Abstract
Trimethoprim-sulfamethoxazole (SXT)-resistant Staphylococcus aureus thymidine-dependent small-colony variants (TD-SCVs) are frequently isolated from the airways of cystic fibrosis (CF) patients, often in combination with isogenic normal strains if patients were treated with SXT for extended periods. As SXT inhibits the synthesis of tetrahydrofolic acid, which acts as a cofactor for thymidylate synthase (thyA), the survival of TD-SCVs depends exclusively on the availability of external thymidine. Since the underlying mechanism for thymidine dependency is unknown, we investigated if alterations in the thyA nucleotide sequences were responsible for this phenomenon. Sequence analysis of several clinical TD-SCVs and their isogenic normal strains with reference to previously published S. aureus thyA nucleotide sequences was performed. Three clinical TD-SCVs were complemented by transforming TD-SCVs with the vector pCX19 expressing ThyA from S. aureus 8325-4. Transcriptional analysis of metabolic and virulence genes and regulators (agr, hla, spa, citB, thyA, and nupC) was performed by quantitative reverse transcription-PCR. The previously published sequences of thyA and two normal clinical strains were highly conserved, while thyA of four normal strains and four SCVs had nonsynonymous point mutations. In 8/10 SCVs, deletions occurred, resulting in stop codons which were located in 4/10 SCVs close to or within the active site of the protein (dUMP binding). Complementation of TD-SCVs with thyA almost fully reversed the phenotype, growth characteristics, and transcription patterns. In conclusion, we demonstrated that mutations of the thyA gene were responsible for the phenotype of TD-SCVs. Complementation of TD-SCVs with thyA revealed that a functional ThyA protein is necessary and sufficient to change the SCV phenotype and behavior back to normal.
Cystic fibrosis (CF) is one of the most common hereditary diseases in the Caucasian population. Patients with CF suffer from chronic suppurative airway infections, which eventually lead to progressive lung insufficiency and premature death. Only a few bacterial species are associated with CF (17). Interestingly, there is an age-related sequence of colonizing and infecting pathogens (6). In most patients, during early infancy and adolescence the leading pathogens are Staphylococcus aureus and Haemophilus influenzae, which are later replaced by Pseudomonas aeruginosa, which, especially in its mucoid form, indicates deterioration of the disease (17).
Colonization and infection with S. aureus are usually monoclonal in CF patients (14). The same clone can be isolated for months or years in spite of appropriate antibiotic treatment. Due to the hostile environment and repeated exposure to antibiotics, especially during long-term treatment with trimethoprim-sulfamethoxazole (SXT), in CF patients, particularly SXT-resistant, thymidine-dependent small-colony variants (TD-SCVs) emerge (2, 8, 11). Menadione-dependent SCVs, hemin-dependent SCVs, and TD-SCVs have been recovered from patients with other chronic infections, such as osteomyelitis (21). Compared to normal S. aureus, SCVs produce much smaller pinpoint or fried-egg colonies, are less pigmented, and are less hemolytic on conventional Columbia blood agar plates (13). The transcription patterns of important virulence regulators and metabolic and stress-related genes of TD-SCVs are dramatically changed, and these strains have a less virulent but more persistent phenotype (12) with decreased expression of α-hemolysin and increased expression of cell wall-associated proteins, such as protein A. Moreover, SCVs are more resistant to antibiotics and defensins (21, 22, 24) and survive intracellularly in various cell types, such as keratinocytes and epithelial and endothelial cells (11, 32, 33). The intracellular locus provides protection against antibiotic treatment and host defense.
Recently, we showed that TD-SCVs exhibit superior stationary-phase survival during long-term culture compared to normal S. aureus due to a lack of a functional tricarboxylic acid (TCA) cycle (5). Reversible inactivation of the TCA cycle in wild-type organisms has been suggested to be a survival strategy used to circumvent oxidative stress induced during host-pathogen interactions (29).
Many bacteria depend on the synthesis of tetrahydrofolic acid (THF). THF is a required cofactor for the conversion of dUMP to dTMP by thymidylate synthase (encoded by thyA), which is essential for DNA synthesis. TMP interferes with THF synthesis at two different steps, thereby inhibiting THF synthesis; sulfamethoxazole as a sulfonamide competitively inhibits the bacterial modification of p-aminobenzoic acid to dihydrofolate, whereas trimethoprim inhibits bacterial dihydrofolate reductase (34). If THF is not synthesized, dUMP is not converted to dTMP. Thus, the bacteria die due to a lack of thymidine (23). However, if external thymidine is available, SXT-resistant TD-SCVs emerge from wild-type S. aureus strains during long-term SXT treatment by an unknown mechanism. In the airways of CF patients, TD-SCVs survive for extended periods due to the occurrence of abundant pus and destroyed cells, which provide sufficient amounts of thymidine.
