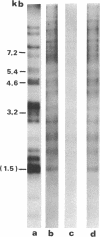
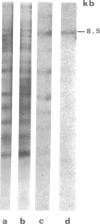
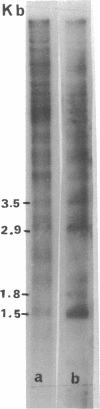
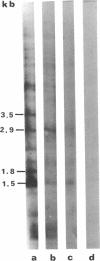
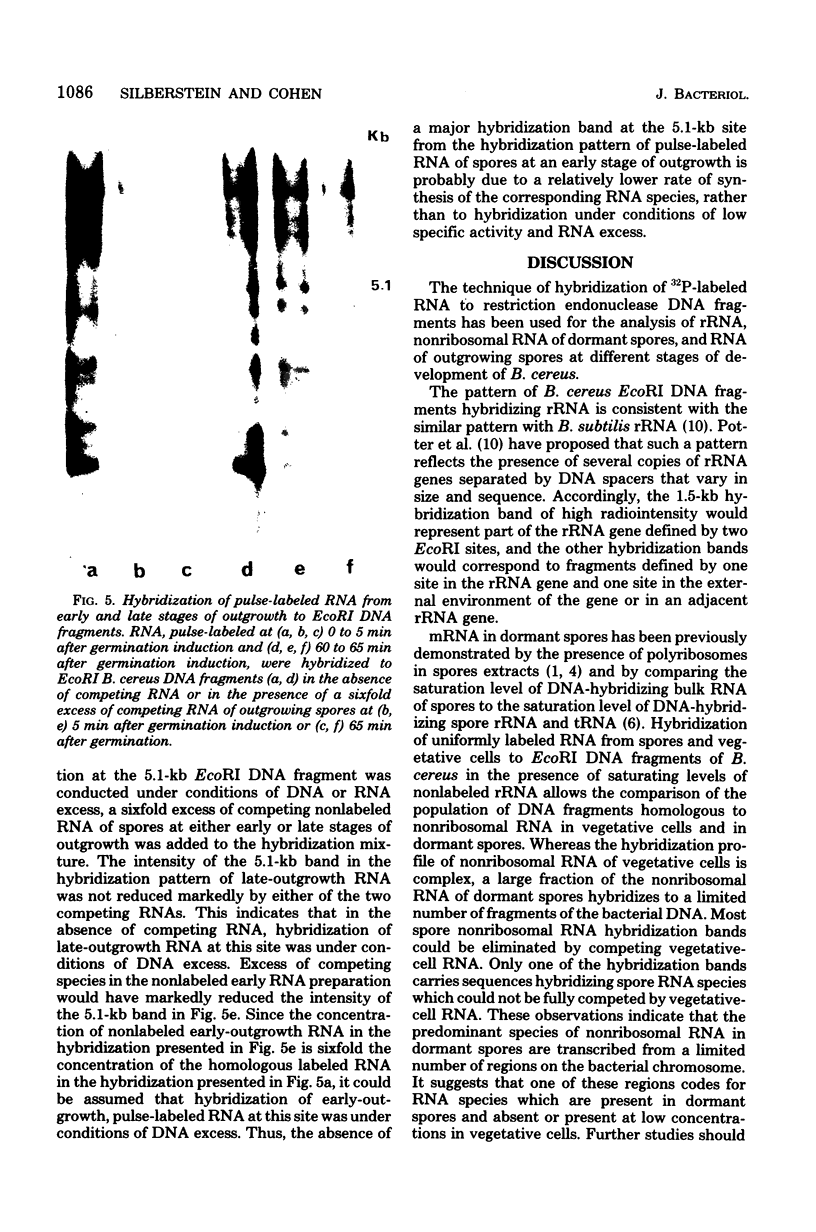
Abstract
Transcribing Bacillus cereus DNA was visualized by means of autoradiography of electrophoretically separated EcoRI restriction endonuclease DNA fragments hybridizing 32P-labeled RNA. Hybridization of RNA of dormant spores, vegetative cells, and outgrowing spores indicates the following. (i) A large fraction of the nonribosomal RNA in dormant spores is transcribed at a limited number of regions on the bacterial chromosome. (ii) After induction of spore germination, transcription activity is not limited to a single short region on the chromosome, but rather is distributed along the chromosome. The DNA/RNA hybridization technique has been used to identify restriction endonuclease DNA fragments homologous to RNA species that are present in dormant spores but absent from vegetative cells, RNA species that are synthesized immediately after germination induction and are present at a relatively low concentration in vegetative cells, and RNA species that are transcribed at a late stage of outgrowth but are absent or present at low concentration at an early stage of outgrowth.
