fig. S1 in the supplemental material). While a 5-nucleotide region is a relatively short 5′ untranslated region (UTR), we did observe an ATG codon 102 bp distal to the currently annotated start site of translation. Hence, it is possible that SigG protein translation initiates at this alternative site.
Gene expression profiles of a ΔsigG strain.
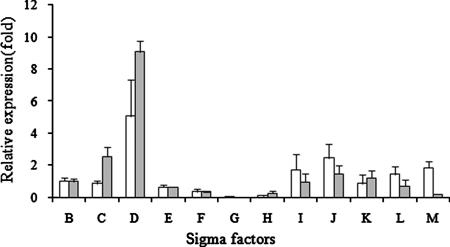
We monitored changes in sigG expression during the growth cycle, comparing log-phase (optical density [OD], 0.5), early-stationary-phase (OD, 1.0), and late-stationary-phase (OD, 2.0) levels by real-time reverse transcription PCR (RT-PCR) with an RT primer set (see Table S1 in the supplemental material). sigG expression was 4.3- ± 0.3-fold greater at an OD of 1 and 4.1- ± 1.2-fold greater at an OD of 2. Therefore, we compared the ΔsigG mutant to the wild type to determine expression changes in early stationary phase (OD, 1) and late stationary phase (OD, 2) that might be attributable to the absence of sigG expression at these growth phases. The ΔsigG mutant was investigated by real-time RT-PCR with the RT primer set (see Table S1 in the supplemental material). The threshold cycle (CT) value was normalized to the value for sigA, and the expression level of the wild type was compared with that of the ΔsigG strain. As shown in Fig. 2, the ΔsigG mutant was confirmed not to express sigG mRNA. For the other sigma factors, sigH and sigF gene expression was decreased, while the expression of sigD was increased. It is known that the expression of the M. tuberculosis sigma factors is interdependent. SigH regulates sigE and sigB, and SigL and SigF regulate sigB (3, 10, 16), while other sigma factors, such as sigD, sigK, and sigM, have autotranscription properties (18). The effect of deletion of the sigG gene on the expression of sigH and sigD represents another example of cascade gene regulation among sigma factors in M. tuberculosis.
FIG. 2.
Expression profiles of M. tuberculosis sigma factors in the ΔsigG mutant strain. The relative expression profile of each sigma factor in the sigG-deficient mutant was determined by real-time RT-PCR at OD of 1 and 2. The CT value of each gene of interest (GOI) was normalized with the housekeeping gene (HKG), sigA. The relative expression value was obtained by the ΔΔCt method: 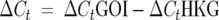 . Regulation of individual genes was calculated using the following term:
. Regulation of individual genes was calculated using the following term: 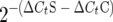 , where S is the ΔsigG strain and C is the wild-type strain. Open bars, OD of 1; shaded bars, OD of 2.
, where S is the ΔsigG strain and C is the wild-type strain. Open bars, OD of 1; shaded bars, OD of 2.
To identify the sigG-dependent genes, a microarray study was performed as described previously (21). The slides were scanned using an Axon 400B scanner, and the image data were quantified using Genepix pro 4.0 software. Each data point was then normalized to the total intensity of the spots, and the ratio of Cy5 to Cy3 was calculated. Our microarray expression profile of the ΔsigG mutant compared to that of the wild-type CDC1551 strain revealed over 100 genes that were down-regulated in the ΔsigG mutant relative to the wild type (see Table S2 in the supplemental material). Several genes which may be involved in fatty acid metabolism were found to be down-regulated, including aceA, the isocitrate lyase gene (Rv0467), fadE5, the acyl-coenzyme A dehydrogenase gene (Rv0244), and scoA, the succinyl-coenzyme A gene (Rv2504c). Other genes that were down-regulated have been shown to be induced in the murine macrophage model; these genes include the heat shock protein gene, Rv0251c (which is induced during exposure to heat, H2O2, and sodium dodecyl sulfate), the isocitrate lyase gene, aceA (12, 20), and lat, the l-lysine-epsilon aminotransferase gene (Rv3290c) (which is induced in a starvation model) (22). In the ΔsigG mutant rpfC expression was induced 11- and 7-fold at OD of 1 and 2, respectively, and hsp (Rv0251c) expression was decreased 0.03- and 0.44-fold at OD of 1 and 2, respectively. rpf genes encode resuscitation-promoting factors and may have an extracytoplasmic location. M. tuberculosis has five rpf-like genes, rpfA (Rv0867c), rpfB (Rv1009), rpfC (Rv1884c), rpfD (Rv2389c), and rpfE (Rv2450c) (4, 15). A previous study showed that there was induction of hsp (Rv0251c), which appears to be SigG dependent in the ΔrpfC deletion mutant (4). The microarray profile of the ΔsigG mutant identified several down-regulated genes which have been shown to be under SigH control, including clpB, dnaK, Rv2466c, and trxB2. In addition, two other genes that are directly SigD regulated, Rv1815 and rpfC, were found to be up-regulated in the ΔsigG mutant (Table 1) (15).
