Abstract
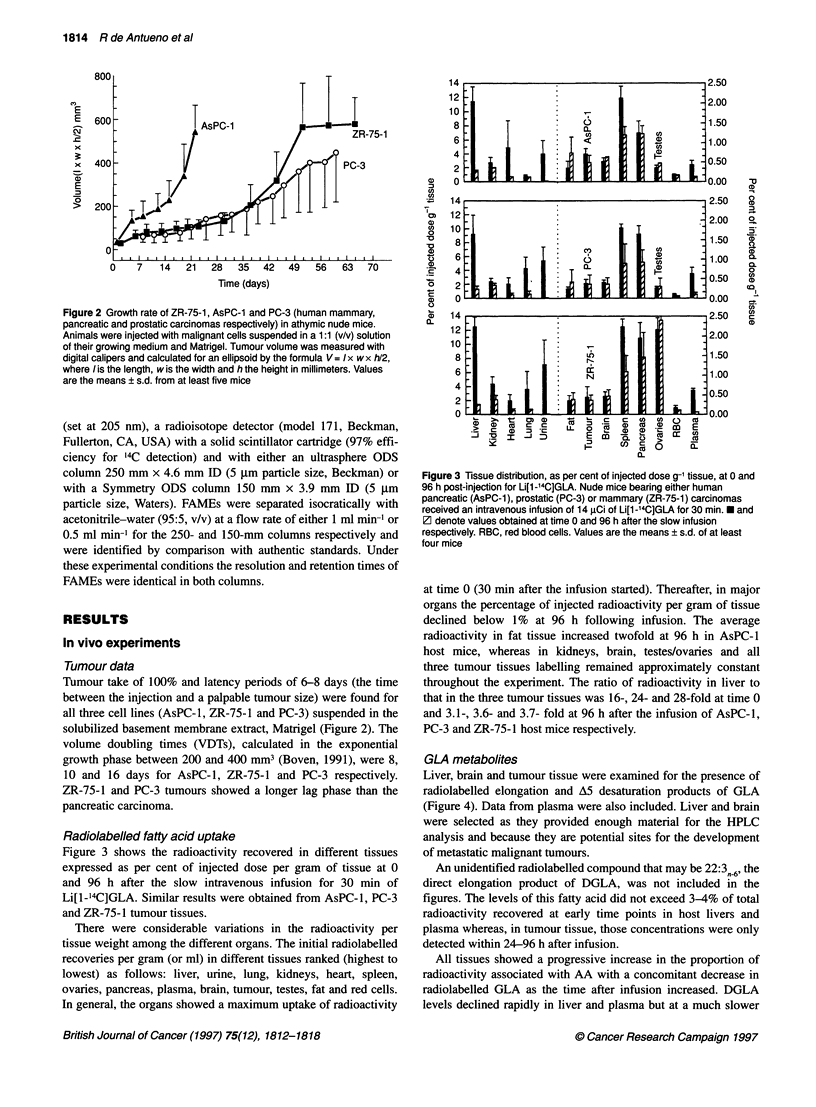
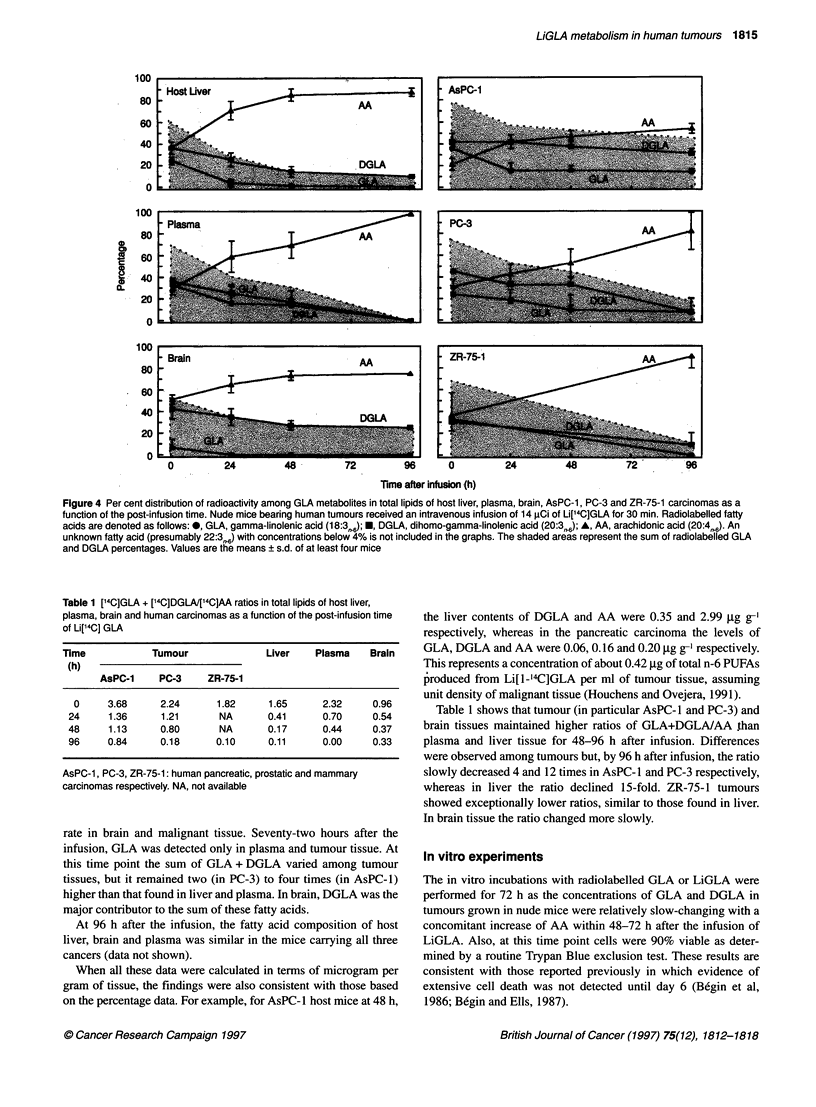
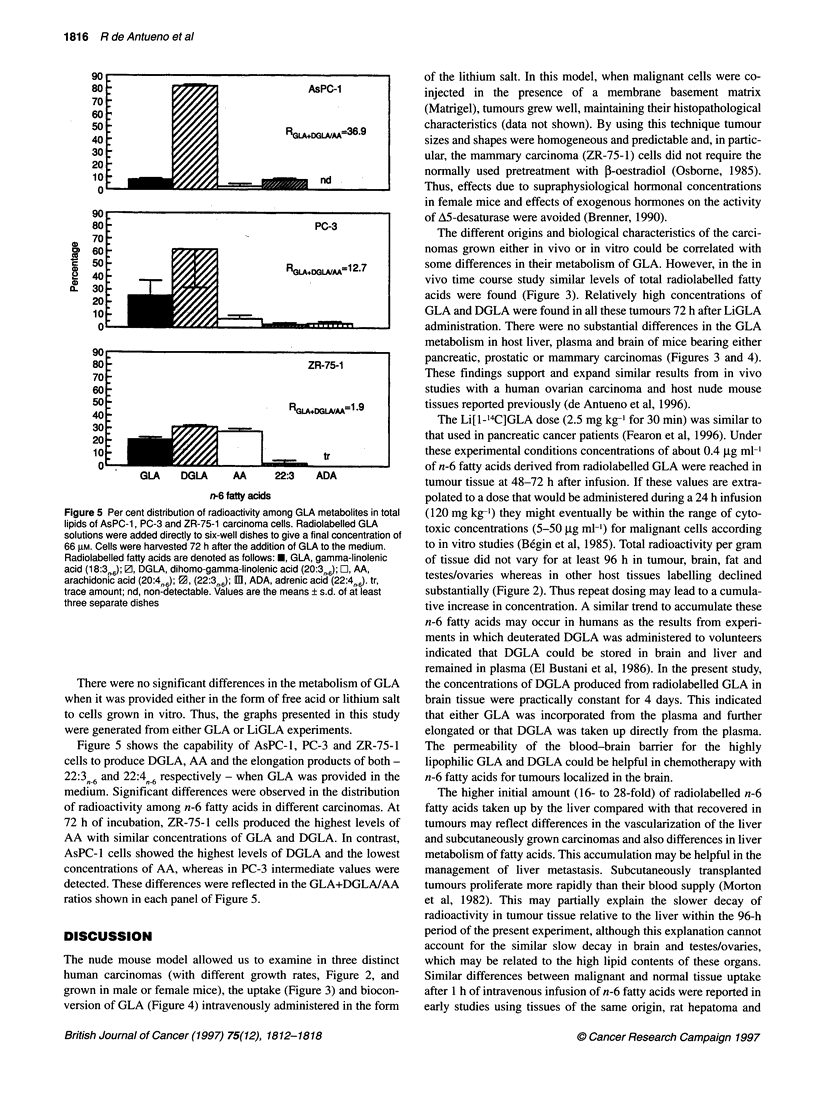
Lipid metabolism has been considered recently as a novel target for cancer therapy. In this field, lithium gamma-linolenate (LiGLA) is a promising experimental compound for use in the treatment of human tumours. In vivo and in vitro studies allowed us to assess the metabolism of radiolabelled LiGLA by tumour tissue and different organs of the host. In vitro studies demonstrated that human pancreatic (AsPC-1), prostatic (PC-3) and mammary carcinoma (ZR-75-1) cells were capable of elongating GLA from LiGLA to dihomo-gamma-linolenic acid (DGLA) and further desaturating it to arachidonic acid (AA). AsPC-1 cells showed the lowest delta5-desaturase activity on DGLA. In the in vivo studies, nude mice bearing the human carcinomas were given Li[1-(14)C]GLA (2.5 mg kg(-1)) by intravenous injection for 30 min. Mice were either sacrificed after infusion or left for up to 96 h recovery before sacrifice. In general, the organs showed a maximum uptake of radioactivity 30 min after the infusion started (t = 0). Thereafter, in major organs the percentage of injected radioactivity per g of tissue declined below 1% 96 h after infusion. In kidney, brain, testes/ovaries and all three tumour tissues, labelling remained constant throughout the experiment. The ratio of radioactivity in liver to tumour tissues ranged between 16- and 24-fold at t = 0 and between 3.1- and 3.7-fold at 96 h. All tissues showed a progressive increase in the proportion of radioactivity associated with AA with a concomitant decrease in radiolabelled GLA as the time after infusion increased. DGLA declined rapidly in liver and plasma, but at a much slower rate in brain and malignant tissue. Seventy-two hours after the infusion, GLA was only detected