Abstract
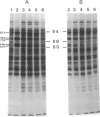
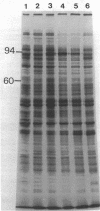
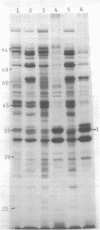
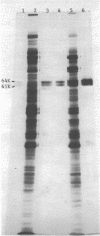
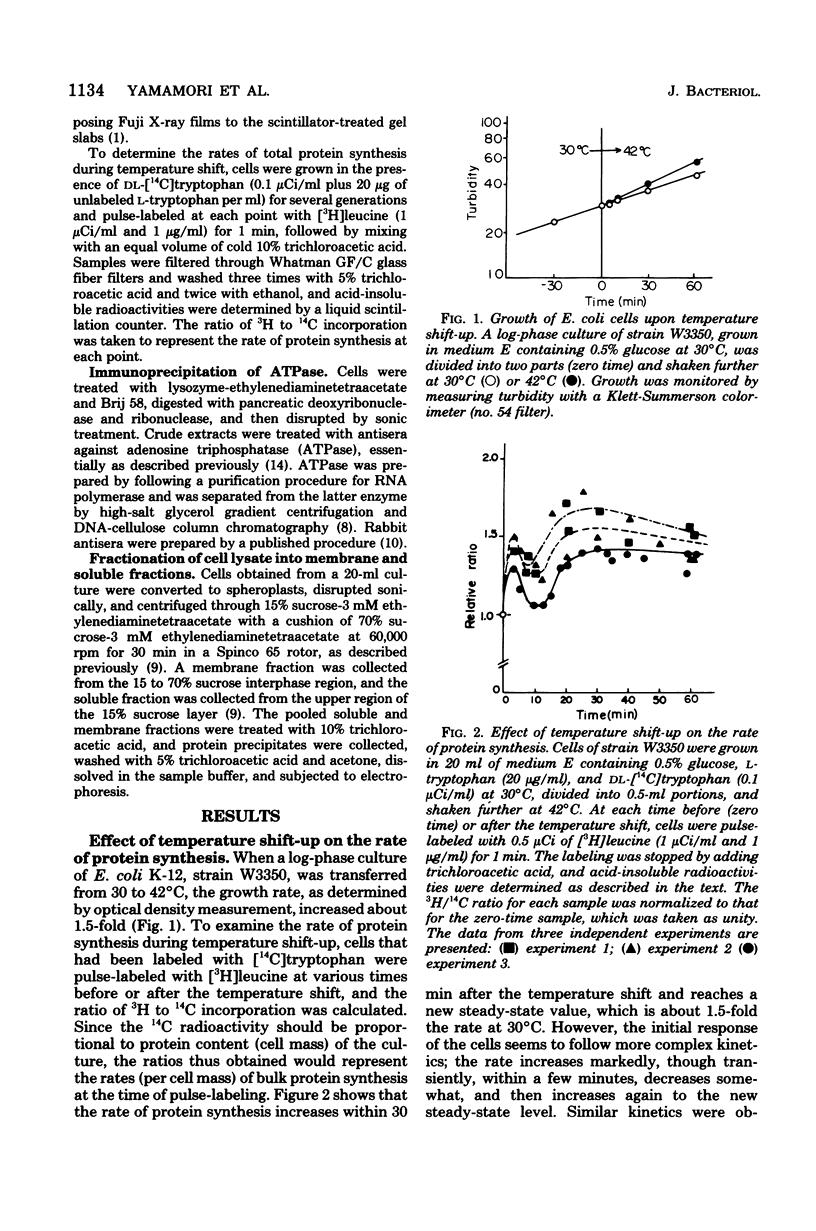
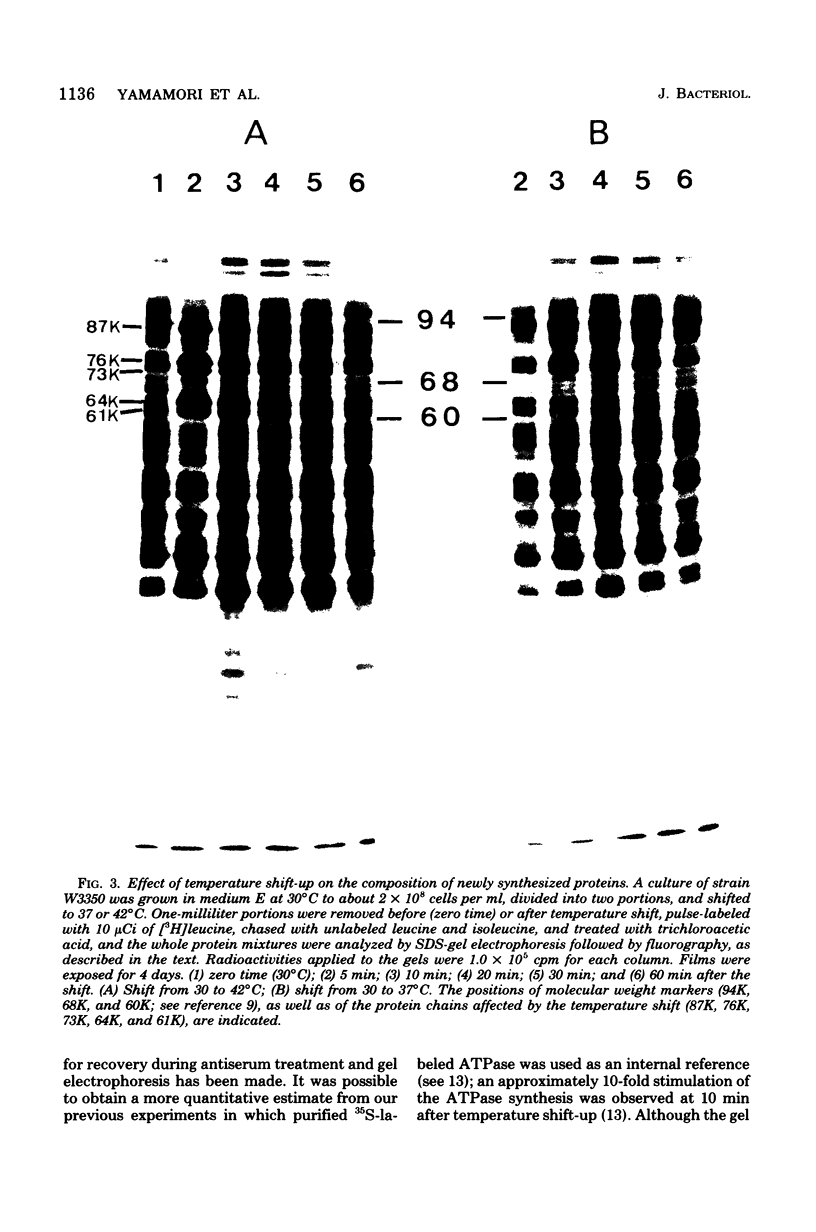
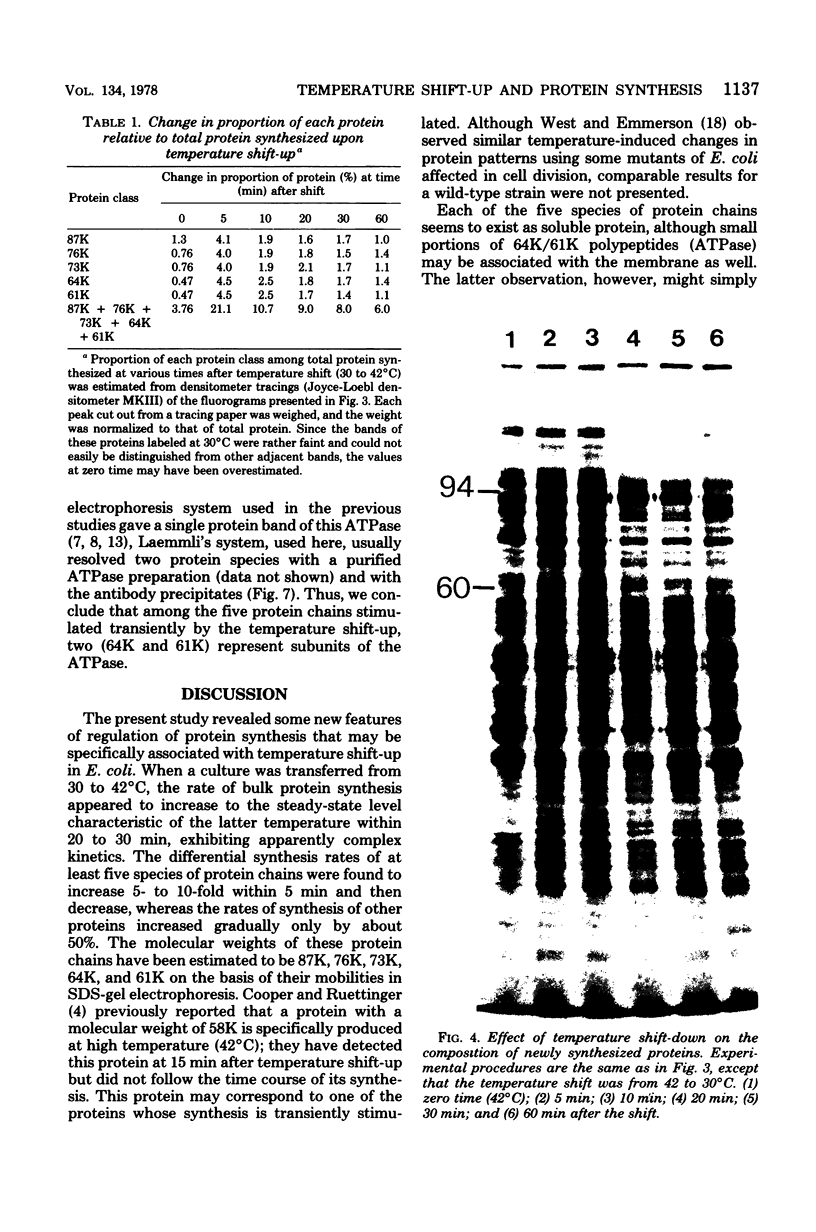
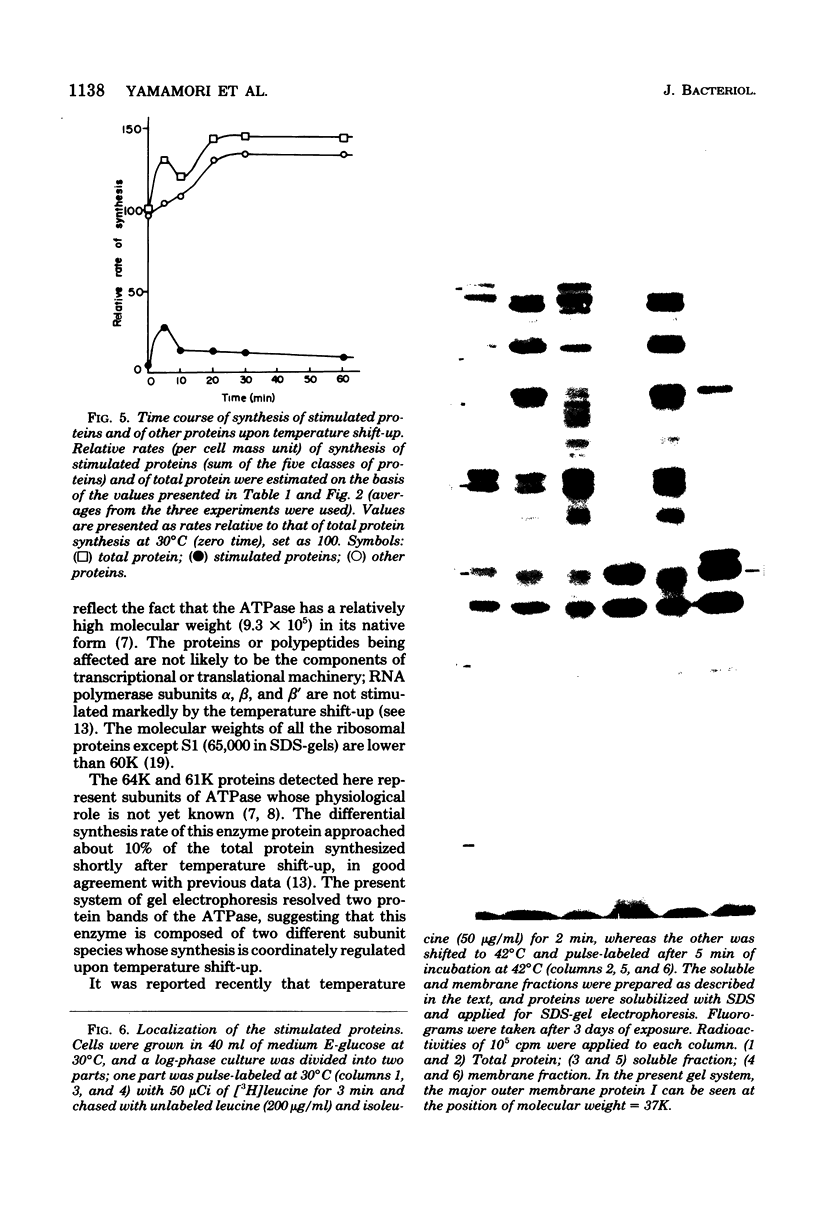
Synthesis of total cellular proteins of Escherichia coli was studied upon transfer of a log-phase culture from 30 (or 37) to 42 degrees C. Cells were pulse-labeled with [3H]leucine, and the labeled proteins were analyzed by gel electrophoresis in the presence of sodium dodecyl sulfate. The rates of synthesis of at least five protein chains were found to increase markedly (5- to 10-fold) within 5 min after temperature shift-up and gradually decrease to the new steady-state levels, in