Abstract
Despite the presence of a lymphocytic infiltrate in solid cancers, the failure for tumour growth to be contained suggests an inadequate immune response to the tumour. Poor cytotoxicity exerted by tumour-infiltrating lymphocytes (TILs) against tumour cells in vitro, combined with continued tumour growth in vivo, suggests deficiencies in TIL function or numbers. Various theories have been postulated to explain how tumour cells may escape immunosurveillance and control. One of the many hypotheses is the failure of production of cytokines, which are necessary for T cells to mediate their function. Thus, the expression of cytokine mRNA in human breast tumour sections was investigated by reverse transcriptase polymerase chain reaction (RT-PCR) with cytokine-specific primers. A relatively consistent finding was detection of interleukin (IL) 10 mRNA among the tumours. No IL-2 and little IL-4 mRNA was detected in the tumours. IL-6 and IL-10 mRNA was detected in only one and two of the normal breast tissues respectively. IL-2, IL-4 and tumour necrosis factor (TNF)-alpha mRNA was not detected in any of the normal breast tissues. The reduced function of TILs may be related to IL-10, which has known inhibitory effects on T-cell activation.
Full text
PDF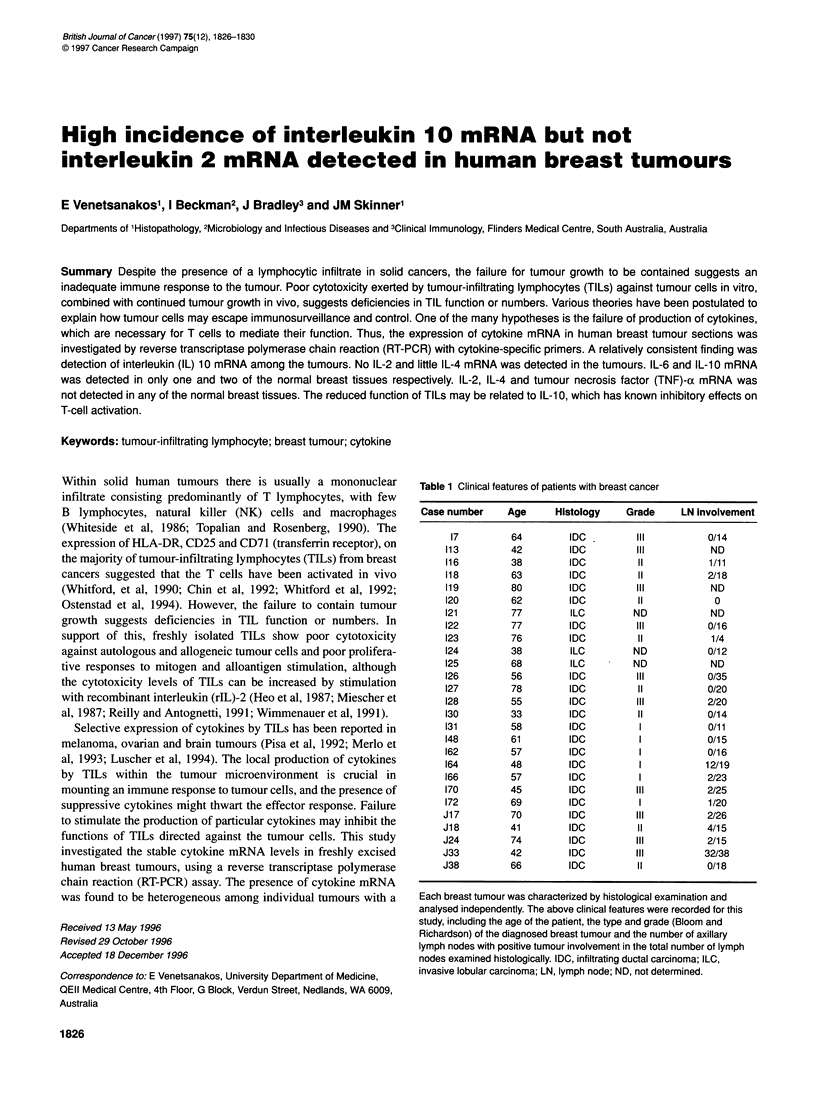


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. C., Brabletz T., Czerny C., Termeer C., Bröcker E. B. Tumor escape mechanisms from immunosurveillance: induction of unresponsiveness in a specific MHC-restricted CD4+ human T cell clone by the autologous MHC class II+ melanoma. Int Immunol. 1993 Dec;5(12):1501–1508. doi: 10.1093/intimm/5.12.1501. [DOI] [PubMed] [Google Scholar]
- Becker J. C., Czerny C., Bröcker E. B. Maintenance of clonal anergy by endogenously produced IL-10. Int Immunol. 1994 Oct;6(10):1605–1612. doi: 10.1093/intimm/6.10.1605. [DOI] [PubMed] [Google Scholar]
- Beckman I., Shepherd K., Dimopoulos K., Ahern M., Firgaira F., Bradley J. Differential expression and regulation of cytokine mRNAs in normal human CD45R T cell subsets. Cytokine. 1994 Mar;6(2):116–123. doi: 10.1016/1043-4666(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Beissert S., Hosoi J., Grabbe S., Asahina A., Granstein R. D. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J Immunol. 1995 Feb 1;154(3):1280–1286. [PubMed] [Google Scholar]
- Chin Y., Janseens J., Vandepitte J., Vandenbrande J., Opdebeek L., Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992 Sep-Oct;12(5):1463–1466. [PubMed] [Google Scholar]
- Ding L., Linsley P. S., Huang L. Y., Germain R. N., Shevach E. M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993 Aug 1;151(3):1224–1234. [PubMed] [Google Scholar]
- Enk A. H., Angeloni V. L., Udey M. C., Katz S. I. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993 Sep 1;151(5):2390–2398. [PubMed] [Google Scholar]
- Gastl G. A., Abrams J. S., Nanus D. M., Oosterkamp R., Silver J., Liu F., Chen M., Albino A. P., Bander N. H. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer. 1993 Aug 19;55(1):96–101. doi: 10.1002/ijc.2910550118. [DOI] [PubMed] [Google Scholar]
- Heo D. S., Whiteside T. L., Johnson J. T., Chen K. N., Barnes E. L., Herberman R. B. Long-term interleukin 2-dependent growth and cytotoxic activity of tumor-infiltrating lymphocytes from human squamous cell carcinomas of the head and neck. Cancer Res. 1987 Dec 1;47(23):6353–6362. [PubMed] [Google Scholar]
