Abstract
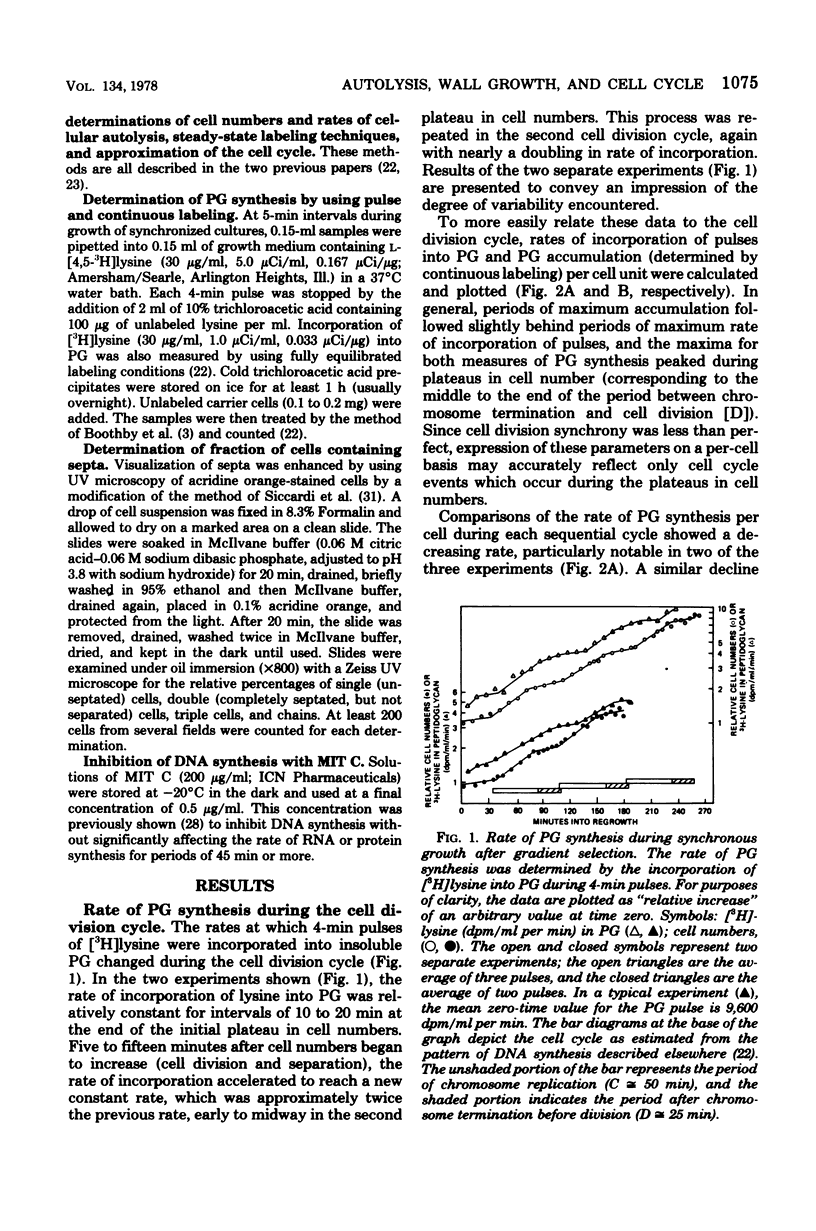
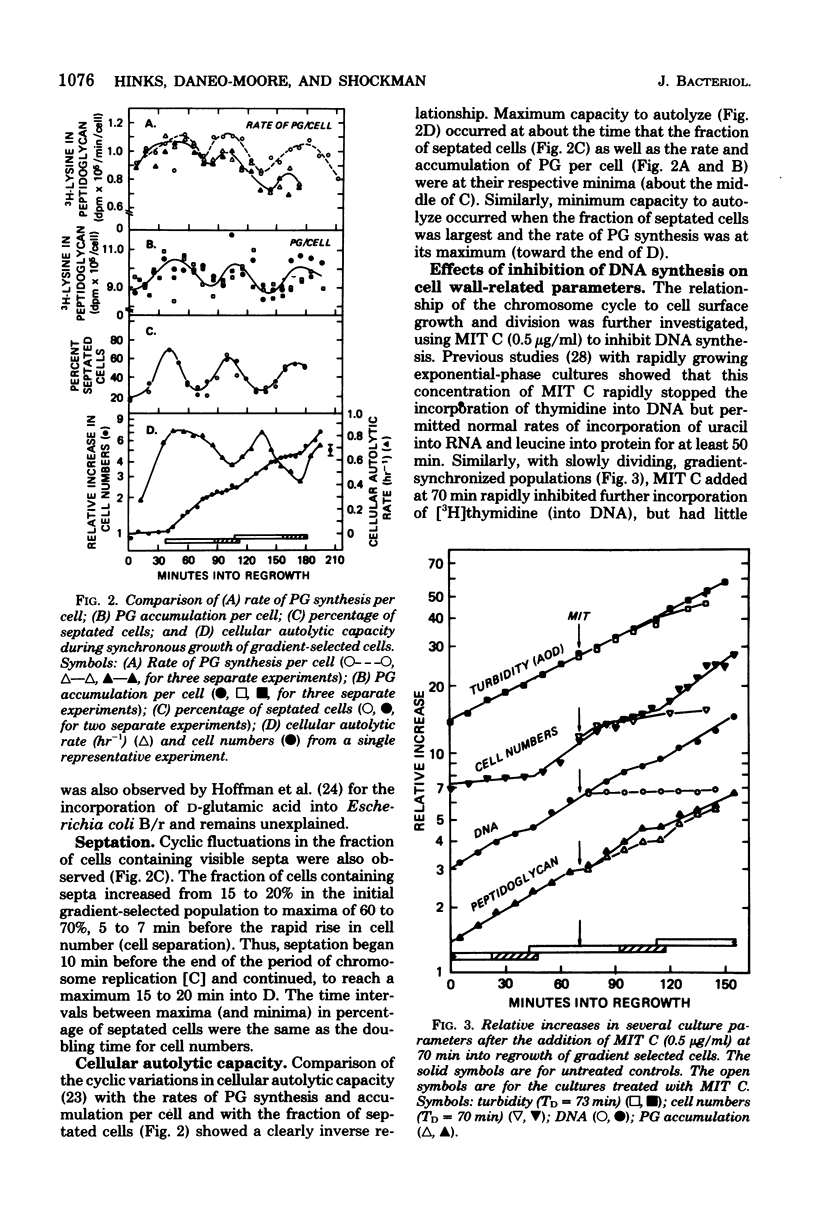
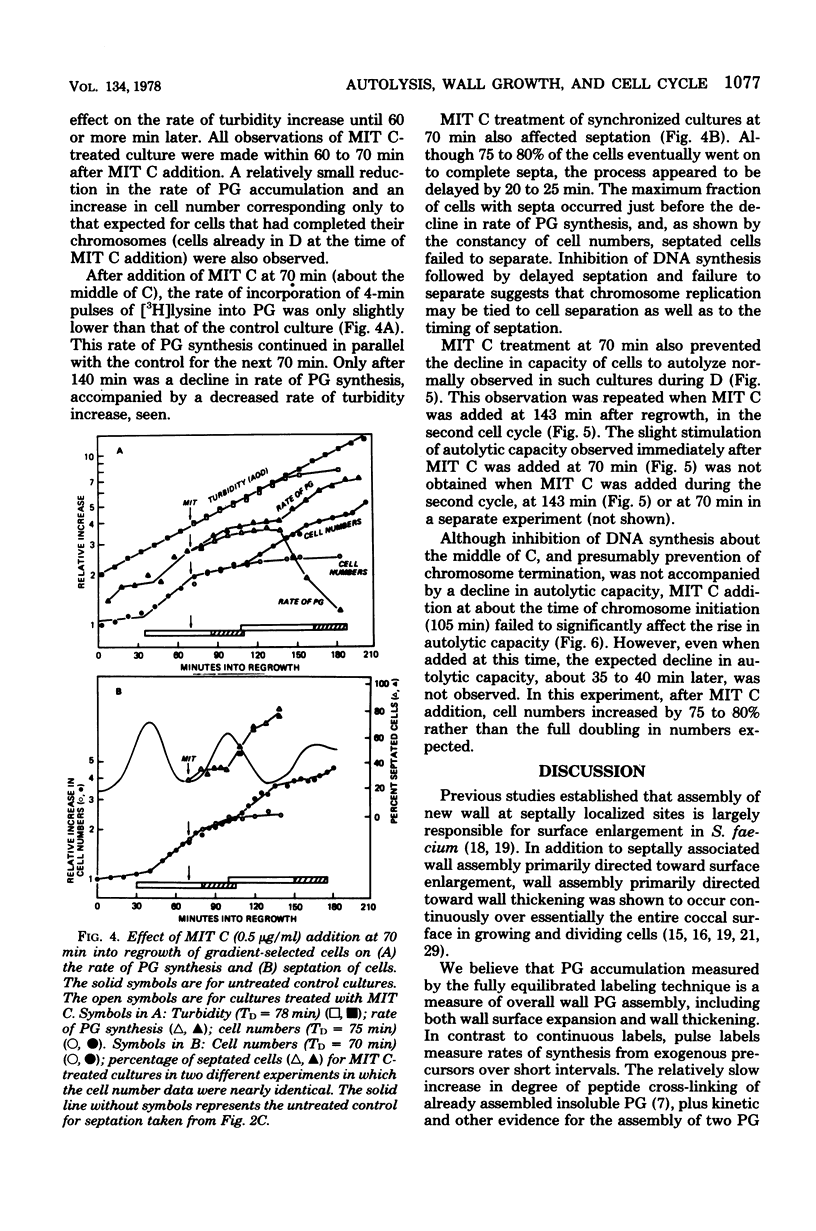
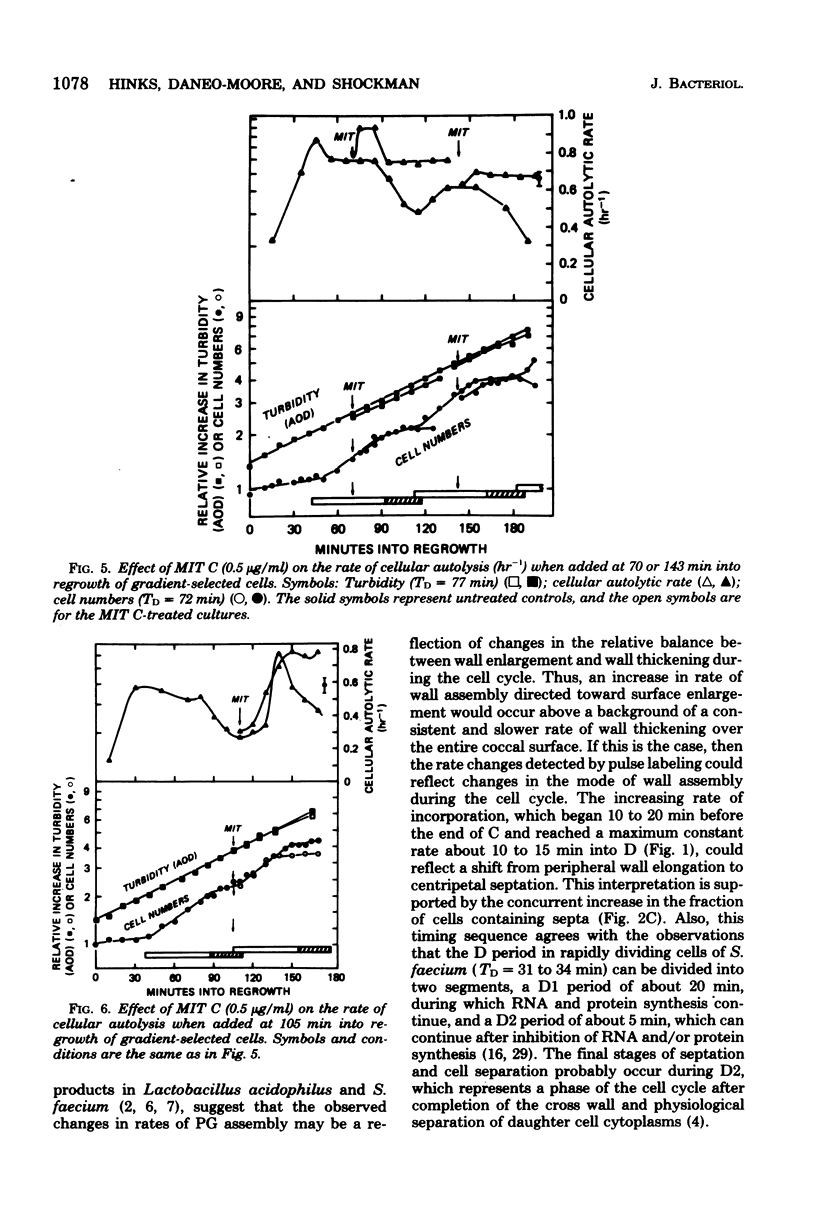
Synchronized, slowly growing (TD = 70 to 80 min) cultures were used to study several wall-associated parameters during the cell cycle: rate of peptidoglycan synthesis, septation, and cellular autolytic activity. The rate of peptidoglycan synthesis per cell declined during most of the period of chromosome replication (C), but increased during the latter part of C and into the period between chromosome termination and cell division (D). An increase in cellular septation was correlated with the increased rate of peptidoglycan synthesis. Cellular autolytic capacity increased during the early portion of C, reached a maximum late in C or early in D, and declined during D. Inhibition of DNA synthesis during C prevented the decline in autolytic capacity at the end of the cell cycle, caused a slight reduction in the rate of peptidoglycan synthesis, delayed but did not prevent septation, and prevented the impending cell division by inhibiting cell separation. Inhibition of DNA synthesis during D did not prevent the increase in autolytic capacity during the next C phase, but, once again, prevented the decline at the end of the subsequent cycle. Thus, increased autolytic capacity at the beginning of the cell cycle did not seem to be related to chromosome initiation, whereas decreased autolytic capacity at the end of the