TABLE 1.
Microarray analysis of sigma factor- and SOS box-related genes in the M. tuberculosis ΔsigG mutant compared to the wild typea
| Gene | Code | Rv no. | Relative signal (mutant/wild type) | q valueb | Description |
|---|---|---|---|---|---|
| sigH-related genes | |||||
| sigH | 2 | Rv3223c | 0.4 ± 0.0 | 0.00 | RNA polymerase sigma factor |
| clpB | 0 | Rv0384c | 0.2 ± 0.0 | 0.00 | Endopeptidase ATP binding protein |
| dnaK | 0 | Rv0350 | 0.3 ± 0.0 | 0.00 | Chaperone protein |
| Rv2466c | 10 | Rv2466c | 0.2 ± 0.0 | 0.00 | Conserved hypothetical protein |
| trxB2 | 7 | Rv3913 | 0.8 ± 0.0 | 0.05 | Thioredoxin reductase |
| sigD-related genes | |||||
| sigD | 2 | Rv3414c | 1.6 ± 0.5 | 0.07 | RNA polymerase sigma factor |
| Rv1815 | 10 | Rv1815 | 5.2 ± 0.7 | 0.07 | Conserved hypothetical protein |
| Rv1884c | 3 | Rv1884c | 11.0 ± 0.3 | 0.07 | Resuscitation-promoting factor |
| SOS box | |||||
| ssb | 2 | Rv0054 | 0.7 ± 0.1 | 0.01 | Single-strand binding protein |
| dnaBc | 2 | Rv0058 | 1.4 ± 0.1 | 0.07 | Replicative DNA helicase |
| ding | 2 | Rv1329c | 0.6 ± 0.0 | 0.07 | ATP-dependent helicase |
| recN | 2 | Rv1696 | 1.0 ± 0.1 | 0.07 | DNA repair protein |
| ruvB | 2 | Rv2592c | 0.6 ± 0.1 | 0.06 | Holliday junction DNA helicase |
| ruvAc | 2 | Rv2593c | 0.8 ± 0.1 | 0.07 | Holliday junction DNA helicase |
| ruvCc | 2 | Rv2594c | 1.6 ± 0.0 | 0.07 | Crossover junction endodeoxyribonuclease |
| lexAc | 9 | Rv2720 | 0.6 ± 0.1 | 0.01 | LexA |
| recAc | 2 | Rv2737c | 1.5 ± 0.1 | 0.07 | RecA |
| dinP | 2 | Rv3056 | 0.7 ± 0.0 | 0.07 | DNA damage-inducible protein |
| dnaE2 | 2 | Rv3370c | 0.6 ± 0.1 | 0.07 | DNA polymerase |
Wild-type and mutant strains were grown to an OD of 1.0. Codes (functional categories) and gene annotation data were obtained from the TubercuList database (http://genolist.pasteur.fr/TubercuList/). The function codes indicate the following functions: 0, virulence, detoxification, and adaptation; 2, information pathways; 3, cell wall and cell processes; 7, intermediary metabolism and respiration; 9, regulatory proteins; 10, conserved hypothetical proteins.