The underlying mechanism of TD-SCVs is not known. Since TD-SCVs are resistant to SXT, we hypothesized that thyA is mutated. Therefore, in this study thyA in several clinical TD-SCVs was sequenced and compared to thyA in isogenic normal S. aureus strains and to thyA sequences in previously published S. aureus genomes. To further strengthen our hypothesis, clinical TD-SCVs were complemented by a vector expressing wild-type ThyA, and then the phenotypes, growth characteristics, and transcription patterns of important virulence regulators and metabolic genes were analyzed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. For sequence analysis, four pairs of normal and SCV strains were used, which were described previously and have been frozen and thawed several times (12). To ensure that similar changes occurred in vivo and were not a result of reculturing of strains, fresh isolated SCVs or pairs of normal and SCV strains from airway secretions of CF patients were analyzed. The newly isolated strains were analyzed by spa typing to determine the clonality of pairs of strains (9), and the members of each pair belonged to the same spa type; Normal-5 and SCV-5 were type t118, Normal-6 and SCV-6 were type t040, SCV-7-1 and SCV-7-2 were type t084, and SCV-8-1 and SCV-8-2 were type t100. All new TD-SCVs were SXT resistant (MIC, >32 μg/ml, as determined by an Etest [AB Biodisk, Solna, Sweden]) and thymidine dependent on chemically defined medium agar (11). Normal S. aureus and TD-SCV strains were grown in brain heart infusion (BHI) (Merck, Darmstadt, Germany) broth, which supports the growth of SCVs, while Escherichia coli was grown in Luria-Bertani broth. E. coli InvαF′ (Invitrogen, Karlsruhe, Germany) was used as a host strain for the TA cloning vector pCR2.1. For bacterial growth, cultures were grown at 35°C on a rotary shaker. Bacterial growth was assessed by measuring the optical density at 568 nm (OD568). Ampicillin was used at a concentration of 50 μg/ml.
TABLE 1.
Bacterial strains used in this study
| Strain(s) or vector | Genotype, relevant characteristics, or nucleotide sequence | Reference |
|---|---|---|
| S. aureus strains | ||
| 8325-4 | Wild-type standard laboratory strain | 20 |
| Normal-1 to Normal-4 | Phenotypically normal S. aureus strains isolated from airway secretions of four individual CF patients | 12 |
| SCV-1 to SCV-4 | SCV S. aureus strains isolated from airway secretions of four individual CF patients | 12 |
| Normal-5 to Normal-6 | Phenotypically normal S. aureus strains isolated from airway secretions of two individual CF patients | This study |
| SCV-5 to SCV-8 | SCV S. aureus strains isolated from airway secretions of four individual CF patients | This study |
| SCV/pCX19thyA | Clinical TD-SCV S. aureus strain harboring pCX19thyA expressing thyA | This study |
| SCV/pCX19empty | Clinical TD-SCV S. aureus strain harboring the empty vector pCX19empty | This study |
| E. coli InvαF′ | Host strain for the TA cloning vector pCR2.1 | |
| Vectors | ||
| pCR2.1 | Cloning vector for sequencing | |
| pCX19 | Xylose-inducible expression vector | 10 |
| pCX19thyA | pCX19 with thyA | This study |
| pCX19empty | pCX19 without thyA | This study |
Sequencing strategy.
For sequencing, thyA of TD-SCVs and isogenic normal strains was amplified with primers which also included regions up- and downstream of thyA (Table 2). The resulting amplicons were cloned into the cloning vector pCR2.1 and sent to MWG Biotech AG, Martinsried, Germany, for sequencing using the M13 vector primers.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ → 3′) |
|---|---|
| Complementation primers | |
| thyEx2-rev | ATATCCCGGGGCAATGACTACACTGCTATTGGa |
| thyEx1-up | ATATGGTACCTGAGGTGTTATCGCATATCTTGb |
| Cloning primers | |
| thy-1475592-upc | CCTACCACATCGTAACGTGA |
| thy-1474240-revc | TGTGCAACTAGAATGGATAAAGTCA |
| Real time RT-PCR primers | |
| yqiL-1 | TGCGATGATGTTAGTCATGT |
| yqiL-2 | TTCAAAGCCTTTTCTACAGC |
| nupC-1 | CTATTTAGCTCAGACAGGAAAAA |
| nupC-2 | AGCAAGATAAATGCAAAGATAAG |
| RNAIII-5 | TGAATTTGTTCACTGTGTCG |
| RNAIII-6 | AGGAAGGAGTGATTTCAATG |
| hla-3 | TTTCACCAGACTTCGCTACAG |
| hla-4 | CCAATTTGTTGAAGTCCAATG |
| citB-1 | TCAAAATCCATCATTCTTCC |
| citB-2 | TCACCGAATTTACCCATAAC |
| spa-1 | TGGTTTTATCCAAAGCCTTA |
| spa-2 | TTTGGAGCTTGAGAGTCATT |
| thyA-3 | TGTGGACTTGAAGTAGGAGAA |
| thyA-4 | ACGTGCTAATTGTGTTTGAAT |
The SmaI restriction site is underlined.
The BamHI restriction site is underlined.
The number in the designation is the number of base pairs in the genome of strain COL.
Complementation of clinical TD-SCVs.
