Abstract
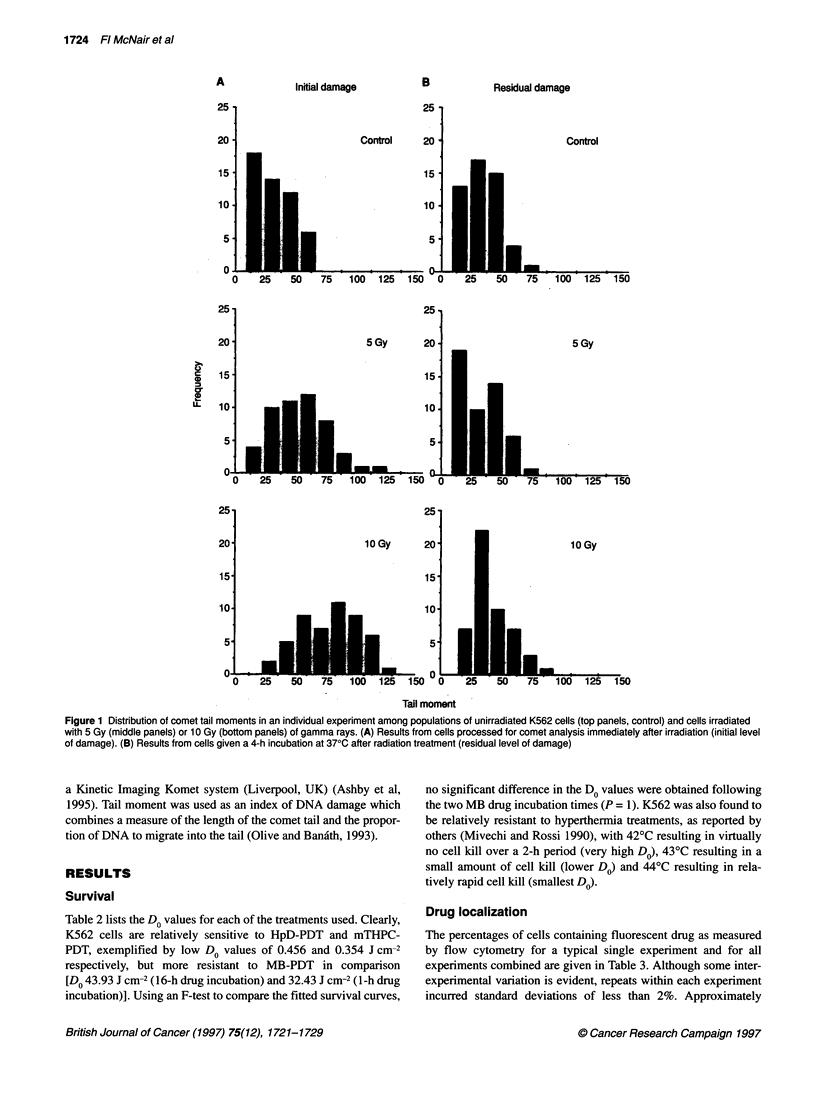
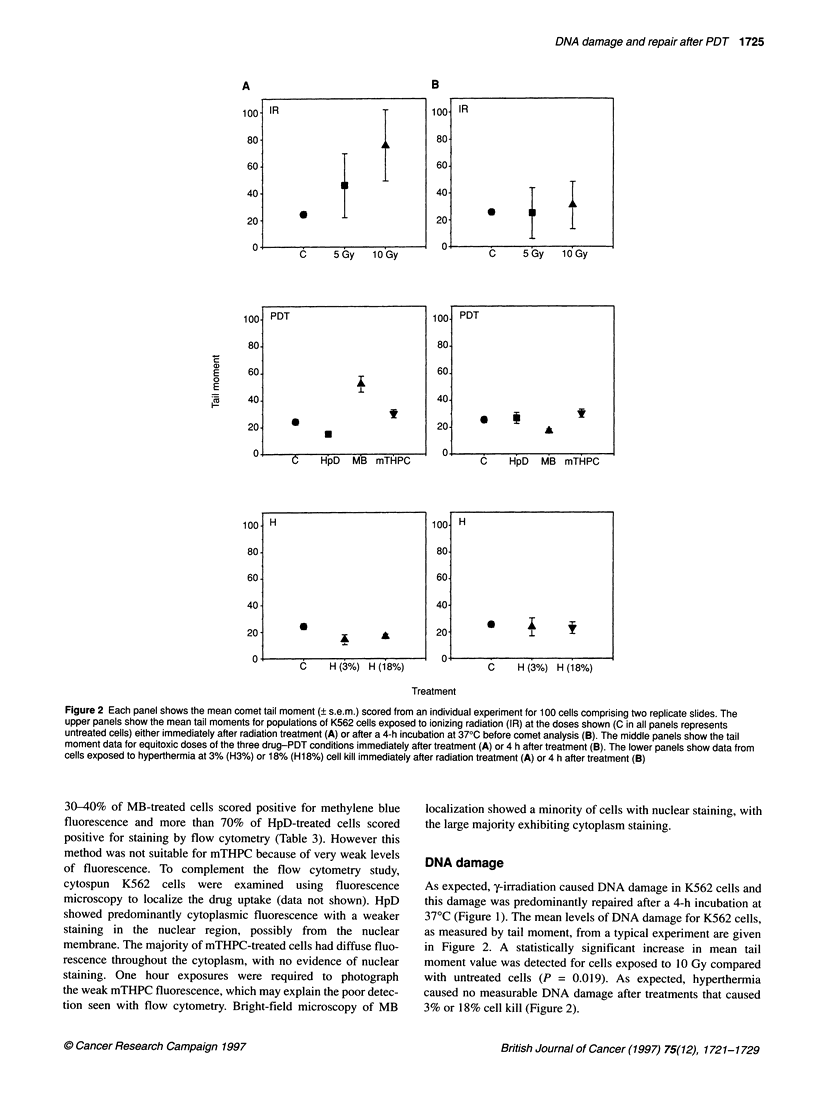
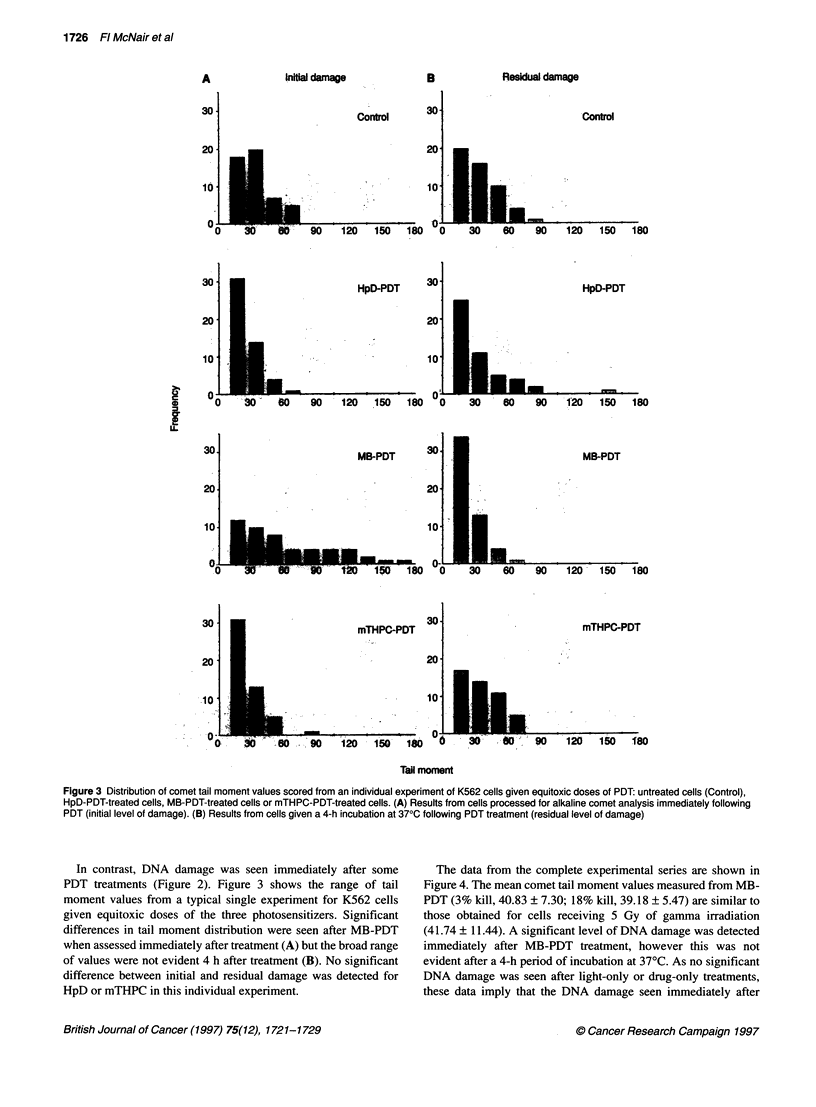
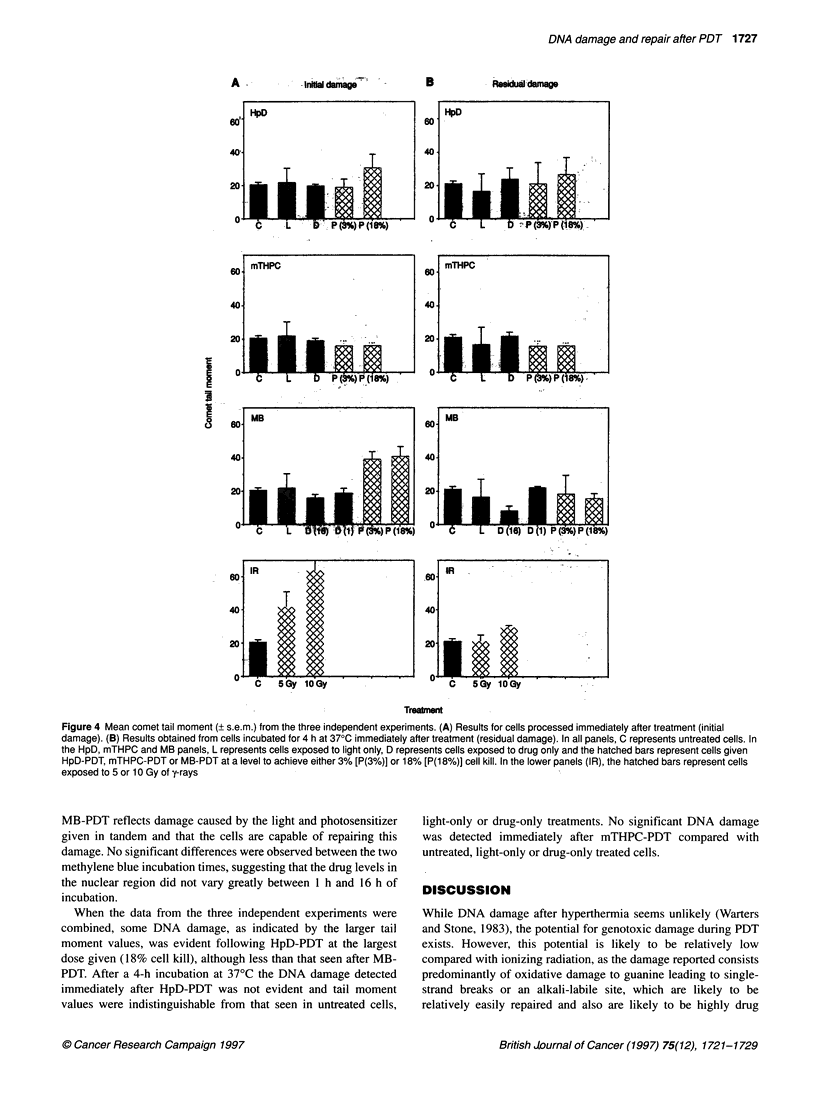
Single-cell electrophoresis (comet assay) has been used to evaluate DNA damage and repair in the human myeloid leukaemia cell line K562 after low-dose (predominantly sub-lethal) treatments of hyperthermia and photodynamic therapy (PDT). Three different photosensitizers were examined: haematoporphyrin derivative (HpD), methylene blue (MB) and meso-tetrahydroxyphenylchlorin (mTHPC). None of the drugs in the absence of light, nor in light alone, resulted in detectable DNA damage. However, a significant amount of DNA damage was detected immediately after treatment with haematoporphyrin derivative or methylene blue PDT compared with drug-only or light-only treatments; no residual level of DNA damage was evident for either drug following a 4-h post-treatment incubation at 37 degrees C. No significant DNA damage was detected after meso-tetrahydroxyphenylchlorin PDT or hyperthermia either immediately or 4 h after treatment. We conclude that the alkaline comet assay can be applied as an effective screening assay for DNA damage induced by a range of laser therapies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Tinwell H., Lefevre P. A., Browne M. A. The single cell gel electrophoresis assay for induced DNA damage (comet assay): measurement of tail length and moment. Mutagenesis. 1995 Mar;10(2):85–90. doi: 10.1093/mutage/10.2.85. [DOI] [PubMed] [Google Scholar]
