Abstract
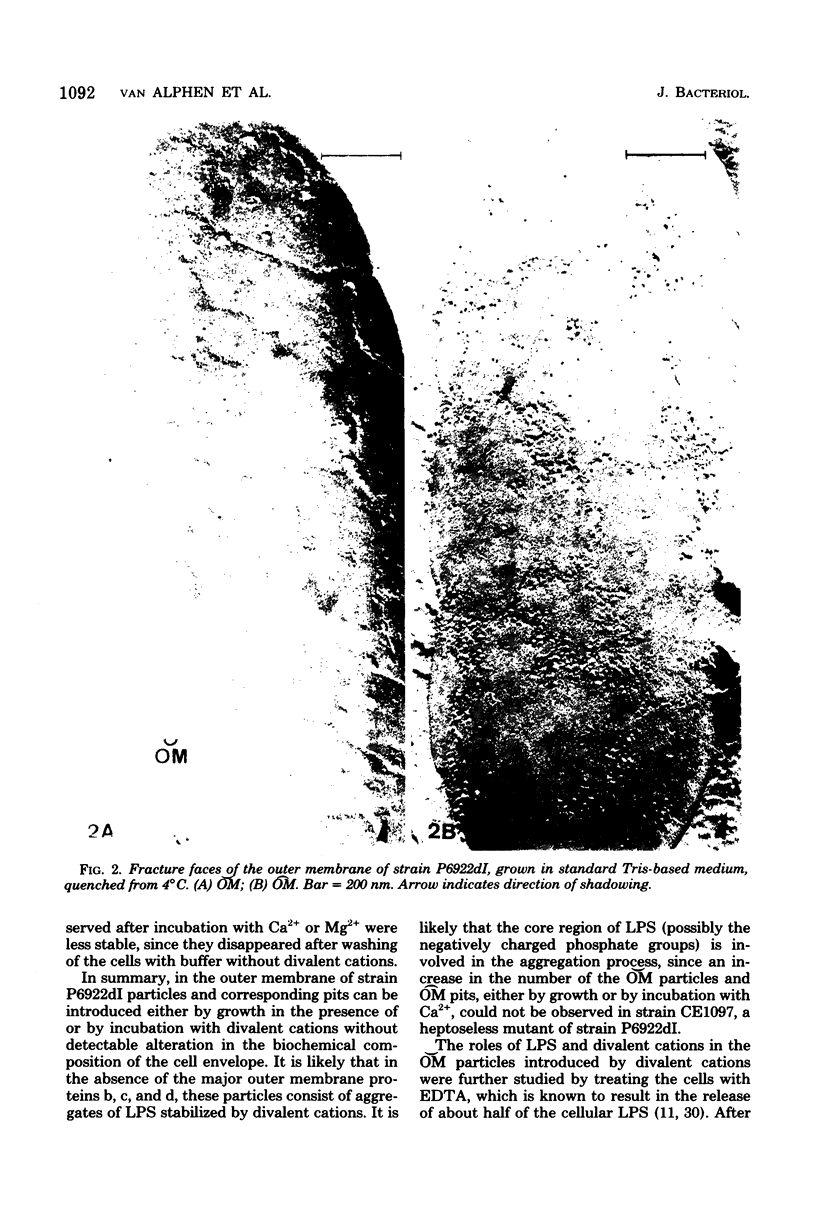
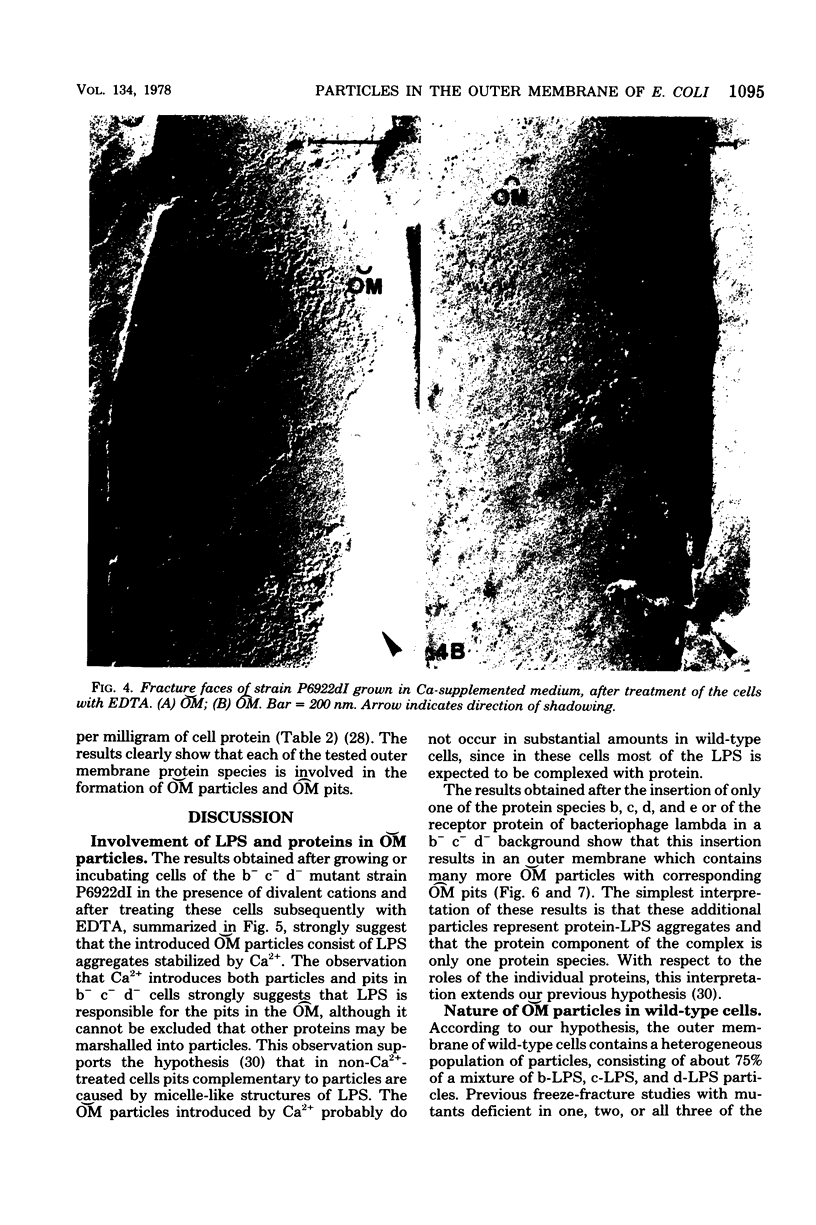
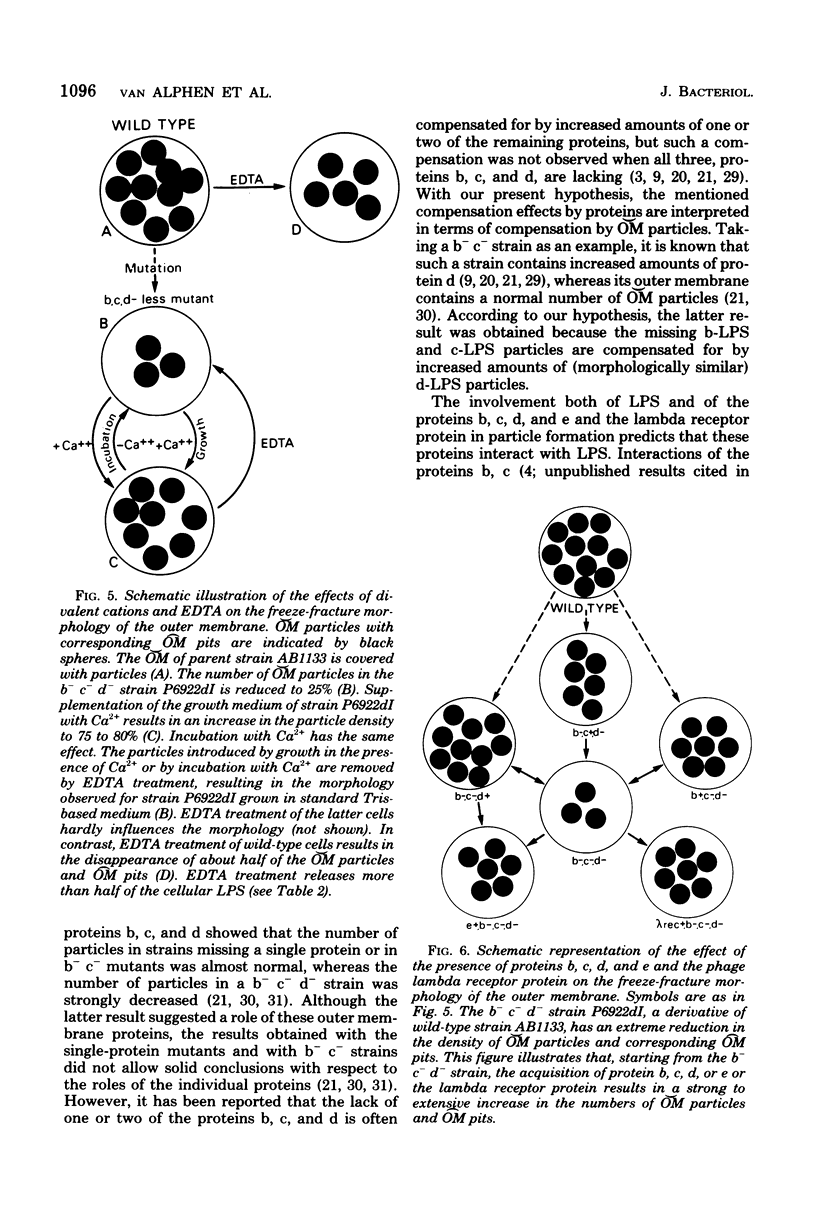
In a previous paper (A. Verkleij, L. van Alphen, J. Bijvelt, and B. Lugtenberg, Biochim. Biophys. Acta 466:269-282, 1977) we have hypothesized that particles on the outer fracture face of the outer membrane ([Formula: see text]), with corresponding pits on the inner fracture face of the outer membrane ([Formula: see text]), consist of lipopolysaccharide (LPS) aggregates stabilized by divalent cations and that they might contain protein and/or phospholipid. In the present paper the roles of LPS, cations, and proteins in these [Formula: see text] particles are described more extensively, using a strain that lacks the major outer membrane proteins, b, c, and d (b− c− d−), and has a reduction in the number of [Formula: see text] particles of 75%. To study the role of divalent cations in the formation of [Formula: see text] particles, these b− c− d− cells were grown or incubated with Ca2+, Mg2+, or putrescine. The presence of Ca2+ resulted in the appearance of many [Formula: see text] particles and [Formula: see text] pits. Mg2+ and putrescine were less effective than Ca2+. Introduction of these particles was not accompanied by alterations in the relative amounts of LPS and cell envelope proteins. Ca2+ treatment of a heptoseless derivative of a b− c− d− strain did not result in morphological changes. Incubation of Ca2+-treated cells with ethylenediaminetetraacetate caused the disappearance of the introduced particles as well as the release of more than 60% of the cellular LPS. These results strongly support the hypothesis that LPS is involved in the formation of [Formula: see text] particles and [Formula: see text] pits. The roles of various