For complementation, the pCX19 vector was used, which is a derivative of staphylococcal low-copy-number plasmid pC194 with lipase cloned into BamHI and SmaI restriction sites under the control of an xylA promoter and a chloramphenicol resistance cassette (15). To complement TD-SCVs, thyA was amplified from S. aureus 8325-4 using the primers listed in Table 2 and cloned into the expression vector pCX19. Prior to ligation of thyA into pCX19, the lipase gene was removed from the vector by restriction with SmaI and BamHI. The resulting plasmid, pCX19thyA, was transformed into three clinical TD-SCVs by protoplast transformation. For complementation with thyA, SCV-1, SCV-2, and SCV-4 were used (Table 3). The thyA mutations of these TD-SCVs are indicated in Table 3. To eliminate sequencing mistakes introduced by PCR or transformation procedures, thyA was sequenced. To ensure that the observed phenotypic changes were due to thyA and not to the plasmid itself, a vector without thyA (pCX19empty) was constructed and also transformed into the three TD-SCVs.
TABLE 3.
Sequencing results and mutations in thyA in clinical normal strains and TD-SCVs
| Strain | Alteration(s) in nucleotide sequence | Predicted results |
|---|---|---|
| Normal-1a | ||
| SCV-1a | Δ247AAT249 | 3-bp deletion → deletion of Asn |
| Normal-2a | ||
| SCV-2a | Δ247AAT249 | 3-bp deletion → deletion of Asn |
| Normal-3b | T76C | Point mutation Cys → Arg (amino acid 26) |
| SCV-3b | C139T, G337A, A493G, ΔA565 | Point mutations Leu → Por (amino acid 47), Gly → Ser (amino acid 113), and Asn → Asp (amino acid 165) and 1-bp deletion → stop codon (amino acid 188) |
| Normal-4a | T730G, G835A | Point mutations Ser → Ala (amino acid 244) and Gly → Ser (amino acid 279) |
| SCV-4a | T82A, Δ592CTTCCGCCTTG601 | 11-bp deletion (amino acid 198 → frameshift mutation → truncated protein |
| Normal-5b | G308A, C838T | Point mutations Gly → Asp (amino acid 103) and Leu → Phe (amino acid 280) |
| SCV-5 | ΔA51 | 1-bp deletion → frameshift mutation → stop codon (amino acid 19) |
| Normal-6b | C383T, A482G, G886A | Point-mutations Ser → Phe (amino acid 128), Asp → Gly (amino acid 161), and Val → Ile (amino acid 296) |
| SCV-6b | G359A, ΔG743 | Point-mutation Arg → Gln (amino acid 120) and 1-bp deletion → frameshift mutation → stop codon (amino acid 251) |
| SCV-7-1b | T7A, C521T, G806A, ΔA893 | Point-mutations Tyr → Asn (amino acid 3), Tyr → Ile (amino acid 174), and Gly → Asp (amino acid 269) and 1-bp deletion → frameshift mutation → stop codon (amino acid 311) |
| SCV-7-2b | T7A, T616C | Point-mutation Tyr → Asn (amino acid 3) and point mutation → stop codon (amino acid 206) |
| SCV-8-1 | ΔT121 | 1-bp deletion → frameshift mutation → stop codon (amino acid 50) |
| SCV-8-2b | Δ547ACATCGT553 | 7-bp deletion → frameshift mutation → stop codon (amino acid 212) |
Allele 2 (C at nucleotide position 300, Asn at amino acid position 100, and same thyA sequence as S. aureus strain MW2).
Allele 1 (A at nucleotide position 300, Lys at amino acid position, and same thyA sequence as S. aureus strain COL).
Quantitative RT-PCR.
Prior to RNA extraction, S. aureus was grown in BHI medium until the desired growth phase, either early log phase, late log phase, or stationary phase. Bacteria were mechanically disrupted (Fast Prep FP120 instrument; Qbiogene, Heidelberg, Germany), and RNA was isolated with an RNeasy mini-kit (Qiagen, Hilden, Germany). cDNA was synthesized from RNA using a Quantitect reverse transcription (RT) kit (Qiagen, Hilden, Germany) by following the manufacturer's recommendations.
Real-time amplification was performed using specific primers (Table 2) with 25 ng of cDNA as the template in each reaction mixture, and it was carried out with an iCycler iQ real-time PCR system (Bio-Rad, Hercules, CA) using iQ SYBR green Supermix (Bio-Rad). The levels of mRNA expression of the different genes were normalized using the expression of the internal control gene yqiL (encoding acetyl-coenzyme A acetyltransferase), which is a housekeeping gene successfully used for quantitative RT-PCR (16, 26). The levels of transcripts were expressed as increases (n-fold) relative to the values for the internal control (4).
RESULTS
In silico analysis of the thyA sequence in S. aureus genomes.
thyA in S. aureus consists of 957 bp encoding a 318-amino-acid protein. The previously published sequences of thyA of strains COL, MRSA252, MSSA476, MU50, MW2, and N315 were aligned with the program clustalW (http://www.ebi.ac.uk/clustalW/) and analyzed. thyA was highly conserved in all strains at the base pair level, but some base pair changes resulted in nonsynonymous mutations. Interestingly, two alleles were detected at position 300, which encoded lysine in COL and MRSA252 but asparagine in the other strains. There are two predicted binding sites in thyA, a binding site for folate and dUMP at positions 142 to 174 coding for PLLTTKKVSFL (amino acids 48 to 58) and a binding site at position 586 to 618 coding for MALPPCHTMFQ (amino acids 196 to 206) (3). Both of these binding sites show 82% homology to similar regions in Bacillus subtilis at the protein level (19).