The q value was calculated using SAM (http://wwwstat.stanford.edu/∼tibs/SAM/) with 1% false discovery rate.
The SOS box showed LexA binding in a gel shift assay (1).
Based on data from the microarray study, we also identified a putative SigG promoter recognition site, GCGNGT-N15-18-CGANCA, through gene alignment and comparison of 400 to 500 bp of the 5′ UTR from the start codon of seven down-regulated genes whose expression was decreased more than 40% and which were transcribed divergently from the gene at its 5′ end (Fig. 3).
FIG. 3.
Identification of the SigG promoter recognition sequence. The putative SigG regulon was identified by microarray data analysis. The nucleotide sequences of the 5′ UTR of down-regulated genes in the ΔsigG mutant were aligned using the Clustal W multialignment sequence analysis program. Identical nucleotide sequences are indicated by a black background, and the consensus sequence motif was determined by weblogo software. N indicates the space between the −35 and −10 regions.
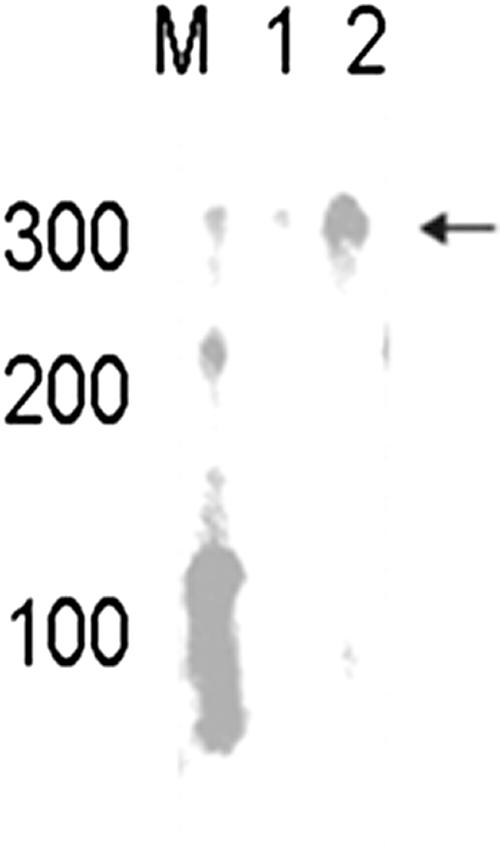
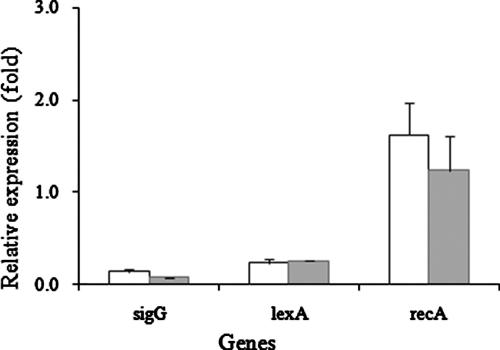
Two interesting findings were the reduction in the repressor lexA (Rv2720) transcription and the increase in recA (Rv2737c) transcription in the ΔsigG mutant. recA is a gene involved in the SOS response and contains a LexA binding motif (SOS box) in the promoter region. We also found that lexA contained a putative SigG consensus binding site in its 5′ UTR 262 bp from the start codon, which was confirmed to be regulated by SigG by in vitro transcription (Fig. 4) and real-time RT-PCR (Fig. 5). For the in vitro transcription assay, the entire open reading frame of the sigG gene was amplified with primers petsigG1 (see Table S1 in the supplemental material) and petsigG2 and cloned into the pET22b(+) vector (Novagen). The construct was transformed into Escherichia coli BL21(DE3), protein expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and the protein was purified by Ni-nitrilotriacetic (Ni-NTA) acid affinity chromatography. In vitro transcription assays were performed as described in a previous study (21). The lexA gene promoter was amplified by PCR using primers lex1 (see Table S1 in the supplemental material) and lex2, the 400-bp PCR product was purified by gel extraction (Qiagen), and 0.09 μg was used as a DNA template. As shown in Fig. 4, we detected the anticipated in vitro transcript with a length of 300 nucleotides.