outer membrane proteins in the formation of [Formula: see text] particles were studied by comparing the freeze-fracture morphology of b− c− d− cells with that of cells which contain one of the outer membrane proteins b, c, d, and e or the receptor protein for bacteriophage lambda. The results showed that the presence of any of these five proteins in a b− c− d− background resulted in a large increase in the number of [Formula: see text] particles and [Formula: see text] pits, indicating that these proteins are, independent of each other, involved in the formation of [Formula: see text] particles and [Formula: see text] pits. The simplest explanation for the results is that in wild-type cells each particle consists of LPS complexed with some molecules of a single protein species, stabilized by either divalent cations or polyamines. It is hypothesized that the outer membrane of the wild-type cell contains a heterogeneous population of particles, of which 75% consists of protein b-LPS, protein c-LPS, and protein d-LPS particles. A function of these particles as aqueous pores is proposed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Leive L. Effect of ethylenediaminetetraacetate upon the surface of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1364–1381. doi: 10.1128/jb.130.3.1364-1381.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Krieger-Brauer H. J. Interrelationship of the phage lambda receptor protein and maltose transport in mutants of Escherichia coli K12. Biochim Biophys Acta. 1977 Aug 15;469(1):89–98. doi: 10.1016/0005-2736(77)90328-5. [DOI] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Ultrastructural study of polymyxin-resistant isolates of Pseudomonas aeruginosa. J Bacteriol. 1976 Jan;125(1):267–281. doi: 10.1128/jb.125.1.267-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Haller I. Mutants of Escherichia coli K12 lacking all 'major' proteins of the outer cell envelope membrane. FEBS Lett. 1975 Jul 15;55(1):161–164. doi: 10.1016/0014-5793(75)80983-5. [DOI] [PubMed] [Google Scholar]