in plasma and tumour tissue. The sum of GLA + DGLA varied among tumour tissues, but it remained 2-4 times higher than in liver and plasma. In brain, DGLA is the major contributor to the sum of these fatty acids. Data showed that cytotoxic GLA and DGLA, the latter provided either by the host or by endogenous synthesis, remained in human tumours for at least 4 days.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner R. R. Endocrine control of fatty acid desaturation. Biochem Soc Trans. 1990 Oct;18(5):773–775. doi: 10.1042/bst0180773. [DOI] [PubMed] [Google Scholar]
- Bégin M. E., Das U. N., Ells G., Horrobin D. F. Selective killing of human cancer cells by polyunsaturated fatty acids. Prostaglandins Leukot Med. 1985 Aug;19(2):177–186. doi: 10.1016/0262-1746(85)90084-8. [DOI] [PubMed] [Google Scholar]
- Bégin M. E. Effects of polyunsaturated fatty acids and of their oxidation products on cell survival. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):269–313. doi: 10.1016/0009-3084(87)90069-7. [DOI] [PubMed] [Google Scholar]
- Bégin M. E., Ells G. Effects of C18 fatty acids on breast carcinoma cells in culture. Anticancer Res. 1987 Mar-Apr;7(2):215–217. [PubMed] [Google Scholar]
- Das U. N., Begin M. E., Ells G., Huang Y. S., Horrobin D. F. Polyunsaturated fatty acids augment free radical generation in tumor cells in vitro. Biochem Biophys Res Commun. 1987 May 29;145(1):15–24. doi: 10.1016/0006-291x(87)91281-2. [DOI] [PubMed] [Google Scholar]
- Das U. N., Prasad V. V., Reddy D. R. Local application of gamma-linolenic acid in the treatment of human gliomas. Cancer Lett. 1995 Aug 1;94(2):147–155. doi: 10.1016/0304-3835(95)03844-m. [DOI] [PubMed] [Google Scholar]