Sequence analysis of thyA in clinical TD-SCVs reveals different mutations compared to the normal clinical strains.
Since there is a strong association between the emergence of TD-SCVs and SXT treatment, we hypothesized that thyA, which is responsible for thymidine synthesis from dUMP, would be affected in TD-SCVs. To test this hypothesis, thyA, including up- and downstream sequences, was amplified from six normal clinical strains and 10 TD-SCVs, cloned into the cloning vector pCR2.1, and sequenced, and the sequences were compared to the thyA sequences of previously sequenced strains. The same alleles found at position 300 in the previously sequenced strains were detected in the clinical strains, and 50% of the clinical strain pairs had one of these alleles and 50% had the other. In two normal strains, the thyA sequence was identical to the thyA sequence of N315. In the other normal strains (n = 4), one to three nonsynonymous point mutations were detected in the gene (Table 3). The thyA sequences of TD-SCVs had several different mutations. In four SCVs, one to three nonsynonymous point mutations occurred. Furthermore, two 3-bp in-frame deletions in two SCVs from two different patients occurred at the same position; six 1-bp (n = 5) or 7-bp (n = 1) deletions and one point mutation resulting in a stop mutation (Table 3) were detected. In one TD-SCV, an 11-bp deletion was observed, which resulted in a frameshift mutation. In 4 of 10 TD-SCVs (SCV-3, SCV-4, SCV-7-2, and SCV-8-2 [Table 3]), mutations were located within or close to the dUMP-binding site (positions 586 to 618), which has been suggested to be the active site of thyA. The in-frame deletion, which was detected in two independent SCVs, occurred at the same position where three Asn residues were encoded in the wild-type gene.
Complementation of clinical TD-SCVs with a functional thyA gene.
The analysis of thyA of 10 clinical TD-SCVs revealed that various mutations occurred in thyA, most of which caused stop mutations in the gene (Table 3). In order to prove that the observed phenotypes of TD-SCVs were due to thyA mutations, the entire thyA gene from S. aureus 8325-4 was cloned into a xylose-inducible expression vector, which was subsequently transformed into three clinical TD-SCVs. The three S. aureus TD-SCVs complemented with the functional thyA gene were indistinguishable from the isogenic normal strains (exemplified by SCV-1) (Fig. 1). Induction by xylose did not affect the phenotype on Columbia blood agar, showing the “leaky” behavior of the pCX19 vector (data not shown). However, if the complemented TD-SCVs lost the plasmid after several subcultures, the strains displayed the SCV phenotype again. To ensure that the altered phenotype depended on the expression of ThyA, an empty vector lacking thyA (Table 1) was transformed into the TD-SCVs. This transformation did not change the SCV phenotype (Fig. 1). Thus, we confirmed that the observed TD-SCV phenotypes were due to the presence of various mutations in the thyA nucleotide sequence, as the SCV phenotypes could be reverted to normal by complementation of the TD-SCVs with functional thyA.
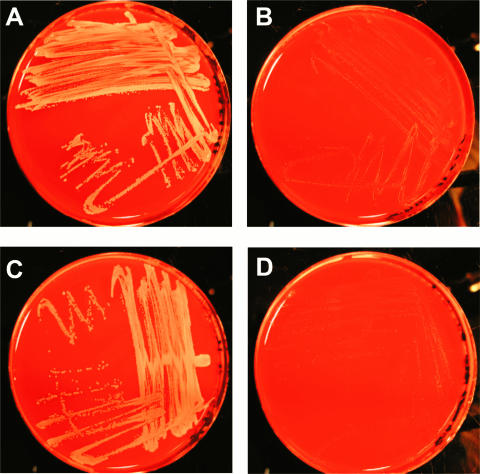
FIG. 1.
Clinical TD-SCV is phenotypically complemented by a functional thyA gene. (A) Normal S. aureus grown on Columbia blood agar. (B) TD-SCV grown on Columbia blood agar exhibiting the SCV phenotype with small, less pigmented colonies. (C) TD-SCV transformed with pCX19thyA expressing functional ThyA, leading to the normal phenotype with large, pigmented colonies. (D) TD-SCV transformed with pCX19empty with the SCV phenotype, indicating the importance of functional ThyA for reversion to the normal phenotype. The images are representative of three sets of TD-SCV, normal S. aureus, and SCV/pCX19thyA strains.
Complementation with thyA reversed the phenotype, growth, and transcription patterns of TD-SCVs.
