Abstract
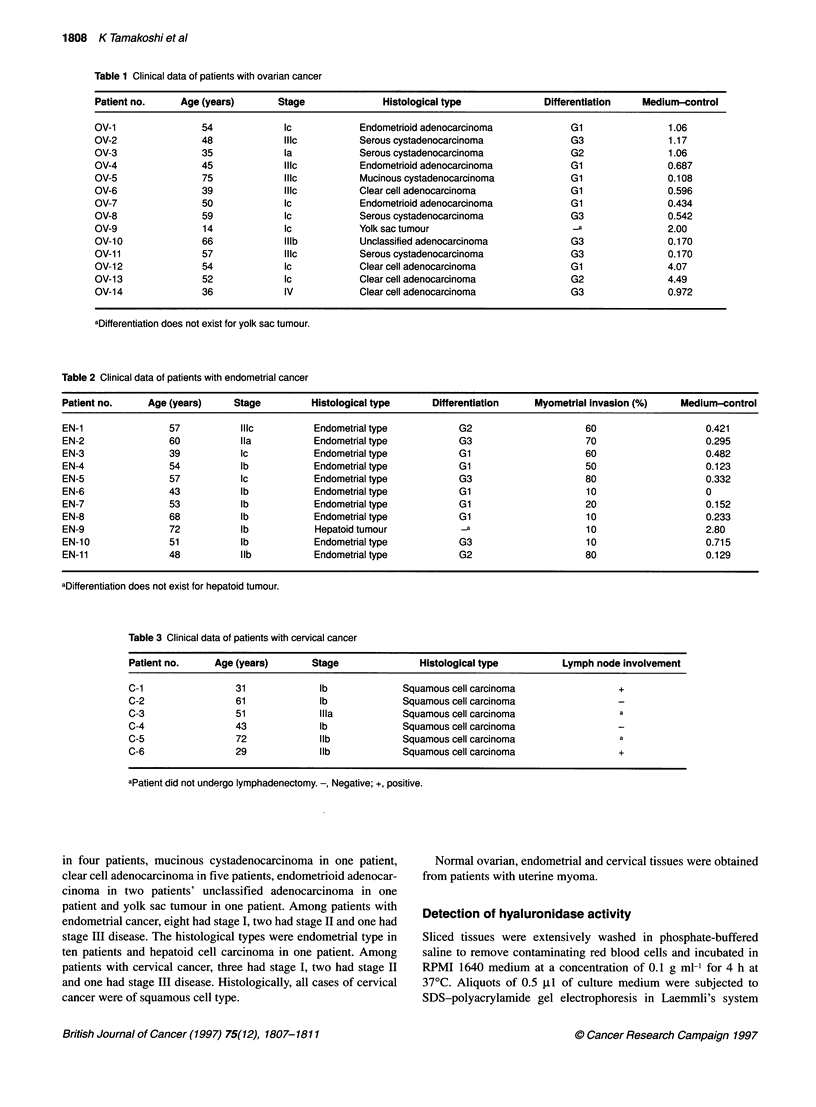
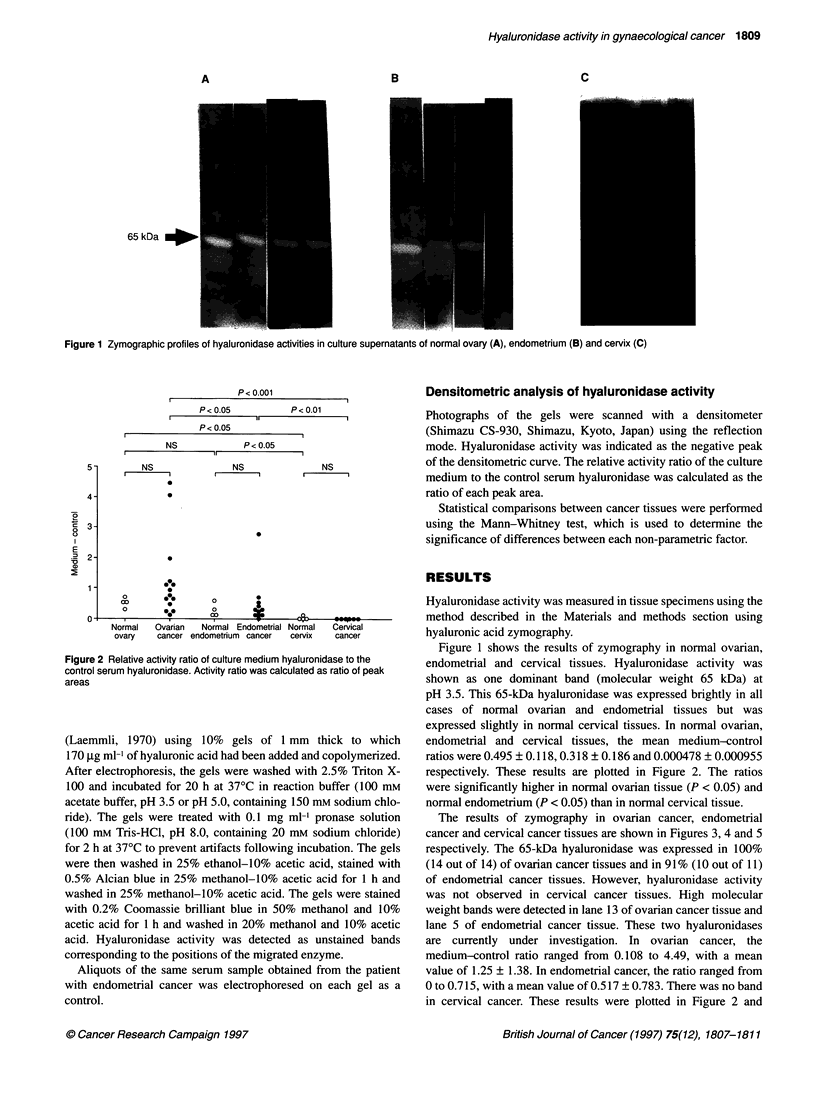
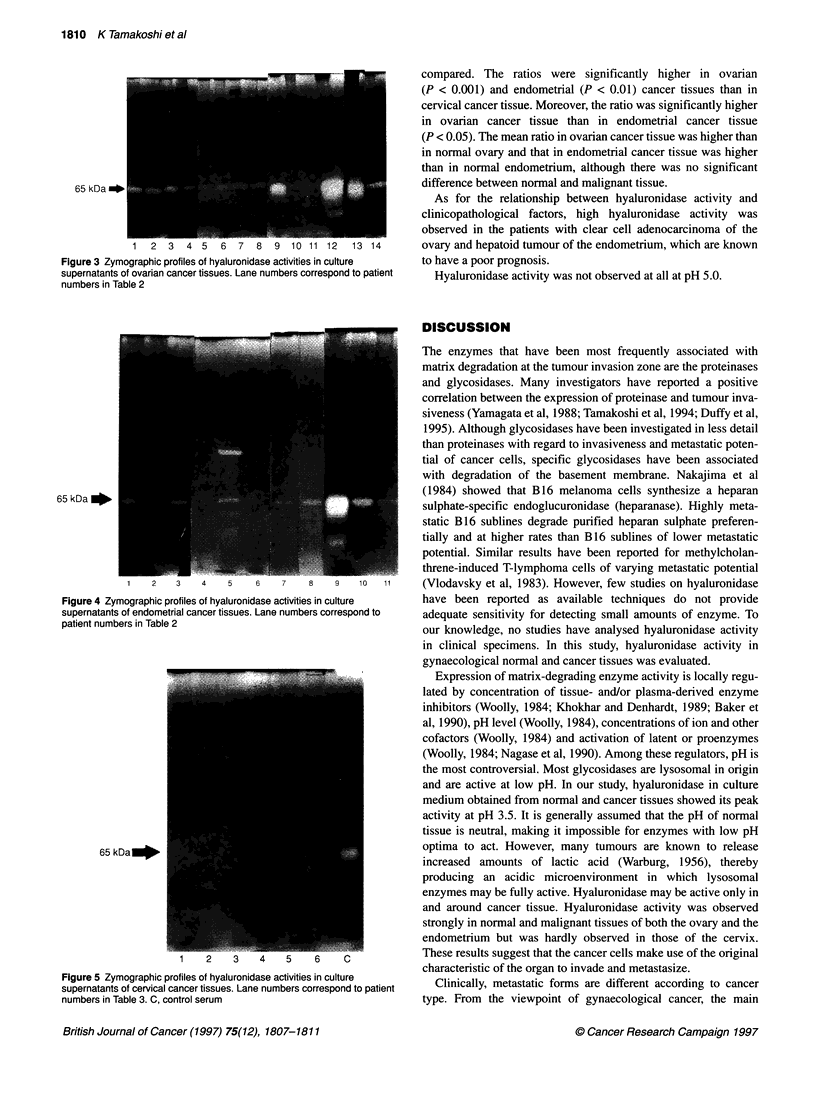
We investigated hyaluronidase activity in gynaecological normal and malignant tissues. Hyaluronidase activity in culture medium of tissue specimens was detected by hyaluronic acid zymography and quantified by densitometry. Hyaluronidase activity was shown as one dominant band (molecular weight 65 kDa) at pH 3.5. Hyaluronidase activity was significantly higher in normal ovary (P < 0.05) and normal endometrium (P< 0.05) than in normal cervix. One dominant 65-kDa hyaluronidase was expressed in 100% (14 out of 14) of ovarian cancer tissues and in 91% (10 out of 11) of endometrial cancer tissues. However, hyaluronidase activity was not observed in cervical cancer tissues. Hyaluronidase activity was significantly higher in ovarian (P < 0.001) and endometrial (P < 0.01) cancer tissues than in cervical cancer tissue and was significantly higher in ovarian cancer tissue than in endometrial cancer tissue (P < 0.05). These facts suggest that the cancer cells make use of the original characteristic of the organ to invade and metastasize. Moreover, these results reflect the difference in metastatic forms and are suggestive of a strong relationship between hyaluronidase activity and invasion and metastasis of ovarian and endometrial cancers compared with cervical cancer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. S., Bleakley P., Woodrow G. C., Doe W. F. Inhibition of cancer cell urokinase plasminogen activator by its specific inhibitor PAI-2 and subsequent effects on extracellular matrix degradation. Cancer Res. 1990 Aug 1;50(15):4676–4684. [PubMed] [Google Scholar]
