Abstract
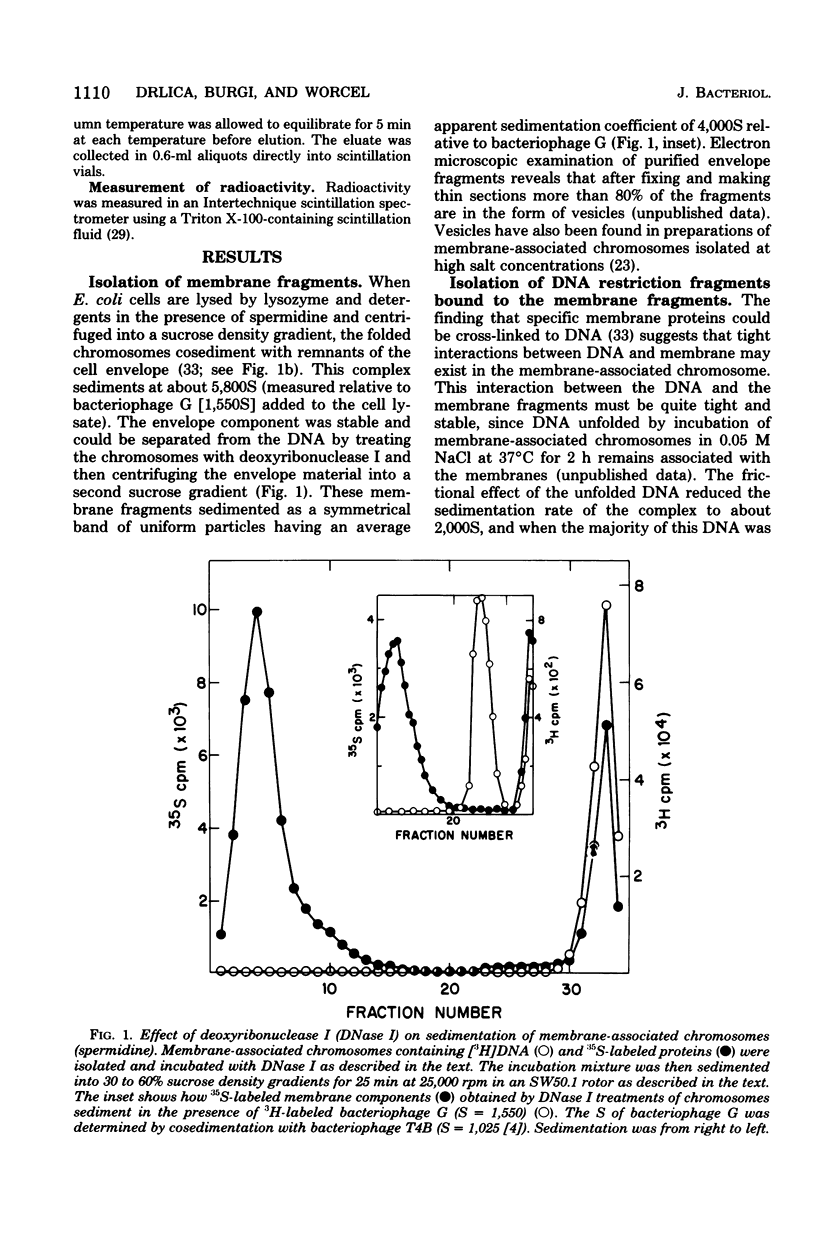
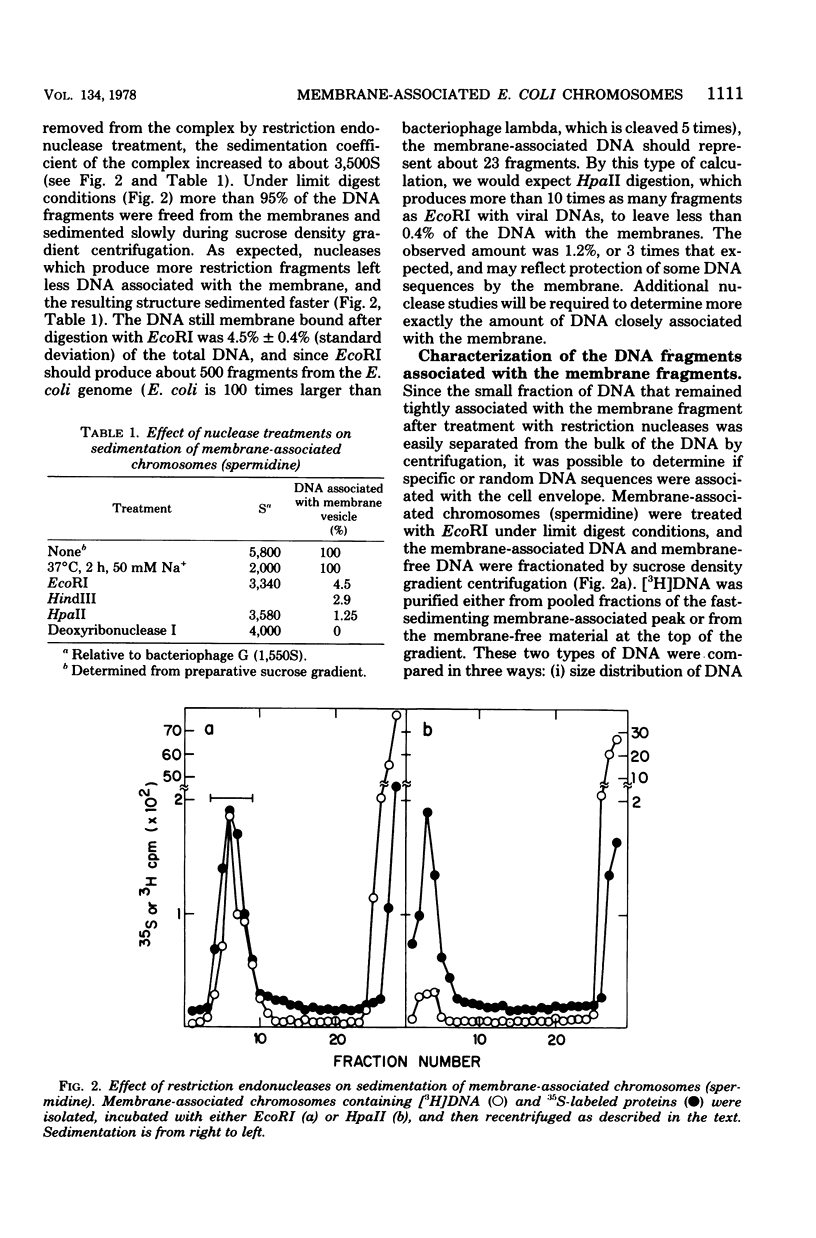
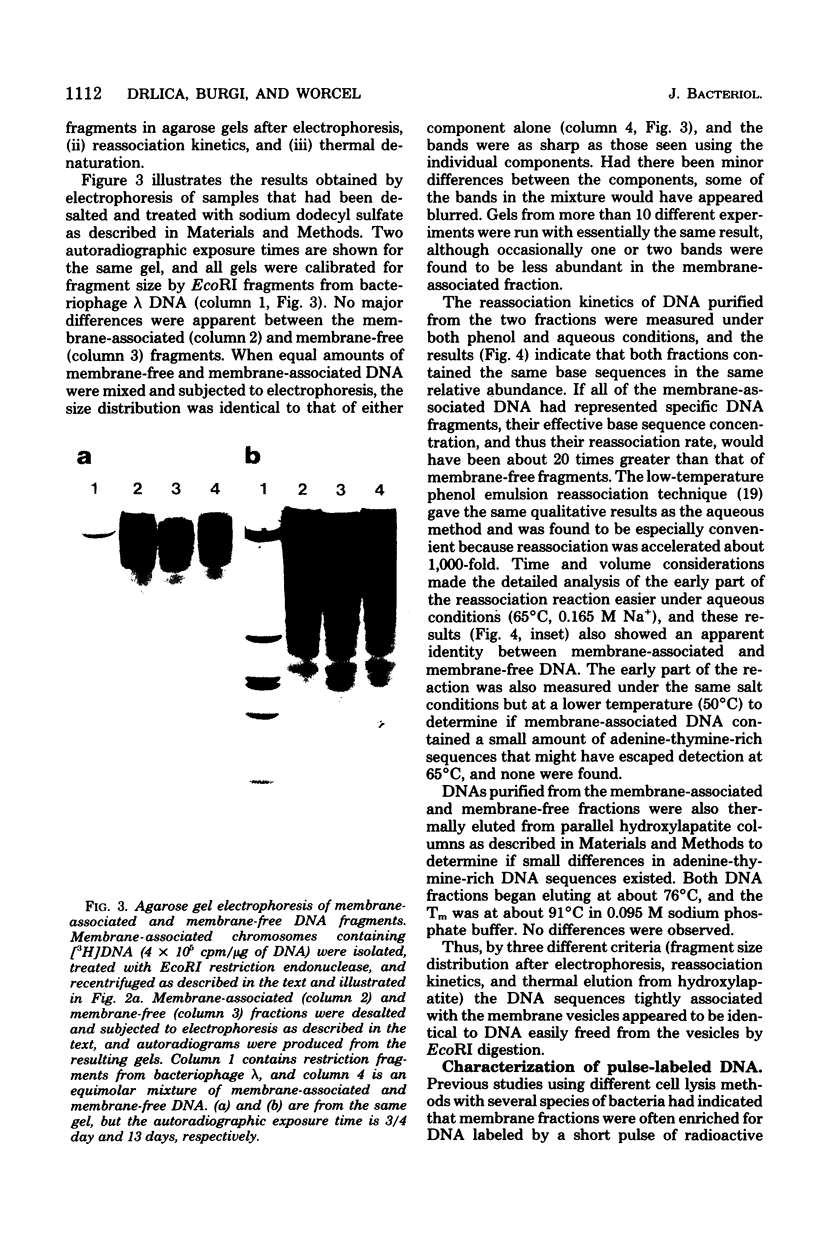
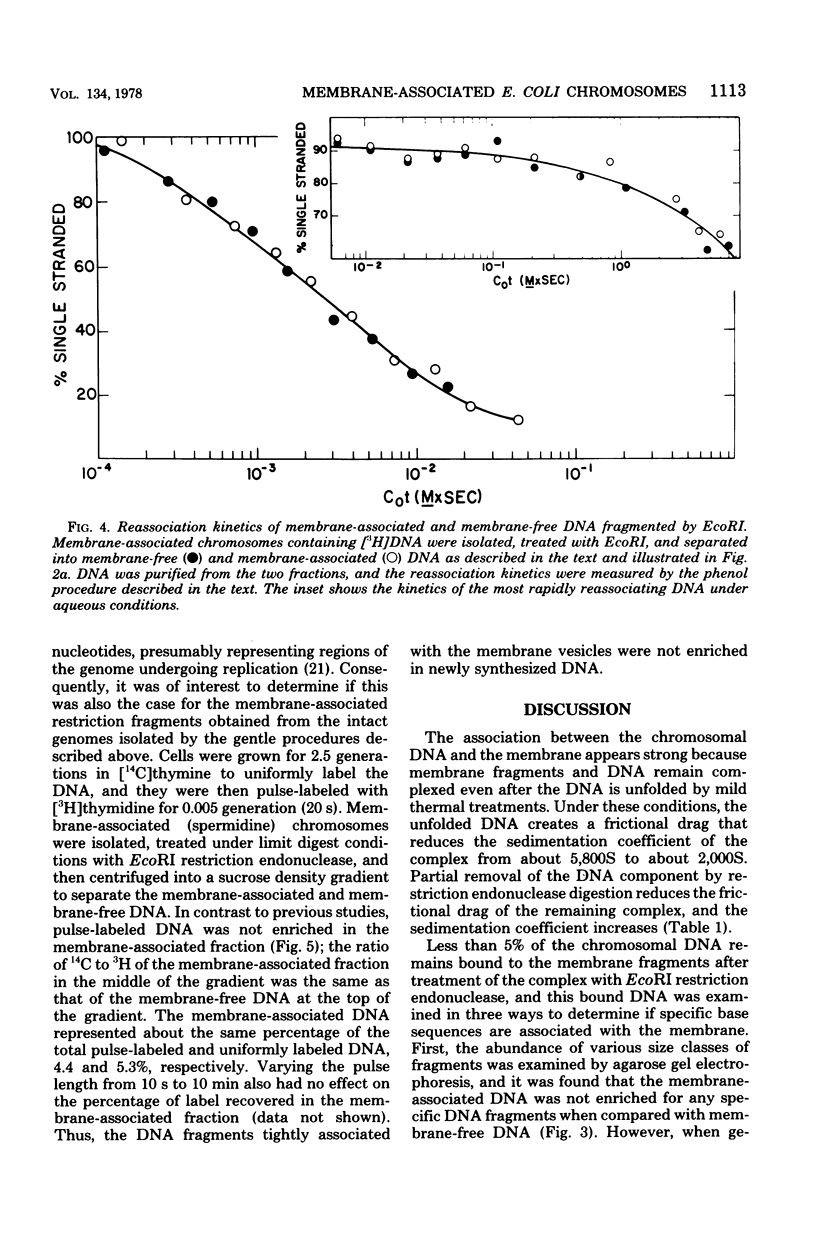
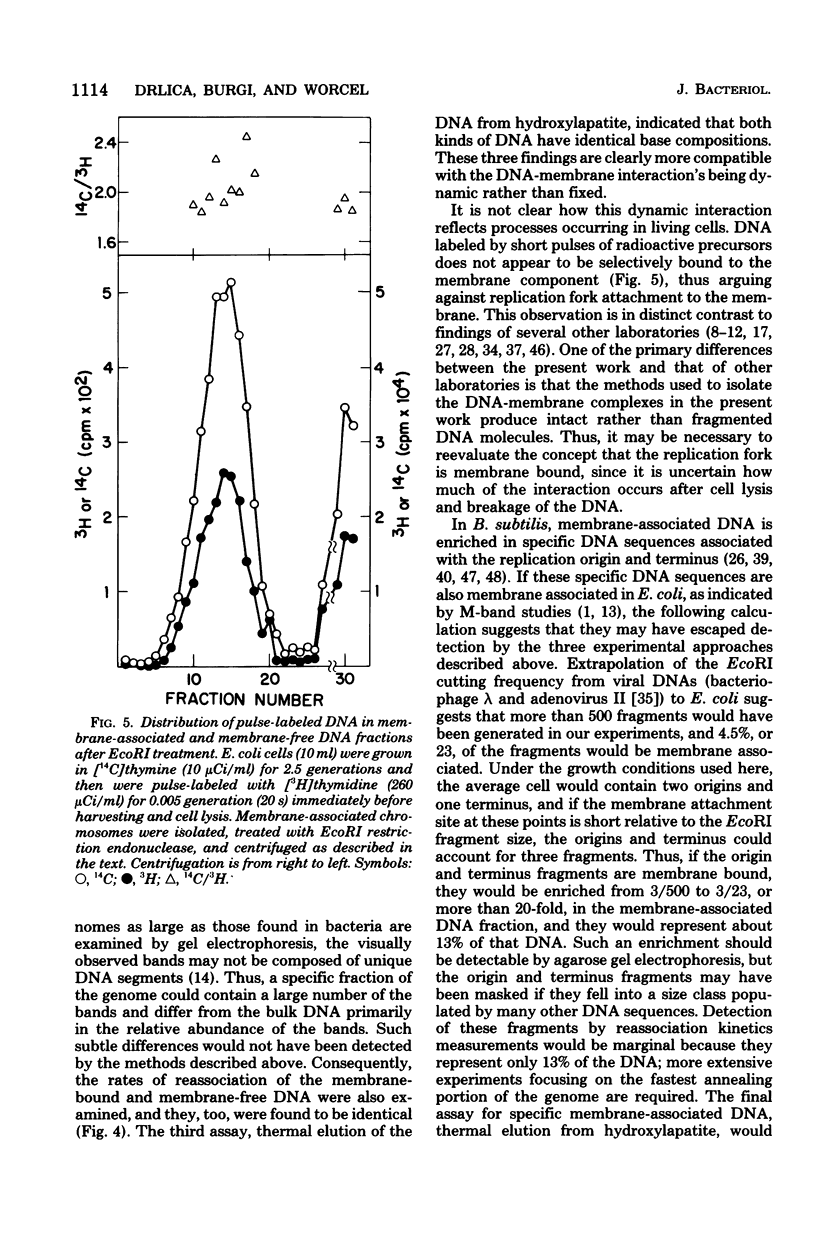
Membrane-associated folded chromosomes isolated from Escherichia coli in the presence of spermidine sedimented at about 5,800S. The folded chromosome and the membrane fragment were each stable in the absence of the other; a 1,700S folded chromosome was obtained after removal of the membrane by a Sarkosyl treatment, and a 4,000S membrane fragment remained after digestion of the chromosomal DNA with deoxyribonuclease I. The interaction between the folded chromosome and the membrane fragment was stable, and, even when the DNA was unfolded, both components remained associated and cosedimented. The large frictional effect of the unfolded DNA reduced the sedimentation rate of the complex to about 2,000S. Partial removal of this unfolded DNA with restriction endonucleases caused the membrane fragments and the remaining associated DNA to sediment faster, at about 3,500S. The DNA remaining associated with the membrane fragments after restriction endonuclease treatment, about 4.5% of the total DNA when EcoRI was used, was indistinguishable from the DNA released from the membranes by three criteria: (i) DNA