cell cycle seemed to be related to chromosome termination. The data presented are consistent with the role of autolytic enzyme activity in the previously proposed model for cell division of S. faecium (G.D. Shockman et al., Ann. N.Y Acad. Sci. 235:161-197, 1974).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck B. D., Park J. T. Activity of three murein hydrolases during the cell division cycle of Escherichia coli K-12 as measured in toluene-treated cells. J Bacteriol. 1976 Jun;126(3):1250–1260. doi: 10.1128/jb.126.3.1250-1260.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Clark D. J. The regulation of DNA replication and cell division in E. coli B-r. Cold Spring Harb Symp Quant Biol. 1968;33:823–838. doi: 10.1101/sqb.1968.033.01.094. [DOI] [PubMed] [Google Scholar]
- Conover M. J., Thompson J. S., Shockman G. D. Autolytic enzyme of Streptococcus faecalis: release of soluble enzyme from cell walls. Biochem Biophys Res Commun. 1966 Jun 13;23(5):713–719. doi: 10.1016/0006-291x(66)90459-1. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Coyette J., Sayare M., Boothby D., Shockman G. D. Turnover of the cell wall peptidoglycan of Lactobacillus acidophilus. The presence of a fraction immune to turnover. J Biol Chem. 1975 Feb 25;250(4):1348–1353. [PubMed] [Google Scholar]