- Baker T., Tickle S., Wasan H., Docherty A., Isenberg D., Waxman J. Serum metalloproteinases and their inhibitors: markers for malignant potential. Br J Cancer. 1994 Sep;70(3):506–512. doi: 10.1038/bjc.1994.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy M. J., Blaser J., Duggan C., McDermott E., O'Higgins N., Fennelly J. J., Tschesche H. Assay of matrix metalloproteases types 8 and 9 by ELISA in human breast cancer. Br J Cancer. 1995 May;71(5):1025–1028. doi: 10.1038/bjc.1995.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy M. J. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992 May;10(3):145–155. doi: 10.1007/BF00132746. [DOI] [PubMed] [Google Scholar]
- Hirabayashi K., Graham J. Genesis of ascites in ovarian cancer. Am J Obstet Gynecol. 1970 Feb 15;106(4):492–497. doi: 10.1016/0002-9378(70)90031-1. [DOI] [PubMed] [Google Scholar]
- Jenks S. Anti-metastasis drug ready for human trials. J Natl Cancer Inst. 1992 Feb 19;84(4):220–220. doi: 10.1093/jnci/84.4.220. [DOI] [PubMed] [Google Scholar]
- Khokha R., Denhardt D. T. Matrix metalloproteinases and tissue inhibitor of metalloproteinases: a review of their role in tumorigenesis and tissue invasion. Invasion Metastasis. 1989;9(6):391–405. [PubMed] [Google Scholar]
- Kobayashi H., Shinohara H., Gotoh J., Fujie M., Fujishiro S., Terao T. Anti-metastatic therapy by urinary trypsin inhibitor in combination with an anti-cancer agent. Br J Cancer. 1995 Nov;72(5):1131–1137. doi: 10.1038/bjc.1995.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miura R. O., Yamagata S., Miura Y., Harada T., Yamagata T. Analysis of glycosaminoglycan-degrading enzymes by substrate gel electrophoresis (zymography). Anal Biochem. 1995 Mar 1;225(2):333–340. doi: 10.1006/abio.1995.1163. [DOI] [PubMed] [Google Scholar]