- Bachmann B., Knüver-Hopf J., Lambrecht B., Mohr H. Target structures for HIV-1 inactivation by methylene blue and light. J Med Virol. 1995 Oct;47(2):172–178. doi: 10.1002/jmv.1890470211. [DOI] [PubMed] [Google Scholar]
- Betti C., Davini T., Giannessi L., Loprieno N., Barale R. Comparative studies by comet test and SCE analysis in human lymphocytes from 200 healthy subjects. Mutat Res. 1995 Jul;343(4):201–207. doi: 10.1016/0165-1218(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Black A. R., Subjeck J. R. Involvement of rRNA synthesis in the enhanced survival and recovery of protein synthesis seen in thermotolerance. J Cell Physiol. 1989 Mar;138(3):439–449. doi: 10.1002/jcp.1041380302. [DOI] [PubMed] [Google Scholar]
- Blazek E. R., Hariharan P. V. Alkaline elution studies of hematoporphyrin-derivative photosensitized DNA damage and repair in Chinese hamster ovary cells. Photochem Photobiol. 1984 Jul;40(1):5–13. doi: 10.1111/j.1751-1097.1984.tb04546.x. [DOI] [PubMed] [Google Scholar]
- Boegheim J. P., Dubbelman T. M., Mullenders L. H., Van Steveninck J. Photodynamic effects of haematoporphyrin derivative on DNA repair in murine L929 fibroblasts. Biochem J. 1987 Jun 15;244(3):711–715. doi: 10.1042/bj2440711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchko G. W., Wagner J. R., Cadet J., Raoul S., Weinfeld M. Methylene blue-mediated photooxidation of 7,8-dihydro-8-oxo-2'-deoxyguanosine. Biochim Biophys Acta. 1995 Jul 25;1263(1):17–24. doi: 10.1016/0167-4781(95)00078-u. [DOI] [PubMed] [Google Scholar]