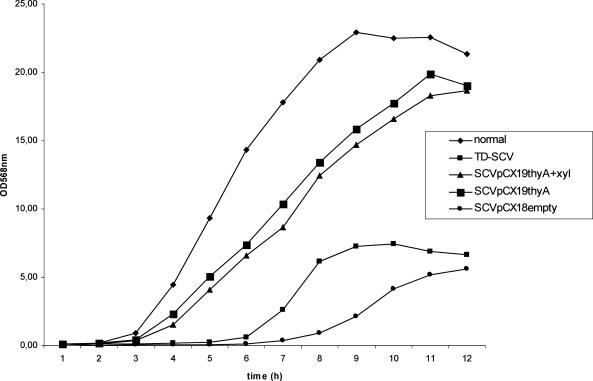
Since in TD-SCVs there are significant changes in important virulence regulators and their target genes (12), transcription of the important virulence regulator agr (encoding accessory gene regulator) and its target virulence genes, hla (encoding alpha-toxin) and spa (encoding protein A), was determined in normal, TD-SCV, the TD-SCV/pCXthyA, and TD-SCV/pCXthyA strains induced by xylose. Prior to RNA extraction, a bacterial growth analysis of the complemented strain with and without xylose was performed, and this strain displayed the same characteristics as the normal strain (Fig. 2). Interestingly, the log phase of the SCV/pCXthyA strain without xylose and the xylose-induced SCV/pCXthyA strain started after 2 h at the same time as the log phase of the normal strain, and the bacteria reached almost the same cell density (OD568, 20) as the normal strain. In contrast, the log phase of the SCV/pCXempty strain was even more delayed than the log phase of the TD-SCV strain, and the SCV/pCXempty strain reached only the cell density of the TD-SCV strain (OD568, 5), indicating the importance of functional ThyA for growth and replication.
FIG. 2.
Analysis of growth of normal S. aureus, TD-SCV, SCV/pCX19thyA, SCV/pCXthyA induced with xylose, and SCV/pCX19empty. Bacteria were grown in BHI broth with or without 0.5% xylose on a rotary shaker at 35°C. Every hour, the OD568 of the cultures were determined. The data are representative of two sets of TD-SCV, normal S. aureus, and SCV/pCX19thyA strains.
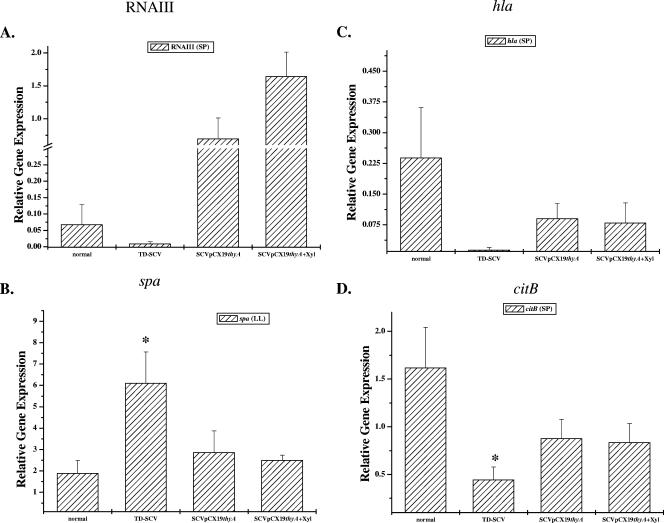
As the transcription of agr, the transcription of hla, and the transcription of spa are differentially regulated (the transcript levels of agr and hla increase during stationary phase, while spa transcription is inhibited), the results for agr and hla transcription were analyzed during stationary phase and the results for spa were analyzed during late log phase using real-time RT-PCR. The transcription levels of RNAIII, the effector gene of agr (Fig. 3A), and hla (Fig. 3C) were increased in the SCV/pCX19thyA strain compared to the TD-SCV strain, while the level of spa was decreased compared to the level in the TD-SCV strain (Fig. 3B). These transcription patterns were very similar to that of the normal strain, indicating that there was complementation of the TD-SCV strain by thyA for these virulence genes. Recently, we showed that enhanced stationary-phase survival of TD-SCVs was associated with an impaired TCA cycle, which is important for entry into the bacterial death phase (4, 5, 28). To analyze if thyA is also able to complement this important metabolic pathway, we determined the transcriptional levels of aconitase (citB), the first enzyme of the TCA cycle in S. aureus. As shown previously (5), citB transcription was significantly decreased in the TD-SCVs compared to the normal strain (Fig. 3D). As expected, the transcriptional level of citB in the SCV/pCX19thyA strain partially reverted back to the level in the normal strain, indicating that there was complementation of this important metabolic pathway by thyA. Xylose induction of the TD-SCV/pCX19thyA strain had an effect only on RNAIII transcription. All other genes tested showed the same transcriptional levels in the TD-SCV/pCX19thyA strain with xylose as in the TD-SCV/pCX19thyA strain without xylose.
FIG. 3.
Real-time RT-PCR quantification of expression in normal, TD-SCV, and SCV/pCX19thyA with or without 0.5% xylose of RNAIII as the effector gene for agr (A), hla (C), and citB (D) in stationary phase (SP) and of spa in late log phase (LL) (B). The levels of mRNA expression of the different genes were normalized using the expression of an internal control, yqiL. The transcript quantities are expressed as increases (n-fold) relative to the values for the internal control. The data are means and standard errors of the means obtained in three independent experiments. Asterisk, P < 0.05 compared to the normal strain (t test).