- Henning U., Schmidmayr W., Hindennach I. Major proteins of the outer cell envelope membrane of Escherichia coli K-12: multiple species of protein I. Mol Gen Genet. 1977 Sep 9;154(3):293–298. doi: 10.1007/BF00571285. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Pugsley A. P., Reeves P. Defective growth functions in mutants of Escherichia coli K12 lacking a major outer membrane protein. J Mol Biol. 1977 Oct 25;116(2):285–300. doi: 10.1016/0022-2836(77)90217-0. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M., Lounatmaa K., Sarvas M., Mäkelä P. H., Nakae T. Bacteriophage-resistant mutants of Salmonella typhimurium deficient in two major outer membrane proteins. J Bacteriol. 1976 Aug;127(2):941–955. doi: 10.1128/jb.127.2.941-955.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Schwarz H., Sonntag I., Henning U. Mutational change of membrane architecture. Mutants of Escherichia coli K12 missing major proteins of the outer cell envelope membrane. Biochim Biophys Acta. 1976 Oct 19;448(3):474–491. doi: 10.1016/0005-2736(76)90301-1. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Thornley M. J., Glauert A. M. Location of the fracture faces within the cell envelope of Acinetobacter species strain MJT-F5-5. J Bacteriol. 1974 May;118(2):693–707. doi: 10.1128/jb.118.2.693-707.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Gilleland H. E., Jr, Eagon R. G. Proteins released from cell envelopes of Pseudomonas aeruginosa on exposure to ethylenediaminetetraacetate: comparison with dimethylformamide-extractable proteins. J Bacteriol. 