FIG. 4.
In vitro transcription assay of the M. tuberculosis lexA gene promoter region with purified SigG protein. The 5′ UTR of the lexA gene was amplified using PCR and was transcribed in vitro with E. coli RNA polymerase core and purified SigG protein. Negative controls lacked SigG protein. The transcript was analyzed on a 5% denaturing gel. Lane M, RNA century marker (Ambion); lane 1, lexA promoter region template without SigG; lane 2: lexA promoter region template with SigG.
FIG. 5.
Expression of lexA and recA genes in the ΔsigG mutant strain. Expression of lexA and recA in the mutant strain was determined by real-time RT-PCR methods. The cDNA was amplified with primers RT-lexA and RT-recA (see Table S1 in the supplemental material), and the CT value was normalized using the sigA value. Open bars, OD of 1; shaded bars, OD of 2.
Role of sigG in SOS response and mitomycin C (MMC) susceptibility.
Upon DNA damage, the SOS response is induced through the RecA-LexA regulon. The LexA protein binds to the SOS box upstream of genes and acts as a repressor of SOS genes. Following DNA damage LexA is cleaved in an autocatalytic reaction stimulated by RecA. The resulting fragment of LexA cannot bind efficiently to the SOS box and thereby cannot repress the transcription of SOS genes (17). This RecA-LexA regulon has also been identified in M. tuberculosis. The M. tuberculosis recA gene also has two promoter sequences. One sequence is a typical sigma 70-like promoter sequence, and the other is a sigma 32-like sequence. In these two promoter sequences, the SOS box is located between the −35 and −10 regions of the sigma 32-like promoter sequence (13). The promoter sequence upstream of sigG shares close similarity to this sigma 32-like sequence, implying that recA and sigG may be transcribed by the same sigma factor in response to SOS stress conditions.
In a previous study lexA was found to contain an SOS box with the sequence TCGAACACATGTTTGA upstream of its promoter region (1). The SigG consensus binding site identified in this study was located upstream of this SOS box. This suggests that lexA transcription by SigG is limited to the SOS response and is controlled by sigG rather than the principal sigma factor. Many M. tuberculosis genes, such as dnaB (Rv0058), ruvA (Rv2593c), ruvC (Rv2594c), lexA (Rv2720), and recA (Rv2737c), have a putative LexA binding site which showed positive binding in a gel shift assay. However, the LexA protein does not bind the same consensus binding site in the promoter region of ssb (Rv0054), dinG (Rv1329c), recN (Rv1696), ruvB (Rv2592c), dinP (Rv3056), or dnaE2(Rv3370c) (1). These results are consistent with our microarray data (Table 1) since many of the same genes were underexpressed in the ΔsigG mutant.
The M. tuberculosis ΔsigG mutant is resistant to mitomycin C.
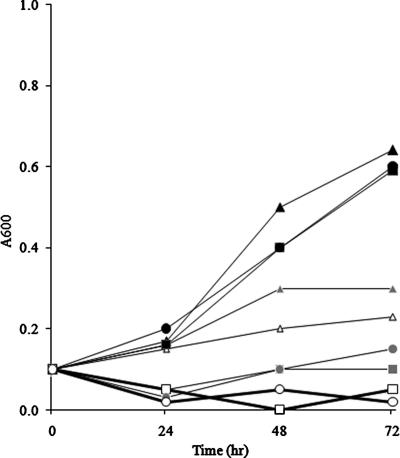
MMC is a potent SOS response inducer and has antibacterial activity against Mycobacterium bovis (14). MMC causes cell death during DNA damage, but there is also rescue due to DNA repair and the SOS response (error-prone response system). However, in the absence of LexA repression, the SOS system is constitutively expressed, and therefore lexA mutants have been demonstrated to display relative MMC resistance compared to the wild type (24). Therefore, we tested the MMC resistance of the M. tuberculosis ΔsigG strain. The ΔsigG mutant strain was treated with 0.2 and 1 μg/ml MMC. Using A600 to monitor growth, we found that the wild-type and complemented strains were more susceptible than the mutant to both 0.2 and 1.0 μg/ml MMC (Fig. 6). This study was repeated two times, and a similar pattern was observed. This indicates that deletion of sigG confers resistance to MMC. The CFU of bacteria were also counted after serial dilution (103- to 105-fold). For the ΔsigG strain the bacterial counts were 107 CFU/ml without MMC and 104 CFU/ml in the presence of 0.2 μg/ml MMC after 2 and 3 days. We did not detect the wild-type and complemented strains at this concentration. With a higher concentration of MMC, we did not detect any bacteria on the plates with the same dilution ratios. These data indicate that deletion of sigG confers resistance to MMC in M. tuberculosis.