Full text
PDF

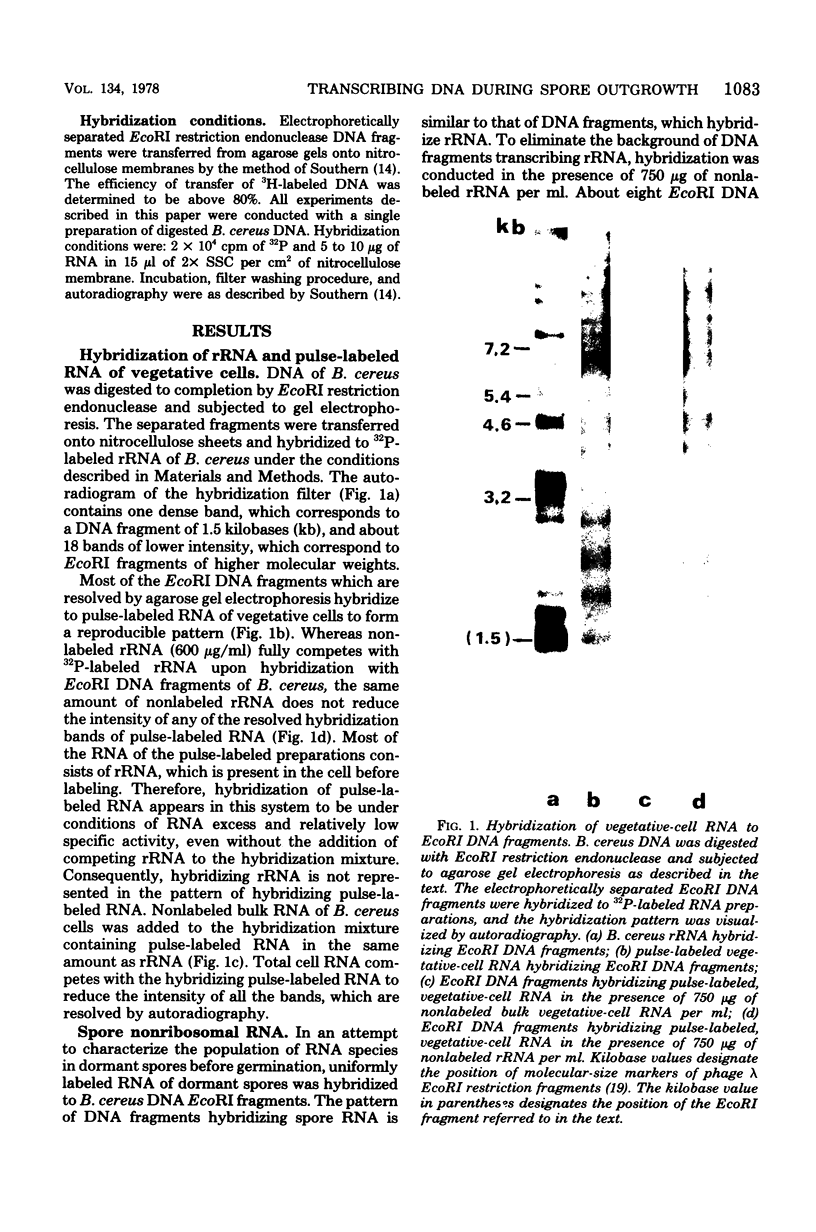


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambon P., Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. X. Ribosomes and nucleic acids of vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5110–5116. [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope mapping of the distribution of ribosomal genes of the Bacillus subtilis chromosome. J Mol Biol. 1973 Apr 5;75(2):265–279. doi: 10.1016/0022-2836(73)90020-x. [DOI] [PubMed] [Google Scholar]
- Feinsod F. M., Douthit H. A. Ribosomes from spores of Bacillus cereus T. Science. 1970 May 22;168(3934):991–991. doi: 10.1126/science.168.3934.991. [DOI] [PubMed] [Google Scholar]
- Hansen J. N., Spiegelman G., Halvorson H. O. Bacterial spore outgrowth: its regulation. Science. 1970 Jun 12;168(3937):1291–1298. doi: 10.1126/science.168.3937.1291. [DOI] [PubMed] [Google Scholar]
- Jeng Y. H., Doi R. H. Messenger ribonucleic acid of dormant spores of Bacillus subtilis. J Bacteriol. 1974 Aug;119(2):514–521. doi: 10.1128/jb.119.2.514-521.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Sueoka N. Gene expression during outgrowth of Bacillus subtilis spores. The relationship between gene order on the chromosome and temporal sequence of enzyme synthesis. J Mol Biol. 1971 Aug 28;60(1):31–44. doi: 10.1016/0022-2836(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Bott K. F., Newbold J. E. Two-dimensional restriction analysis of the Bacillus subtilis genome: gene purification and ribosomal ribonucleic acid gene organization. J Bacteriol. 