- De Tomás M. E., Mercuri O. Biosynthesis of lipids in tumoral cells. Adv Exp Med Biol. 1977;83:119–125. doi: 10.1007/978-1-4684-3276-3_11. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fearon K. C., Falconer J. S., Ross J. A., Carter D. C., Hunter J. O., Reynolds P. D., Tuffnell Q. An open-label phase I/II dose escalation study of the treatment of pancreatic cancer using lithium gammalinolenate. Anticancer Res. 1996 Mar-Apr;16(2):867–874. [PubMed] [Google Scholar]
- Hassam A. G., Crawford M. A. The incorporation of orally administered radiolabeled dihomo gamma-linolenic acid (20 : 3 omega 6) into rat tissue lipids and its conversion of arachidonic acid. Lipids. 1978 Nov;13(11):801–803. doi: 10.1007/BF02533479. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Tang D. G., Gao X., Butovich I. A., Liu B., Timar J., Hagmann W. 12-lipoxygenases and 12(S)-HETE: role in cancer metastasis. Cancer Metastasis Rev. 1994 Dec;13(3-4):365–396. doi: 10.1007/BF00666105. [DOI] [PubMed] [Google Scholar]
- Jiang W. G., Hiscox S., Hallett M. B., Horrobin D. F., Mansel R. E., Puntis M. C. Regulation of the expression of E-cadherin on human cancer cells by gamma-linolenic acid (GLA). Cancer Res. 1995 Nov 1;55(21):5043–5048. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Marra C. A., de Alaniz M. J. Incorporation and metabolic conversion of saturated and unsaturated fatty acids in SK-Hep1 human hepatoma cells in culture. Mol Cell Biochem. 1992 Nov 18;117(2):107–118. doi: 10.1007/BF00230749. [DOI] [PubMed] [Google Scholar]
- Morton R. E., Hartz J. W., Reitz R. C., Waite B. M., Morris H. P. The acyl-CoA desaturases of microsomes from rat liver and the Morris 7777 hepatoma. Biochim Biophys Acta. 1979 May 25;573(2):321–331. doi: 10.1016/0005-2760(79)90065-1. [DOI] [PubMed] [Google Scholar]
- Morton R. E., Waite M., King V. L., Morris H. P. Uptake and metabolism of free fatty acids by the Morris 7777 hepatoma and host rat liver. Lipids. 1982 Aug;17(8):529–537. doi: 10.1007/BF02535380. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Hobbs K., Clark G. M. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985 Feb;45(2):584–590. [PubMed] [Google Scholar]