FIG. 6.
Effect of MMC on the growth of the ΔsigG mutant. Wild-type and ΔsigG mutant strains were exposed to various concentrations of MMC, and the growth rate was determined using the OD at 600 nm at the time points indicated. Black circles, wild-type strain without MMC; black triangles, ΔsigG strain without MMC; black squares, complemented strain without MMC; gray circles, wild-type strain with MMC (0.2 μg/ml); gray triangles, ΔsigG strain with MMC (0.2 μg/ml); gray squares, complemented strain with MMC (0.2 μg/ml); open circles, wild-type strain with MMC (1 μg/ml); open triangles, ΔsigG strain with MMC (1 μg/ml); open squares, complemented strain with MMC (1 μg/ml).
Macrophage infection.
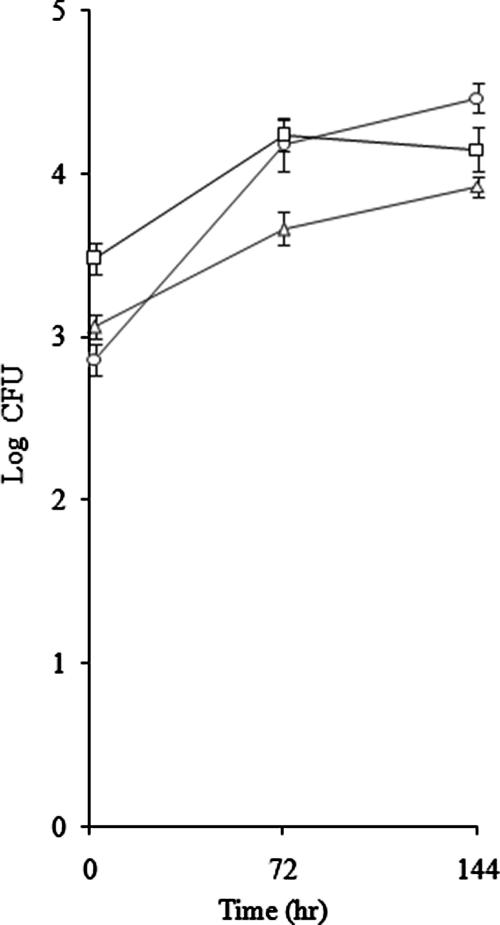
To assess the role of sigG in macrophage infection, we compared the ability of the ΔsigG mutant to invade and survive within J774A.1 murine macrophages with that of its parental wild-type strain (CDC1551). The initial intracellular inoculum after 2 h of infection was about 103 CFU. As shown in Fig. 7, the ΔsigG mutant displayed impaired survival in the macrophage infection model at 3 days (P = 0.05) and 6 days (P = 0.01) postinfection. This result is consistent with the results of a previous study in which sigG was found to be one of the most induced genes during macrophage infection, with a relative increase in expression of 9.7-fold (2). The environment of the macrophage phagosome appears to be deficient in carbohydrate and imposes oxidative, nitrosative, and nutritional depletion stress conditions on bacteria (11, 19). We found that hsp (Rv0251c) expression was reduced in the ΔsigG mutant and that this gene is part of the sigG regulon (see Table S2 in the supplemental material). hsp genes are also induced in macrophages, and deletion of the hsp gene in M. tuberculosis causes attenuation in monocyte-derived macrophages (23). The hsp gene (Rv0251c) was reported to belong to the sigE regulon, and its expression was reduced by sigE deletion. However, we identified the sigG consensus promoter recognition sequence in the upstream region of the hsp gene, whose expression was also reduced by deletion of sigG. As determined by real-time RT-PCR, deletion of sigG led to a reduction in sigH expression, but it did not change the expression of sigE. These observations support the hypothesis that hsp is also regulated by sigG. Another sigG regulon gene, aceA, was induced in macrophages, and the deletion mutant showed attenuation in the macrophage infection model.
