Abstract
Phylogenetic analysis confirmed that two genes required for acetoclastic methanogenesis, ackA and pta, were horizontally transferred to the ancestor of Methanosarcina from a derived cellulolytic organism in the class Clostridia. This event likely occurred within the last 475 million years, causing profound changes in planetary methane biogeochemistry.
Methanogenesis from acetate (acetoclastic methanogenesis) is responsible for approximately two-thirds of the biogenic methane produced annually on Earth (20), primarily in aquatic environments such as lake, river, and marine sediments, wetlands, soils, and the gastrointestinal tracts of animals (5, 9, 10). This metabolic process is performed exclusively by methanogenic euryarchaea of the order Methanosarcinales by activating acetate into acetyl-coenzyme A (acetyl-CoA), from which a methyl group is then transferred into the central methanogenic pathway (9). While members of the family Methanosaetaceae perform this step using the acetyl-CoA synthetase pathway (20), members of the genus Methanosarcina use a different pathway consisting of acetate kinase (AckA) and phosphoacetyl transferase (Pta) for the synthesis of acetyl-CoA:
 |
(1) |
 |
(2) |
The wider environmental distribution and greater physiological and metabolic diversity of Methanosarcina and the favorable kinetics of the AckA/Pta pathway at high concentrations of acetate (13) imply that this pathway is responsible for the majority of acetoclastic methanogenesis on Earth.
Using acetyl-CoA, the carbon monoxide dehydrogenase complex then catalyzes the release of CoA and the transfer of CH3 to tetrahydromethanopterin or tetrahydrosarcinapterin. The methyl group is then transferred to CoM by CoM methyltransferase and reduced to CH4 by methyl-CoM reductase (17). Carbon monoxide dehydrogenase, also known as CO dehydrogenase/acetyl-CoA synthase (Cdh), is widely distributed across the archaeal and bacterial domains and is present in nonacetoclastic methanogens; however, in these organisms it typically catalyzes a reaction in the reverse direction, synthesizing acetyl-CoA to be used in anabolic pathways.
Surprisingly, neither AckA nor Pta has any homologs in other methanogens or even in any other archaeal species. However, the genes encoding these proteins are widely distributed among the bacteria and have also been reported to be present in various eukaryotic lineages (12), presumably due to transfer from diverse groups of bacteria in several independent events. In bacteria, AckA and Pta are used to activate acetate for use as a carbon source or to produce acetate as an electron sink during energy production via fermentation (20), specifically using cellulose-derived sugars in the case of cellulolytic clostridia (6, 20).
Using several phylogenetic techniques, we showed that ackA and pta were acquired by the ancestor of Methanosarcina in a single horizontal gene transfer event, likely from a clade of cellulolytic bacteria belonging to the class Clostridia. So far, this is the only component of a methanogenic pathway found to have evolved via horizontal gene transfer.
For global phylogenetic analysis, 539 homologs of AckA and 303 homologs of Pta were identified via a BLAST (2) search of all completed microbial genomes in the GenBank database (4), using the corresponding homologs from Methanosarcina acetivorans (gi 19917661 and gi 20092407) as queries. All sequences annotated as “acetate kinase” or “phosphate acetyltransferase” were used, and all of them showed highly significant E values (E < 10−5).
For local trees of AckA and Pta within Clostridia, corresponding gene homologs were identified via a BLAST search of all Clostridia genomes in the GenBank database, using the methodology indicated above. Archaeal homologs of AckA and Pta from available Methanosarcina genomes (Methanosarcina acetivorans, Methanosarcina mazei, Methanosarcina barkeri) were also included. AckA and PtaA homologs from the firmicute Fusobacterium nucleatum subsp. nucleatum ATCC 25586 were included in each case as an outgroup.