- Pritchard G. A., Jones D. L., Mansel R. E. Lipids in breast carcinogenesis. Br J Surg. 1989 Oct;76(10):1069–1073. doi: 10.1002/bjs.1800761028. [DOI] [PubMed] [Google Scholar]
- Sakai T., Yamaguchi N., Shiroko Y., Sekiguchi M., Fujii G., Nishino H. Prostaglandin D2 inhibits the proliferation of human malignant tumor cells. Prostaglandins. 1984 Jan;27(1):17–26. doi: 10.1016/0090-6980(84)90217-x. [DOI] [PubMed] [Google Scholar]
- Voss A. C., Sprecher H. Metabolism of 6,9,12-octadecatrienoic acid and 6,9,12,15-octadecatetraenoic acid by rat hepatocytes. Biochim Biophys Acta. 1988 Feb 4;958(2):153–162. doi: 10.1016/0005-2760(88)90172-5. [DOI] [PubMed] [Google Scholar]
- de Antueno R. J., Cantrill R. C., Huang Y. S., Ells G. W., Elliot M., Horrobin D. F. Metabolism of n-6 fatty acids by NIH-3T3 cells transfected with the ras oncogene. Mol Cell Biochem. 1994 Oct 12;139(1):71–81. doi: 10.1007/BF00944205. [DOI] [PubMed] [Google Scholar]
- de Antueno R. J., Niedfeld G., De Tomás M. E., Mercuri O. F., Montoro L. Microsomal fatty acid desaturation and elongation in a human lung carcinoma grown in nude mice. Biochem Int. 1988 Mar;16(3):413–420. [PubMed] [Google Scholar]
- de Kock M., Lottering M. L., Seegers J. C. Differential cytotoxic effects of gamma-linolenic acid on MG-63 and HeLa cells. Prostaglandins Leukot Essent Fatty Acids. 1994 Aug;51(2):109–120. doi: 10.1016/0952-3278(94)90086-8. [DOI] [PubMed] [Google Scholar]
- du Toit P. J., van Aswegen C. H., du Plessis D. J. The effect of gamma-linolenic acid and eicosapentaenoic acid on urokinase activity. Prostaglandins Leukot Essent Fatty Acids. 1994 Aug;51(2):121–124. doi: 10.1016/0952-3278(94)90087-6. [DOI] [PubMed] [Google Scholar]