FIG. 7.
Growth of the M. tuberculosis ΔsigG mutant in J774A.1 macrophages. The growth of wild-type and ΔsigG strains in macrophages was examined. J774A.1 alveolar macrophages were cultivated in cRPMI (2 mM glutamine, 10% fetal bovine serum) with 5% CO2 at 37°C and activated with gamma interferon (500 U/ml) for 12 h, followed by lipopolysaccharide (200 ng/ml) for 3 h before infection, and 105 cells were infected with wild-type and mutant M. tuberculosis at a multiplicity of infection of 1:1. At 2 h, 3 days, and 6 days macrophage lysates were plated on 7H10 medium to count the surviving bacteria. ○, wild-type strain; ▵, ΔsigG strain; □, complemented strain.
Previous studies with M. tuberculosis indicated that sigG may play a role in the stress response during macrophage infection and in the SOS response. sigG is one of the most highly induced genes during macrophage infection (2). The promoter region of the sigG gene showed similarity with a known recA P1 promoter sequence upstream of the M. tuberculosis recA gene, which has been shown to be a key factor in the SOS response (5). This suggests that sigG is cotranscribed with the recA gene by the same RNA polymerase-sigma factor holoenzyme and that sigG may be a key regulator in the stress response in M. tuberculosis.
In this study, we identified the significance of sigG during macrophage infection and in the SOS response. Genes regulated by the SigG sigma factor are important for M. tuberculosis survival inside macrophages and for SOS stress response-related functions.
Supplementary Material
Acknowledgments
Support by NIH grants AI36973, AI37856, and AI43846 is gratefully acknowledged.
Footnotes
Published ahead of print on 26 November 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Brooks, P. C., F. Movahedzadeh, and E. O. Davis. 2001. Identification of some DNA damage-inducible genes of Mycobacterium tuberculosis: apparent lack of correlation with LexA binding. J. Bacteriol. 1834459-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappelli, G., E. Volpe, M. Grassi, B. Liseo, V. Colizzi, and F. Mariani. 2006. Profiling of Mycobacterium tuberculosis gene expression during human macrophage infection: upregulation of the alternative sigma factor G, a group of transcriptional regulator, and proteins with unknown function. Res. Microbiol. 157445-455. [DOI] [PubMed] [Google Scholar]
- 3.Dainese, E., S. Rodrigue, G. Delogu, R. Provvedi, L. Laflamme, R. Brzezinski, G. Fadda, I. Smith, L. Gaudreau, G. Palu, and R. Manganelli. 2006. Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic function sigma factor L and roles in virulence and in global regulation of gene expression. Infect. Immun. 742457-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downing, K. J., J. C. Betts, D. I. Young, R. A. McAdam, F. Kelly, M. Young, and V. Mizrahi. 2004. Global expression profiling of strain harboring null mutants reveals that the five rpf-like genes of Mycobacterium tuberculosis show functional redundancy. Tuberculosis 84167-179. [DOI] [PubMed] [Google Scholar]
- 5.Gamulin, V., H. Cetkovic, and I. Ahel. 2004. Identification of a promoter motif regulating the major DNA damage response mechanism of Mycobacterium tuberculosis. FEMS Microbiol. 23857-63. [DOI] [PubMed] [Google Scholar]
- 6.Harries, A., and C. Dye. 2006. Tuberculosis. Ann. Trop. Med. Parasitol. 100415-431. [DOI] [PubMed] [Google Scholar]