Global phylogenetic trees for both Pta (Fig. 1A) and AckA (Fig. 1B) show that the Pta and AckA genes are distributed throughout the bacterial domain, clustering into major groups that suggest an evolutionary history largely dominated by vertical inheritance. The distributions are similar for these two proteins, although the different numbers of homologs identified in each BLAST search indicate that in many lineages independent gene losses and/or duplications have occurred. In both cases, homologs from Methanosarcina were shown to root within the bacterial class Clostridia on a relatively short branch, suggesting that there was a horizontal gene transfer event from within this clade.
FIG. 1.
Maximum likelihood phylogenetic trees of Pta and AckA homologs. Homologs found in Methanosarcina are red and in both cases are found in a monophyletic group corresponding to the Clostridia (blue). Sequences were aligned using the MUSCLE algorithm (default settings) (7). Phylogenetic reconstruction was performed using Phyml (11), with estimated portions of invariable sites and a single substitution rate category.
Consensus trees of both Pta (Fig. 2A) and AckA (Fig. 2B) homologs within the clostridia show strong support for horizontal gene transfer from a group of cellulolytic clostridia including Clostridium cellulolyticum and Clostridium thermocellum. None of the sequences included in Fig. 2 failed the test for compositional homogeneity as implemented in Tree-Puzzle (15). Phylogenetic analysis using PhyloBayes 2.3 (13), which incorporates amino acid frequency vectors into its substitution models, produced results in agreement with the results of these methods, supporting the hypothesis that the resulting grouping is not an artifact due to biases in amino acid usage (data not shown). Furthermore, the multiple-sequence alignment generated for Pta shows several signature positions shared by Methanosarcina and cellulolytic clostridia (see Fig. S1 in the supplemental material). The consensus tree for Pta also provides strong support for the hypothesis that Clostridium phytofermentans and Caldicellulosiruptor saccharolyticus belong to the same monophyletic group within the Clostridia. Internal nodes in the AckA consensus tree have much lower support values, although a similar group (including C. saccharolyticus but not C. phytofermentans) has a high posterior probability.
FIG. 2.
Consensus trees of Pta and AckA homologs within the Clostridia. Branches in which there were postulated horizontal gene transfers to Methanosarcina are indicated by an asterisk. Homologs from the firmicute F. nucleatum were used as outgroups to root the trees. The numbers associated with each clade indicate bootstrap values for maximum likelihood and neighbor joining and the posterior probability from Bayesian inference, respectively. Sequence alignment was performed using M-Coffee (18) with default parameters. Maximum likelihood phylogenetic reconstruction was performed with Phyml (11), with estimated portions of invariable sites, four substitution rate categories, and an estimated gamma distribution parameter. For neighbor joining, 100 bootstrapped replicates were generated from the M-Coffee alignment using SEQBOOT from the PHYLIP package (8). Trees were then generated using PROTDIST (using an exponential rate distribution), followed by NEIGHBOR (default settings). Results were combined into a tree with bootstrap support using CONSENSE. Posterior probability trees were generated using MrBayes, version 3.1.2 (1, 14), a fixed jones amino acid substitution model, four rate categories following a gamma distribution, four chains, an alpha of 1, a swap frequency of 1, a random starting tree, and 500,000 generations, sampling every 50th generation. The first 2,000 generations were removed from the analysis as burn-in. The trees were then combined into a consensus tree using the CONSENSE program. Bootstrap (and posterior probability) values for each node from the previously described analyses were then applied. Tests of sequence heterogeneity and branch lengths for the consensus trees were calculated using TreePuzzle (15) with the following settings: user-defined trees, exact parameter estimates, parameters estimated by quartet sampling+neighbor joining, estimated gamma distribution rates, and four categories.