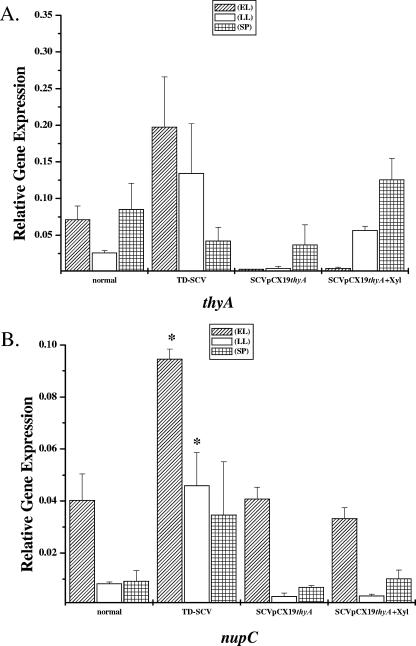
Furthermore, we wanted to know if thyA is expressed in the TD-SCVs. The levels of thyA transcript expression were highest during stationary phase (Fig. 4A). Compared to the normal strain, thyA expression of the TD-SCV strain was higher during the early log and late log growth phases, with the highest level of expression during early log phase (Fig. 4A). As in the normal strain, transcription of thyA in the SCV/pCX19thyA strain was highest during stationary phase but reached the level of thyA transcription in the late log and stationary phases in the normal strain only if it was induced with xylose (Fig. 4A).
FIG. 4.
Real-time RT-PCR quantification of expression in normal, TD-SCV, and SCV/pCX19thyA with or without 0.5% xylose of thyA (A) and nupC (B) in early log phase (EL), late log phase (LL), and stationary phase (SP). The levels of mRNA expression of the different genes were normalized using the expression of an internal control, yqiL, and the transcript quantities are expressed as increases (n-fold) relative to the values for the internal control. The data are means and standard errors of the means obtained in three independent experiments Asterisk, P < 0.05 compared to the normal strain (t test).
Since thyA is essential for survival, TD-SCVs need to take up thymidine from an extracellular site. To accomplish this, thymidine has to be transported into the bacterial cell. One important transporter for nucleotides is NupC (25). As assessed by real-time RT-PCR, expression of nupC peaked during early log phase in the normal strain (Fig. 4B). Compared to the normal strain, nupC in the TD-SCV strain showed increased expression during all growth phases, with the highest level during late log phase (Fig. 4B). In contrast, nupC expression in the SCV/pCX19thyA strain exhibited the same pattern as nupC expression in the normal strain, with the highest level of expression during early log phase and low levels of expression during the late log and stationary phases (Fig. 4B). Xylose induction of the SCV/pCX19thyA strain did not affect the levels of nupC expression.
DISCUSSION
TD-SCVs emerge during long-term treatment with SXT in the airways of CF patients, are resistant to SXT, and are often isolated together with the normal S. aureus strains. Often, TD-SCVs survive with the isogenic normal S. aureus strains for years even in the absence of antibiotic pressure, most likely due to optimized adaptation to the hostile environment (14). In this study, we tried to determine the underlying mechanism of TD-SCVs. To do this, we sequenced thyA, the gene which we hypothesized is affected in TD-SCVs due to a lack of THF, as THF is not synthesized in bacteria if patients are treated with SXT.
Sequence analysis of thyA in TD-SCVs and the isogenic normal strains revealed that various mutations occurred at different positions in the gene. These data are in line with data of Besier et al., who recently described a great variety of mutations in thyA in TD-SCVs (1). Unexpectedly, in four normal strains one to three point mutations occurred at different loci compared to the TD-SCVs, and all of them resulted in nonsynonymous changes in amino acids. Since the normal strains were also under antibiotic pressure during SXT therapy of the patients, such changes probably occur as a response to this selective pressure. It is not known if the activity of the protein is affected by these changes. Surprisingly, in our study, most mutations in TD-SCVs were scattered around the dUMP-binding site. This may be due to the fact that the dUMP-binding site has been described as the active site of the protein in other bacteria and species (7). Therefore, we assume that mutations occurring at this site have the most dramatic effects on the activity of the protein. Although thyA is severely mutated in TD-SCVs, this gene is transcribed at high levels. These results could be explained as follows. First, thymidine synthesis is tightly regulated, and increasing thyA expression occurs in TD-SCVs due to the missing negative feedback by thymidine. Second, mutations in the dUMP-binding site in TD-SCVs prevent binding of dUMP, which then is not captured by the more abundant nonfunctional protein and thus is available for other important pathways. Finally, increased transcription of thyA leading to increased translation of the protein could also be important to overcome the inactivity of the protein.
Interestingly, we detected one in-frame deletion, which occurred at the same site in two independent SCVs and occurred in a region where three asparagines were encoded in the gene. Such deletions are most likely due to slipped-strand mispairing, which seems to occur in combination with inadequate DNA mismatch repair systems (31). In this way, repeats can be deleted or inserted during DNA polymerase-mediated DNA duplication depending on the orientation of the strand. However, it is not clear if this in-frame deletion leads to an inactive protein.