- Dezélée P., Shockman G. D. Studies of the formation of peptide cross-links in the cell wall peptidoglycan of Streptococcus faecalis. J Biol Chem. 1975 Sep 10;250(17):6806–6816. [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Activity of murein hydrolases and membrane synthesis in synchronized Escherichia coli B/r. Ann Microbiol (Paris) 1974 Sep;125 B(2):163–166. [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Activity of murein hydrolases in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1239–1244. doi: 10.1128/jb.129.3.1239-1244.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Oscillations in the synthesis of cell wall components in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1234–1238. doi: 10.1128/jb.129.3.1234-1238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. I. Requirements for RNA and protein synthesis at different growth rates. J Mol Biol. 1974 Mar 25;84(1):1–19. doi: 10.1016/0022-2836(74)90209-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. II. Analysis of the control mechanism. J Mol Biol. 1974 Mar 25;84(1):21–36. doi: 10.1016/0022-2836(74)90210-1. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. Cell division during inhibition of deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1968 May;95(5):1627–1633. doi: 10.1128/jb.95.5.1627-1633.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Reinitiation of cell wall growth after threonine starvation of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):1175–1183. doi: 10.1128/jb.105.3.1175-1183.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Site of initiation of cellular autolysis in Streptococcus faecalis as seen by electron microscopy. J Bacteriol. 