- Dickson J. A., Calderwood S. K. Effects of hyperglycemia and hyperthermia on the pH, glycolysis, and respiration of the Yoshida sarcoma in vivo. J Natl Cancer Inst. 1979 Dec;63(6):1371–1381. [PubMed] [Google Scholar]
- Elyan S. A., West C. M., Roberts S. A., Hunter R. D. Use of low-dose rate irradiation to measure the intrinsic radiosensitivity of human T-lymphocytes. Int J Radiat Biol. 1993 Oct;64(4):375–383. doi: 10.1080/09553009314551561. [DOI] [PubMed] [Google Scholar]
- Epe B., Pflaum M., Boiteux S. DNA damage induced by photosensitizers in cellular and cell-free systems. Mutat Res. 1993 May;299(3-4):135–145. doi: 10.1016/0165-1218(93)90091-q. [DOI] [PubMed] [Google Scholar]
- Fairbairn D. W., Olive P. L., O'Neill K. L. The comet assay: a comprehensive review. Mutat Res. 1995 Feb;339(1):37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- Fowler G. J., Rees R. C., Devonshire R. The photokilling of bladder carcinoma cells in vitro by phenothiazine dyes. Photochem Photobiol. 1990 Sep;52(3):489–494. doi: 10.1111/j.1751-1097.1990.tb01790.x. [DOI] [PubMed] [Google Scholar]
- Konings A. W. Membranes as targets for hyperthermic cell killing. Recent Results Cancer Res. 1988;109:9–21. doi: 10.1007/978-3-642-83263-5_2. [DOI] [PubMed] [Google Scholar]
- Levy J. G. Photodynamic therapy. Trends Biotechnol. 1995 Jan;13(1):14–18. doi: 10.1016/S0167-7799(00)88895-2. [DOI] [PubMed] [Google Scholar]