As we detected various mutations in thyA in the clinical TD-SCVs, we tried to determine the impact of thyA by transforming clinical SCVs with a functional thyA gene. Supporting our hypothesis, transformation of TD-SCVs with a vector expressing ThyA changed the phenotype to that of the normal strain. These results were corroborated by the fact that the SCV phenotype of TD-SCVs did not revert to normal if SCVs were transformed with an empty expressing vector and by the fact that the SCV phenotype emerged again if TD-SCVs were cured of the ThyA-expressing vector. Moreover, replication of TD-SCVs transformed by pCX19thy was fully complemented with the typical growth phenotype of normal strains, and a cell density that was the same as that of the normal strain was reached. Interestingly, these results differ from the growth phenotype of TD-SCVs which were supplemented with thymidine (12). Supplementation of TD-SCVs with thymidine did not affect the lag phase of the SCVs, which was extended in the supplemented SCVs, as it was in the TD-SCVs. Only if the supplemented TD-SCVs reached the log phase did SCVs revert to the normal phenotype and reach the same cell density as the normal strain. This observation indicates that thyA affects more important functions required for proper bacterial growth. Interestingly, most effects analyzed in the SCV/pCX19thyA mutant were present without induction by xylose, indicating that the pCX19 vector is really leaky.
Mutations in essential genes, such as thyA, are usually lethal for bacteria (23). The emergence of TD-SCVs that has been described to occur in vivo for S. aureus, E. coli, and Salmonella after treatment with SXT (8, 11, 18, 27, 30) is possible only if bacteria have access to external thymidine. In the presence of thymidine, thyA mutations can occur due to uptake of extracellular thymidine. Since nucleotides do not pass through bacterial cell walls and membranes by diffusion, uptake is an active process, which is accomplished by nucleotide transporters, such as nupC (25). Accordingly, we studied the expression of nupC using real-time RT-PCR, and this expression was shown to be increased in the TD-SCVs compared to the normal strain and decreased in the SCV/pCX19thyA strain.
In conclusion, mutations in thyA have been shown to be responsible for the SCV phenotype in TD-SCVs. By transforming clinical TD-SCVs with a vector expressing ThyA in trans, we convincingly showed that a functional thyA gene is necessary and sufficient to complement the phenotype, growth characteristics, expression of genes for virulence regulators and virulence genes, and also important metabolic pathways.
Acknowledgments
This work was supported by an Innovative Research Grant from the Medical Faculty, University of Münster (grant KA-1-1-05-02 to B.C.K.) and by grants from Deutsche Forschungsgemeinschaft (grant KA2249/1-3 to B.C.K and grant He1850/8-1 to M.H.).
We thank Barbara Ritzerfeld and Barbara Grünastel for expert and enthusiastic technical assistance.
Footnotes
Published ahead of print on 28 September 2007.
REFERENCES
- 1.Besier, S., A. Ludwig, K. Ohlsen, V. Brade, and T. A. Wichelhaus. 2007. 2007. Molecular analysis of the thymidine-auxotrophic small colony variant phenotype of Staphylococcus aureus. Int. J. Med. Microbiol. 297217-225. [DOI] [PubMed] [Google Scholar]
- 2.Besier, S., M. C. von Smaczny, A. Krahl, H. Ackermann, V. Brade, and T. A. Wichelhaus. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 45168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreras, C. W., and D. V. Santi. 1995. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 64721-762. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee, I., P. Becker, M. Grundmeier, M. Bischoff, G. A. Somerville, G. Peters, B. Sinha, N. Harraghy, R. A. Proctor, and M. Herrmann. 2005. Staphylococcus aureus ClpC is required for stress resistance, aconitase activity, growth recovery, and death. J. Bacteriol. 1874488-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee, I., M. Herrmann, R. A. Proctor, G. Peters, and B. C. Kahl. 2007. Enhanced post-stationary-phase survival of a clinical thymidine-dependent small-colony variant of Staphylococcus aureus results from lack of a functional tricarboxylic acid cycle. J. Bacteriol. 1892936-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation. 2004. Patient registry. Annual data report. Cystic Fibrosis Foundation, Bethesda, MD.
- 7.Finer-Moore, J. S., A. C. Anderson, R. H. O'Neil, M. P. Costi, S. Ferrari, J. Krucinski, and R. M. Stroud. 2005. The structure of Cryptococcus neoformans thymidylate synthase suggests strategies for using target dynamics for species-specific inhibition. Acta Crystallogr. D 611320-1334. [DOI] [PubMed] [Google Scholar]
- 8.Gilligan, P. H., P. A. Gage, D. F. Welch, M. J. Muszynski, and K. R. Wait. 1987. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J. Clin. Microbiol. 251258-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmsen, D., H. Claus, W. Witte, J. Rothgänger, H. Claus, D. Turnwald, and U. Vogel. 2003. Typing of methicillin-resistant Staphylococcus aureus in the university hospital setting using a novel software for spa-repeat determination and database management. J. Clin. Microbiol. 415442-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain, M., K. Becker, C. von Eiff, J. Schrenzel, G. Peters, and M. Herrmann. 2001. Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. J. Bacteriol. 1836778-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahl, B., M. Herrmann, A. Schulze Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 1771023-1029. [DOI] [PubMed] [Google Scholar]
- 12.Kahl, B. C., G. Belling, P. Becker, I. Chatterjee, K. Wardecki, K. Hilgert, A. L. Cheung, G. Peters, and M. Herrmann. 2005. Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive changes in regulator and virulence gene expression profiles. Infect. Immun. 734119-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahl, B. C., G. Belling, R. Reichelt, M. Herrmann, R. A. Proctor, and G. Peters. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahl, B. C., A. Duebbers, G. Lubritz, J. Haeberle, H. G. Koch, B. Ritzerfeld, M. Reilly, E. Harms, R. A. Proctor, M. Herrmann, and G. Peters. 2003. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 414424-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krismer, B. A. 1999. Studium der Funktion der sekretierten Proteine SceA und SceB, Analyse des Galaktoseoperons galRKET und Konstruktion von Sekretions- und Expressionsvektoren in Staphylococcus carnosus. Thesis, Lienz, Austria.