1970 Aug;103(2):504–512. doi: 10.1128/jb.103.2.504-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Approximation of the cell cycle in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Jun;134(3):1188–1191. doi: 10.1128/jb.134.3.1188-1191.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Cellular autolytic activity in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Feb;133(2):822–829. doi: 10.1128/jb.133.2.822-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Marunouchi T., Messer W. Deoxyribonucleic acid replication and cell division in Escherichia coli at 33 C. J Bacteriol. 1972 Dec;112(3):1431–1432. doi: 10.1128/jb.112.3.1431-1432.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Peptidoglycan biosynthesis in a thermosensitive division mutant of Escherichia coli. Biochemistry. 1976 May 4;15(9):1781–1790. doi: 10.1021/bi00654a001. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayare M., Daneo-Moore L., Shockman G. D. Influence of macromolecular biosynthesis on cellular autolysis in Streptococcus faecalis. J Bacteriol. 1972 Oct;112(1):337–344. doi: 10.1128/jb.112.1.337-344.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siccardi A. G., Galizzi A., Mazza G., Clivio A., Albertini A. M. Synchronous germination and outgrowth of fractionated Bacillus subtilis spores: tool for the analysis of differentiation and division of bacterial cells. J Bacteriol. 1975 Jan;121(1):13–19. doi: 10.1128/jb.121.1.13-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]