- 7.Kaushal, D., B. G. Schroeder, S. Tyagi, T. Yoshimatsu, C. Scott, C. Ko, L. Carpenter, J. Mehrotra, Y. C. Manabe, R. D. Fleischmann, and W. R. Bishai. 2002. Reduced immunpathology and mortality despite tissue persistence in Mycobacterium tuberculosis mutant lacking alternative sigma factor, sigH. Proc. Natl. Acad. Sci. USA 998300-8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, M. H., L. Pascopella, W. R. Jacobs, Jr., and G. F. Hatfull. 1991. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacilli Calmette-Guerin. Proc. Natl. Acad. Sci. USA 883111-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manganelli, R., E. Dubnau, S. Tyagi, F. R. Kramer, and I. Smith. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31715-724. [DOI] [PubMed] [Google Scholar]
- 10.Manganelli, R., M. Voskuil, G. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor E: role in global gene expression and survival in macrophages. Mol. Microbiol. 41423-437. [DOI] [PubMed] [Google Scholar]
- 11.McKinney, J. D., and J. E. Gomez. 2003. Life on inside for Mycobacterium tuberculosis. Nat. Med. 91356-1357. [DOI] [PubMed] [Google Scholar]
- 12.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 17735-738. [DOI] [PubMed] [Google Scholar]
- 13.Movahedzadeh, F., M. J. Colston, and E. O. Davis. 1997. Determination of DNA sequences required for regulated Mycobacterium tuberculosis RecA expression in response to DNA-damaging agents suggests that two modes of regulation exist J. Bacteriol. 1793509-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peh, H. L., A. Toh, B. Murugasu-Oei, and T. Dick. 2001. In vitro activities of mitomycin C against growing and hypoxic dormant tubercle bacilli. Antimicrob. Agents Chemother. 452403-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raman, S., R. Hazra, C. C. Dascher, and R. N. Husson. 2004. Transcription regulation by Mycobacterium tuberculosis alternative sigma factor SigD and its role in virulence. J. Bacteriol. 1866605-6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raman, S., T. Song, X. Puyang, S. Bardarov, W. R. Jacob, Jr., and R. N. Husson. 2001. The alternative sigma factor sigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J. Bacteriol. 1836119-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rand, L., J. Hinds, B. Springer, P. Sander, R. S. Buxton, and E. O. Davis. 2003. The majority of inducible DNA repair genes in Mycobacterium tuberculosis are induced independently of RecA. Mol. Microbiol. 501031-1042. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigue, S., J. Brodeur, P. E. Jacques, A. L. Gervais, R. Brzezinski, and L. Gaudreau. 2007. Identification of mycobacterial sigma factor binding sites by chromatin immunoprecipitation assays. J. Bacteriol. 1891505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Lin, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma, V., S. Sharma, K. Hoener zu Bentrup, J. D. McKinney, D. G. Russell, W. R. Jacobs, Jr., and J. C. Sacchettini. 2000. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol. 7663-668. [DOI] [PubMed] [Google Scholar]
- 21.Sun, R., P. J. Converse, C. Ko, S. Tyagi, N. E. Morrison, and W. R. Bishai. 2004. Mycobacterium tuberculosis sigC is an ECF sigma factor required for lethality in mice and for the conditional expression of a set of genes. Mol. Microbiol. 5225-38. [DOI] [PubMed] [Google Scholar]
- 22.Tripathi, S. M., and R. Ramachandran. 2006. Overexpression, purification and crystallization of lysine epsilon-aminotransferase (Rv3290c) from Mycobacterium tuberculosis H37Rv. Acta Crystallogr. Sect. F. 62572-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson, K. A., G. R. Stewart, S. M. Newton, H. M. Vordermeier, J. R. Wain, H. N. Murphy, K. Horner, D. B. Young, and R. J. Wilkinson. 2005. Infection biology of a novel alpha-crystallin of Mycobacterium tuberculosis: Acr2. J. Immunol. 1744237-4243. [DOI] [PubMed] [Google Scholar]
- 24.Yang, M. K., S. R. Su, and V. L. Sung. 2005. Identification and characterization of a second lexA gene of Xanthomonas axonopodis pathovar citri. Appl. Environ. Microbiol. 713589-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.