size distribution in agarose gels after electrophoresis, (ii) reassociation kinetics, and (iii) thermal elution from hydroxylapatite. This finding, that random DNA sequences rather than specific ones were responsible for the majority of the DNA-membrane interactions, argues against the folded chromosome's being a static structure with specific DNA sequences interacting with the cell envelope.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Brown C., Hendrickson W. G., Boyd D. H., Clifford P., Cote R. H., Schaechter M. Release of Escherichia coli DNA from membrane complexes by single-strand endonucleases. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2756–2760. doi: 10.1073/pnas.74.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgi A. W., Robinton J., Carlson C. L. Studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:43–51. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J. SEDIMENTATION AND BIOLOGICAL PROPERTIES OF T-PHAGES OF ESCHERICHIA COLI. Virology. 1964 Jul;23:408–418. doi: 10.1016/0042-6822(64)90264-8. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Drlica K., Worcel A. Conformational transitions in the Escherichia coli chromosome: analysis by viscometry and sedimentation. J Mol Biol. 1975 Oct 25;98(2):393–411. doi: 10.1016/s0022-2836(75)80126-4. [DOI] [PubMed] [Google Scholar]
- Dworsky P., Schaechter M. Effect of rifampin on the structure and membrane attachment of the nucleoid of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1364–1374. doi: 10.1128/jb.116.3.1364-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firshein W. The DNA-membrane fraction of Pneumococcus contains a DNA replication complex. J Mol Biol. 1972 Oct 14;70(3):383–397. doi: 10.1016/0022-2836(72)90547-5. [DOI] [PubMed] [Google Scholar]
- Firshein W. Two membrane sites for DNA synthesis in Pneumococcus. Mol Gen Genet. 1976 Nov 17;148(3):323–335. doi: 10.1007/BF00332907. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Hanawalt P. Isolation and characterization of the DNA replication complex from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):301–322. doi: 10.1016/0022-2836(70)90032-x. [DOI] [PubMed] [Google Scholar]
- Fujita H., Komano T., Maruyama Y. Dynamic aspects of membrane-bound DNA in Bacillus subtilis during the course of synchronous growth. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1361–1367. doi: 10.1016/0006-291x(73)90651-7. [DOI] [PubMed] [Google Scholar]
- Harmon J. M., Taber H. W. Some properties of a membrane-deoxyribonucleic acid complex isolated from Bacillus subtilis. J Bacteriol. 1977 Feb;129(2):789–795. doi: 10.1128/jb.129.2.