- Ma L., Moan J., Berg K. Evaluation of a new photosensitizer, meso-tetra-hydroxyphenyl-chlorin, for use in photodynamic therapy: a comparison of its photobiological properties with those of two other photosensitizers. Int J Cancer. 1994 Jun 15;57(6):883–888. doi: 10.1002/ijc.2910570618. [DOI] [PubMed] [Google Scholar]
- Mivechi N. F., Rossi J. J. Use of polymerase chain reaction to detect the expression of the Mr 70,000 heat shock genes in control or heat shock leukemic cells as correlated to their heat response. Cancer Res. 1990 May 15;50(10):2877–2884. [PubMed] [Google Scholar]
- Moan J., Berg K., Kvam E., Western A., Malik Z., Rück A., Schneckenburger H. Intracellular localization of photosensitizers. Ciba Found Symp. 1989;146:95–111. doi: 10.1002/9780470513842.ch7. [DOI] [PubMed] [Google Scholar]
- Musarrat J., Wani A. A. Quantitative immunoanalysis of promutagenic 8-hydroxy-2'-deoxyguanosine in oxidized DNA. Carcinogenesis. 1994 Sep;15(9):2037–2043. doi: 10.1093/carcin/15.9.2037. [DOI] [PubMed] [Google Scholar]
- Nordén B., Tjerneld F. Structure of methylene blue-DNA complexes studied by linear and circular dichroism spectroscopy. Biopolymers. 1982 Sep;21(9):1713–1734. doi: 10.1002/bip.360210904. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Banáth J. P., Durand R. E. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the "comet" assay. Radiat Res. 1990 Apr;122(1):86–94. [PubMed] [Google Scholar]
- Olive P. L., Banáth J. P. Induction and rejoining of radiation-induced DNA single-strand breaks: "tail moment" as a function of position in the cell cycle. Mutat Res. 1993 Oct;294(3):275–283. doi: 10.1016/0921-8777(93)90010-e. [DOI] [PubMed] [Google Scholar]