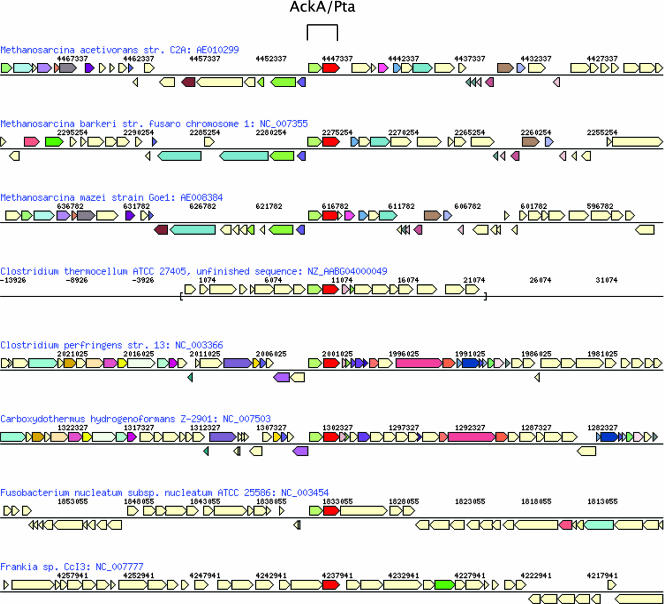
With the exception of Moorella thermoacetica and Desulfitobacterium hafniense Y51, all completed Clostridia genomes contain both ackA and pta homologs (see Table S1 in the supplemental material). In all genomes with both ackA and pta, the genes are adjacent and in the same order as the homologs in Methanosarcina (Fig. 3), although additional lone copies of each gene may be present elsewhere in the genome (e.g., ackA in Thermoanaerobacter ethanolicus X514). Therefore, it is likely that the absence of ackA or pta in some genomes is due to gene loss and not incomplete sequencing. Given this assumption, we can infer that the presence of adjacent ackA/pta genes was very likely the ancestral state of the clade from which these genes were transferred. This agrees with the consensus phylogenies in implicating a single transfer event for the acquisition of ackA and pta by methanogenic archaea.
FIG. 3.
Gene synteny of ackA/pta. Genes encoding homologs of AckA and Pta are adjacent in Methanosarcina (first three rows), as well as in clostridia that contain both genes (all Clostridium species not shown). The genes are not adjacent in most other bacteria, including the firmicute Frankia (bottom row). Another firmicute closely related to the clostridia, F. nucleatum (second row from the bottom), shows a pattern similar to that of the clostridia. This is in agreement with the hypothesis that a single horizontal gene transfer event occurred between these two clades. No other genes adjacent to ackA or pta in clostridia appeared to have been transferred into Methanosarcina.
As suggested by our phylogenetic analyses, the source of the transferred ackA and pta genes in the ancestor of Methanosarcina was likely a cellulolytic clostridium belonging to a sister group of the C. thermocellum/C. cellulolyticum clade. Close physical interactions between methanogens and clostridia are common (16), and gene transfer between them is abundant (3). Furthermore, modern representatives of Methanosarcina and cellulolytic clostridia often coexist in the same environment, especially in freshwater systems. For these reasons, horizontal gene transfer between these two groups is biologically plausible.
Cellulolytic enzymatic activity is a shared derived characteristic of the clade from which ackA and pta were transferred to Methanosarcina. This allows speculative dating of the transfer event. While cellulose synthesis is found in some bacteria and algae, the gene transfer event occurred after the cellulolytic clostridia had undergone significant diversification. This suggests the existence of a diverse, cellulose-rich, freshwater environment, which presumably required the availability of significant terrestrial plant biomass for fermentation, probably no earlier than the Mid-Ordovician or about 475 million years ago (19).
The ancestral state of the methanogenic pathway in Methanosarcina before the transfer event was likely one of the following two possibilities: (i) absence of an acetoclastic pathway and instead utilization of the numerous other methanogenic substrates available to Methanosarcinales (10); or (ii) presence of an acetoclastic pathway utilizing acetyl-CoA synthetase, which is still found in Methanosaetaceae (13). A further complication is the possibility that Methanosarcina and Methanosaetaceae coevolved after the transfer event, optimizing growth with different acetate concentrations (13). Regardless of the evolutionary scenario, the evolution of the AckA/Pta acetoclastic methanogenic pathway via horizontal gene transfer likely resulted in a significant net increase in the amount of biogenic methane produced, which has had global biogeochemical consequences, possibly including climate change.