- 16.Kuhn, G., P. Francioli, and D. S. Blanc. 2006. Evidence for clonal evolution among highly polymorphic genes in methicillin-resistant Staphylococcus aureus. J. Bacteriol. 188169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy, L. R., H. Chmel, G. Bell, and D. Armstrong. 1977. Thymidine-dependent strain of Salmonella oslo selected by trimethoprim-sulfamethoxazole therapy. Am. J. Clin. Pathol. 68307-311. [DOI] [PubMed] [Google Scholar]
- 19.Montorsi, M., K. Islam, and R. Lorenzetti. 1995. Comparison between thymidylate synthase B of Bacillus subtilis ATCC6633 and 168. Biochem. Mol. Biol. Int. 351245-1251. [PubMed] [Google Scholar]
- 20.Novick, R. P. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33155-166. [DOI] [PubMed] [Google Scholar]
- 21.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. J. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4295-305. [DOI] [PubMed] [Google Scholar]
- 22.Sadowska, B., A. Bonar, C. von Eiff, R. A. Proctor, M. Chmiela, W. Rudnicka, and B. Rozalska. 2002. Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. FEMS Immunol. Med. Microbiol. 32191-197. [DOI] [PubMed] [Google Scholar]
- 23.Samanta, H. K., and S. B. Bhattacharjee. 1977. Thymineless death in Escherichia coli. Z. Naturforsch. C 32835-838. [DOI] [PubMed] [Google Scholar]
- 24.Samuelsen, O., H. H. Haukland, B. C. Kahl, C. von Eiff, R. A. Proctor, H. Ulvatne, K. Sandvik, and L. H. Vorland. 2005. Staphylococcus aureus small colony variants are resistant to the antimicrobial peptide lactoferricin B. J. Antimicrob. Chemother. 561126-1129. [DOI] [PubMed] [Google Scholar]
- 25.Saxild, H. H., L. N. Andersen, and K. Hammer. 1996. dra-nupC-pdp operon of Bacillus subtilis: nucleotide sequence, induction by deoxyribonucleosides, and transcriptional regulation by the deoR-encoded DeoR repressor protein. J. Bacteriol. 178424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seggewiss, J., K. Becker, O. Kotte, M. Eisenacher, M. R. Yazdi, A. Fischer, P. McNamara, L. N. Al, R. Proctor, G. Peters, M. Heinemann, and C. von Eiff. 2006. Reporter metabolite analysis of transcriptional profiles of a Staphylococcus aureus strain with normal phenotype and its isogenic hemB mutant displaying the small-colony-variant phenotype. J. Bacteriol. 1887765-7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert, H., C. von Eiff, and G. Fatkenheuer. 1999. Fatal case due to methicillin-resistant Staphylococcus aureus small colony variants in an AIDS patient. Emerg. Infect. Dis. 5450-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somerville, G. A., M. S. Chaussee, C. I. Morgan, J. R. Fitzgerald, D. W. Dorward, L. J. Reitzer, and J. M. Musser. 2002. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 706373-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somerville, G. A., A. Cockayne, M. Durr, A. Peschel, M. Otto, and J. M. Musser. 2003. Synthesis and deformylation of Staphylococcus aureus delta-toxin are linked to tricarboxylic acid cycle activity. J. Bacteriol. 1856686-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanner, E. I., and C. H. Bullin. 1974. Thymidine-dependent Escherichia coli infection and some associated laboratory problems. J. Clin. Pathol. 27565-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Belkum, A., A. Scherer, L. van Alphen, and H. Verbrugh. 1998. Short-sequence DNA repeats in prokaryotic genomes. Microbiol. Mol. Biol. Rev. 62275-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vann, J. M., and R. A. Proctor. 1988. Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: role of S. aureus alpha-hemolysin. Microb. Pathog. 4443-453. [DOI] [PubMed] [Google Scholar]
- 33.von Eiff, C., K. Becker, D. Metze, G. Lubritz, J. Hockmann, T. Schwarz, and G. Peters. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 321643-1647. [DOI] [PubMed] [Google Scholar]
- 34.Yao, J. D. C., and R. C. Moellering. 2003. Antibacterial agents and susceptibility test methods, p. 1039-1073. In P. R. Murray, E. J. Baron, J. H. Jorgensen M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.