- Powell S. N., McMillan T. J. The repair fidelity of restriction enzyme-induced double strand breaks in plasmid DNA correlates with radioresistance in human tumor cell lines. Int J Radiat Oncol Biol Phys. 1994 Jul 30;29(5):1035–1040. doi: 10.1016/0360-3016(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Wagner S. J., Cifone M. A., Murli H., Dodd R. Y., Myhr B. Mammalian genotoxicity assessment of methylene blue in plasma: implications for virus inactivation. Transfusion. 1995 May;35(5):407–413. doi: 10.1046/j.1537-2995.1995.35595259151.x. [DOI] [PubMed] [Google Scholar]
- Ward J. F. DNA damage and repair. Basic Life Sci. 1991;58:403–421. doi: 10.1007/978-1-4684-7627-9_15. [DOI] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Warters R. L., Stone O. L. Macromolecule synthesis in HeLa cells after thermal shock. Radiat Res. 1983 Dec;96(3):646–652. [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J Cell Biol. 1985 Oct;101(4):1198–1211. doi: 10.1083/jcb.101.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M. Size-dependent resistance of human tumour spheroids to photodynamic treatment. Br J Cancer. 1989 Apr;59(4):510–514. doi: 10.1038/bjc.1989.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodek D., Olive P. L. Neutral filter elution detects differences in chromatin organization which can influence cellular radiosensitivity. Radiat Res. 1992 Nov;132(2):242–247. [PubMed] [Google Scholar]