Supplementary Material
Acknowledgments
This work was supported through the NASA Applied Information System Research (NNG04GP90G) and Exobiology (NNX07AK15G) Programs.
Footnotes
Published ahead of print on 30 November 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altekar, G., S. Dwarkadas, J. P. Huelsenbeck, and F. Ronquist. 2004. Parallel Metropolis coupled Markov chain Monte Carlo for Bayesian phylogenetic inference. Bioinformatics 20407-415. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 3.Beiko, R. G., T. J. Harlow, and M. A. Ragan. 2005. Highways of gene sharing in prokaryotes. Proc. Natl. Acad. Sci. USA 10214332-14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, and D. L. Wheeler. 2006. GenBank. Nucleic Acids Res. 34D16-D20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boone, D., W. Whitman, and Y. Koga. 2001. Order III. Methanosarcinales, p. 268-294. In D. Boone, G. Castenholz, and G. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer-Verlag, New York, NY. [Google Scholar]
- 6.Desvaux, M. 2005. Clostridium cellulolyticum: model organism of mesophilic cellulolytic clostridia. FEMS Microbiol. Rev. 29741-764. [DOI] [PubMed] [Google Scholar]
- 7.Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 321792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 2005. PHYLIP (Phylogeny Inference Package), 3.6 ed. Department of Genome Sciences, University of Washington, Seattle.
- 9.Ferry, J. G. 1992. Methane from acetate. J. Bacteriol. 1745489-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, P. Macdonald, W. FitzHugh, S. Calvo, R. Engels, S. Smirnov, D. Atnoor, A. Brown, N. Allen, J. Naylor, N. Stange-Thomann, K. DeArellano, R. Johnson, L. Linton, P. McEwan, K. McKernan, J. Talamas, A. Tirrell, W. Ye, A. Zimmer, R. D. Barber, I. Cann, D. E. Graham, D. A. Grahame, A. M. Guss, R. Hedderich, C. Ingram-Smith, H. C. Kuettner, J. A. Krzycki, J. A. Leigh, W. Li, J. Liu, B. Mukhopadhyay, J. N. Reeve, K. Smith, T. A. Springer, L. A. Umayam, O. White, R. H. White, E. Conway de Macario, J. G. Ferry, K. F. Jarrell, H. Jing, A. J. Macario, I. Paulsen, M. Pritchett, K. R. Sowers, R. V. Swanson, S. H. Zinder, E. Lander, W. W. Metcalf, and B. Birren. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52696-704. [DOI] [PubMed] [Google Scholar]
- 12.Ingram-Smith, C., S. R. Martin, and K. S. Smith. 2006. Acetate kinase: not just a bacterial enzyme. Trends Microbiol. 14249-253. [DOI] [PubMed] [Google Scholar]
- 13.Min, H., and S. H. Zinder. 1989. Kinetics of acetate utilization by two thermophilic acetotrophic methanogens: Methanosarcina sp. strain CALS-1 and Methanothrix sp. strain CALS-1. Appl. Environ. Microbiol. 55488-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronquist, F., and J. P. Huelsenbeck. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 191572-1574. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt, H. A., K. Strimmer, M. Vingron, and A. von Haeseler. 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18502-504. [DOI] [PubMed] [Google Scholar]
- 16.Stams, A. 1994. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie van Leeuwenhoek 66271-294. [DOI] [PubMed] [Google Scholar]
- 17.Thauer, R. K. 1998. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture. Microbiology 1442377-2406. [DOI] [PubMed] [Google Scholar]
- 18.Wallace, I. M., O. O'Sullivan, D. G. Higgins, and C. Notredame. 2006. M-Coffee: combining multiple sequence alignment methods with T-Coffee. Nucleic Acids Res. 341692-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellman, C. H., P. L. Osterloff, and U. Mohiuddin. 2003. Fragments of the earliest land plants. Nature 425282-285. [DOI] [PubMed] [Google Scholar]
- 20.White, D. 2000. The physiology and biochemistry of prokaryotes, 2nd ed. Oxford University Press, New York, NY.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.