1973 Apr;114(1):399–407. doi: 10.1128/jb.114.1.399-407.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Lugtenberg E. J., Ververgaert P. H. Freeze etch morphology of outer membrane mutants of Escherichia coli K12. Biochim Biophys Acta. 1976 Mar 19;426(3):581–586. doi: 10.1016/0005-2736(76)90401-6. [DOI] [PubMed] [Google Scholar]
- Verkleij A., van Alphen L., Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli K12. II. Freeze fracture morphology of wild type and mutant strains. Biochim Biophys Acta. 1977 Apr 18;466(2):269–282. doi: 10.1016/0005-2736(77)90224-3. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Yu F., Mizushima S. Stimulation by lipopolysaccharide of the binding of outer membrane proteins O-8 and O-9 to the peptidoglycan layer of Escherichia coli K--12. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1397–1402. doi: 10.1016/0006-291x(77)90597-6. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Lugtenberg B., van Boxtel R., Verhoef K. Architecture of the outer membrane of Escherichia coli K12. I. Action of phospholipases A2 and C on wild type strains and outer membrane mutants. Biochim Biophys Acta. 1977 Apr 18;466(2):257–268. doi: 10.1016/0005-2736(77)90223-1. [DOI] [PubMed] [Google Scholar]
- van Alphen W., Lugtenberg B., Berendsen W. Heptose-deficient mutants of Escherichia coli K12 deficient in up to three major outer membrane proteins. Mol Gen Genet. 1976 Sep 23;147(3):263–269. doi: 10.1007/BF00582877. [DOI] [PubMed] [Google Scholar]
- van Alphen W., van Seim N., Lugtenberg B. Pores in the outer membrane of Escherichia coli K12: involvement of proteins b and e in the functioning of pores for nucleotides. Mol Gen Genet. 1978 Feb 7;159(1):75–83. doi: 10.1007/BF00401750. [DOI] [PubMed] [Google Scholar]