- Nagase H., Enghild J. J., Suzuki K., Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990 Jun 19;29(24):5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Di Ferrante N., Nicolson G. L. Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem. 1984 Feb 25;259(4):2283–2290. [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Nicolson G. L. Heparanases and tumor metastasis. J Cell Biochem. 1988 Feb;36(2):157–167. doi: 10.1002/jcb.240360207. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Lotan D., Baig M. M., Carralero R. M., Wood W. R., Hendrix M. J., Lotan R. Inhibition by retinoic acid of type IV collagenolysis and invasion through reconstituted basement membrane by metastatic rat mammary adenocarcinoma cells. Cancer Res. 1989 Apr 1;49(7):1698–1706. [PubMed] [Google Scholar]
- Rosman M., Hayden C. L., Thiel R. P., Chambers J. T., Kohorn E. I., Chambers S. K., Schwartz P. E. Prognostic indicators for poor risk epithelial ovarian carcinoma. Cancer. 1994 Aug 15;74(4):1323–1328. doi: 10.1002/1097-0142(19940815)74:4<1323::aid-cncr2820740423>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Tamakoshi K., Kikkawa F., Nawa A., Maeda O., Kawai M., Sugamuma N., Yamagata S., Tomoda Y. Different pattern of zymography between human gynecologic normal and malignant tissues. Am J Obstet Gynecol. 1994 Aug;171(2):478–484. doi: 10.1016/0002-9378(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Woolley D. E. Collagenolytic mechanisms in tumor cell invasion. Cancer Metastasis Rev. 1984;3(4):361–372. doi: 10.1007/BF00051460. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Ito Y., Tanaka R., Shimizu S. Gelatinases of metastatic cell lines of murine colonic carcinoma as detected by substrate-gel electrophoresis. Biochem Biophys Res Commun. 1988 Feb 29;151(1):158–162. doi: 10.1016/0006-291x(88)90573-6. [DOI] [PubMed] [Google Scholar]