1977 Jan;129(1):492–500. doi: 10.1128/jb.129.1.492-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E. Multidimensional restriction enzyme analysis of complex genomes. Anal Biochem. 1976 Apr;71(2):452–458. doi: 10.1016/s0003-2697(76)80011-5. [DOI] [PubMed] [Google Scholar]
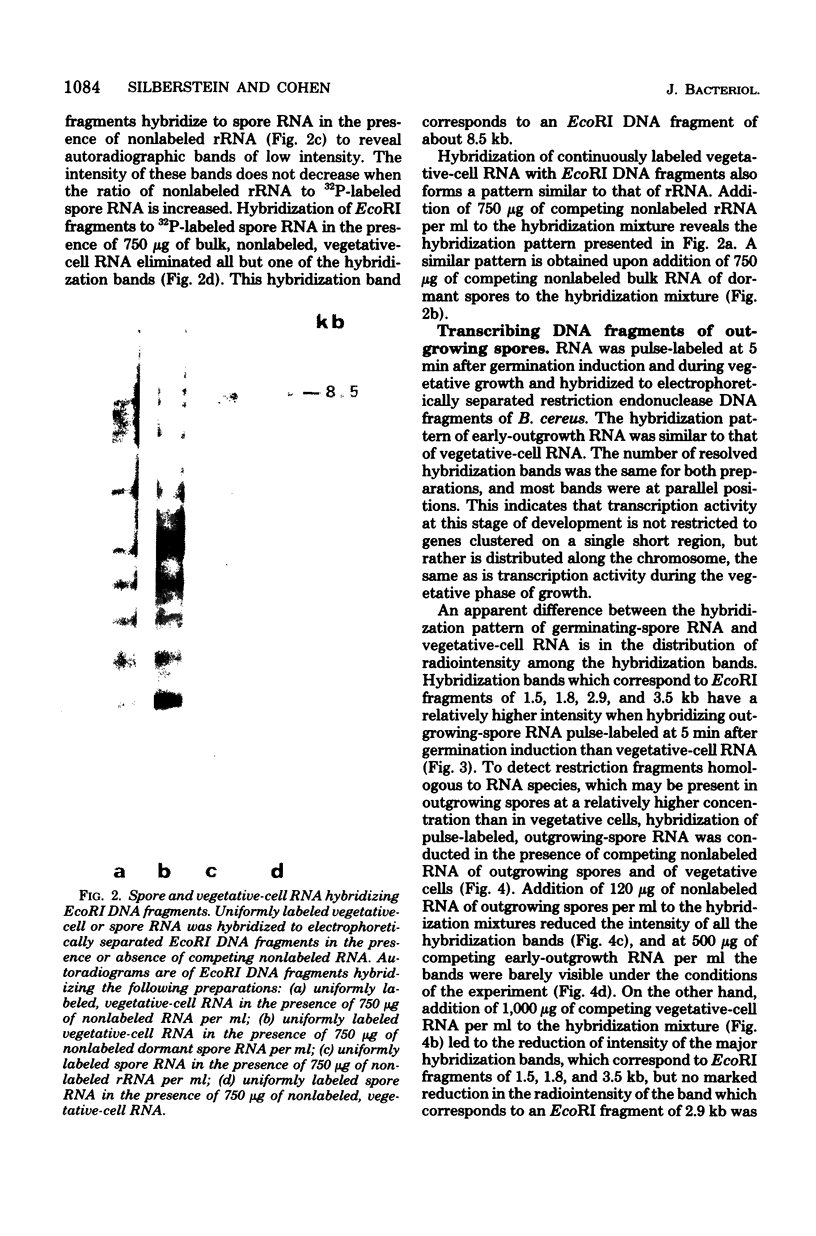
- Segall J., Losick R. Cloned Bacillus subtilis DNA containing a gene that is activated early during sporulation. Cell. 1977 Aug;11(4):751–761. doi: 10.1016/0092-8674(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. I. Ordered enzyme synthesis. J Bacteriol. 1968 Feb;95(2):469–478. doi: 10.1128/jb.95.2.469-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir H., Gilvarg C. Density gradient centrifugation for the separation of sporulating forms of bacteria. J Biol Chem. 1966 Mar 10;241(5):1085–1090. [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Torriani A., Levinthal C. Ordered synthesis of proteins during outgrowth of spores of Bacillus cereus. J Bacteriol. 1967 Jul;94(1):176–183. doi: 10.1128/jb.94.1.176-183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARY J. C., HALVORSON H. O. KINETICS OF GERMINATION OF BACILLUS SPORES. J Bacteriol. 1965 May;89:1340–1347. doi: 10.1128/jb.89.5.1340-1347.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E. C., Steinberg W. The effect of gene position, gene dosage and a regulatory mutation on the temporal sequence of enzyme synthesis accompanying outgrowth of Bacillus subtilis spores. Mol Gen Genet. 1978 Jan 17;158(3):287–296. doi: 10.1007/BF00267200. [DOI] [PubMed] [Google Scholar]