- Lüscher U., Filgueira L., Juretic A., Zuber M., Lüscher N. J., Heberer M., Spagnoli G. C. The pattern of cytokine gene expression in freshly excised human metastatic melanoma suggests a state of reversible anergy of tumor-infiltrating lymphocytes. Int J Cancer. 1994 May 15;57(4):612–619. doi: 10.1002/ijc.2910570428. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Salazar F., Petersson M., Masucci G., Hansson J., Pisa P., Zhang Q. J., Masucci M. G., Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994 Dec 1;180(6):2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo A., Juretic A., Zuber M., Filgueira L., Lüscher U., Caetano V., Ulrich J., Gratzl O., Heberer M., Spagnoli G. C. Cytokine gene expression in primary brain tumours, metastases and meningiomas suggests specific transcription patterns. Eur J Cancer. 1993;29A(15):2118–2125. doi: 10.1016/0959-8049(93)90046-i. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., Moretta L., von Fliedner V. Clonal and frequency analyses of tumor-infiltrating T lymphocytes from human solid tumors. J Immunol. 1987 Jun 1;138(11):4004–4011. [PubMed] [Google Scholar]
- Nakagomi H., Pisa P., Pisa E. K., Yamamoto Y., Halapi E., Backlin K., Juhlin C., Kiessling R. Lack of interleukin-2 (IL-2) expression and selective expression of IL-10 mRNA in human renal cell carcinoma. Int J Cancer. 1995 Nov 3;63(3):366–371. doi: 10.1002/ijc.2910630311. [DOI] [PubMed] [Google Scholar]
- Ostenstad B., Lea T., Schlichting E., Harboe M. Human colorectal tumour infiltrating lymphocytes express activation markers and the CD45RO molecule, showing a primed population of lymphocytes in the tumour area. Gut. 1994 Mar;35(3):382–387. doi: 10.1136/gut.35.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa P., Halapi E., Pisa E. K., Gerdin E., Hising C., Bucht A., Gerdin B., Kiessling R. Selective expression of interleukin 10, interferon gamma, and granulocyte-macrophage colony-stimulating factor in ovarian cancer biopsies. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7708–7712. doi: 10.1073/pnas.89.16.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly E. B., Antognetti G. Increased tumor-specific CTL activity in human tumor-infiltrating lymphocytes stimulated with autologous tumor lines. Cell Immunol. 1991 Jul;135(2):526–533. doi: 10.1016/0008-8749(91)90295-m. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Kunkel S. L., Burdick M. D., Wilke C. A., Orringer M. B., Whyte R. I., Strieter R. M. Production of interleukin-10 by human bronchogenic carcinoma. Am J Pathol. 1994 Jul;145(1):18–25. [PMC free article] [PubMed] [Google Scholar]
- Topalian S. L., Rosenberg S. A. Tumor-infiltrating lymphocytes: evidence for specific immune reactions against growing cancers in mice and humans. Important Adv Oncol. 1990:19–41. [PubMed] [Google Scholar]
- Whiteside T. L., Miescher S., Hurlimann J., Moretta L., von Fliedner V. Separation, phenotyping and limiting dilution analysis of T-lymphocytes infiltrating human solid tumors. Int J Cancer. 1986 Jun 15;37(6):803–811. doi: 10.1002/ijc.2910370602. [DOI] [PubMed] [Google Scholar]
- Whitford P., George W. D., Campbell A. M. Flow cytometric analysis of tumour infiltrating lymphocyte activation and tumour cell MHC class I and II expression in breast cancer patients. Cancer Lett. 1992 Jan 10;61(2):157–164. doi: 10.1016/0304-3835(92)90174-t. [DOI] [PubMed] [Google Scholar]
- Whitford P., Mallon E. A., George W. D., Campbell A. M. Flow cytometric analysis of tumour infiltrating lymphocytes in breast cancer. Br J Cancer. 1990 Dec;62(6):971–975. doi: 10.1038/bjc.1990.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmenauer S., Wintzer H. O., von Kleist S. Phenotyping of human tumor-infiltrating lymphocytes before and after exposure to different in vitro stimulation conditions. Anticancer Res. 1991 May-Jun;11(3):1013–1020. [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., de Vries J. E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993 Jun 1;150(11):4754–4765. [PubMed] [Google Scholar]