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Elkana Y., Ehrlich S. D., Lederberg J. Electrophoretic separation of Bacillus subtilis genes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2207–2211. doi: 10.1073/pnas.72.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht R. M., Stimpson D., Pettijohn D. Sedimentation properties of the bacterial chromosome as an isolated nucleoid and as an unfolded DNA fiber. Chromosomal DNA folding measured by rotor speed effects. J Mol Biol. 1977 Apr 15;111(3):257–277. doi: 10.1016/s0022-2836(77)80051-x. [DOI] [PubMed] [Google Scholar]
- Hecht R. M., Taggart R. T., Pettijohn D. E. Size and DNA content of purfied E. coli nucleoids observed by fluorencence microscopy. Nature. 1975 Jan 3;253(5486):60–62. doi: 10.1038/253060a0. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Pène J. J. Association of the Bacillus subtilis chromosome with the cell membrane: resolution of free and bound deoxyribonucleic acid on renografin gradients. J Bacteriol. 1970 Nov;104(2):839–850. doi: 10.1128/jb.104.2.839-850.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Lockwood A., Worcel A. Replication of the Escherichia coli chromosome with a soluble enzyme system. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3189–3193. doi: 10.1073/pnas.71.8.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., De Jong M. A., Woldringh C. L., Nanninga N. Factors affecting the release of folded chromosomes from Escherichia coli. Eur J Biochem. 1976 Apr 1;63(2):469–475. doi: 10.1111/j.1432-1033.1976.tb10249.x. [DOI] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. A., Sueoka N. Membrane attachment of the replication origins of a multifork (dichotomous) chromosome in Bacillus subtilis. J Mol Biol. 1972 Aug 21;69(2):237–248. doi: 10.1016/0022-2836(72)90228-8. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Parker D. L., Glaser D. A. Effect of growth conditions on DNA-membrane attachment in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2446–2450. doi: 10.1073/pnas.72.6.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Portalier R., Worcel A. Association of the folded chromosome with the cell envelope of E. coli: characterization of the proteins at the DNA-membrane attachment site. Cell. 1976 Jun;8(2):245–255. doi: 10.1016/0092-8674(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Quinlan D. C., Maniloff J. Membrane association of the deoxyribonucleic acid growing-point region in Mycoplasma gallisepticum. J Bacteriol. 1972 Dec;112(3):1375–1379. doi: 10.1128/jb.112.3.1375-1379.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Hammers J. M. Isolation of DNA-membrane complex in Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4787–4791. doi: 10.1073/pnas.71.12.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Godson G. N., Radding C. M. Specificity of the S1 nuclease from Aspergillus oryzae. J Biol Chem. 1975 Nov 25;250(22):8848–8855. [PubMed] [Google Scholar]
- Woldringh C. L., Nanninga N. Organization of the nucleoplasm in Escherichia coli visualized by phase-contrast light microscopy, freeze fracturing, and thin sectioning. J Bacteriol. 1976 Sep;127(3):1455–1464. doi: 10.1128/jb.127.3.1455-1464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Murakami S., Yoshikawa H. Chromosome-membrane association in Bacillus subtilis. I. DNA release from membrane fraction. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1559–1565. doi: 10.1016/s0006-291x(71)80264-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yoshikawa H. Association of the replication terminus of the Bacillus subtilis chromosome to the cell membrane. J Bacteriol. 1975 Nov;124(2):1030–1033. doi: 10.1128/jb.124.2.1030-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Yoshikawa H. Chromosome--Membrane association in Bacillus subtilis. III. Isolation and characterization of a DNA-protein complex carrying replication origin markers. J Mol Biol. 1977 Feb 25;110(2):219–253. doi: 10.1016/s0022-2836(77)80070-3. [DOI] [PubMed] [Google Scholar]