contrast to the majority of proteins which gradually increase to the steady-state levels (about 1.5-fold the rate at 30 degrees C). Temperature shift-down did not cause any appreciable changes in the pattern of protein synthesis as detected by the present method. Among the proteins greatly affected by the temperature shift-up were those with apparent molecular weights fo 87,000 (87K), 76K, 73K, 64K, and 61K. Two of them (64K and 61K) were found to be precipitated with specific antiserum against proteins that had previously been shown to have an adenosine triphosphatase activity. The bearings of these findings on bacterial adaptation to variation in growth temperature are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chaloner-Larsson G., Yamazaki H. Adjustment of RNA content during temperature upshift in Escherichia coli. Biochem Biophys Res Commun. 1977 Jul 25;77(2):503–508. doi: 10.1016/s0006-291x(77)80008-9. [DOI] [PubMed] [Google Scholar]
- Chao L. Gene expression in stringent and relaxed strains of Escherichia coli during amino acid deprivation. Biochem Biophys Res Commun. 1977 Jul 11;77(1):42–49. doi: 10.1016/s0006-291x(77)80162-9. [DOI] [PubMed] [Google Scholar]
- Cooper S., Ruettinger T. Temperature dependent alteration in bacterial protein composition. Biochem Biophys Res Commun. 1975 Feb 3;62(3):584–586. doi: 10.1016/0006-291x(75)90438-6. [DOI] [PubMed] [Google Scholar]
- Gallant J., Palmer L., Pao C. C. Anomalous synthesis of ppGpp in growing cells. Cell. 1977 May;11(1):181–185. doi: 10.1016/0092-8674(77)90329-4. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Ishihama A., Ikeuchi T., Yura T. A novel adenosine triphosphatase isolated from RNA polymerase preparations of Escherichia coli. I. Copurification and separation. J Biochem. 1976 May;79(5):917–925. doi: 10.1093/oxfordjournals.jbchem.a131159. [DOI] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Ikeuchi T., Imai M., Yura T. Escape synthesis of RNA polymerase subunits and termination factor rho following induction of prophage lambda in Escherichia coli. Mol Gen Genet. 1977 Feb 15;150(3):317–324. doi: 10.1007/BF00268131. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Evidence for a positive regulation of RNA polymerase synthesis in Escherichia coli. J Mol Biol. 1975 Oct 5;97(4):621–642. doi: 10.1016/s0022-2836(75)80063-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Hyperproduction of the sigma subunit of RNA polymerase in a mutant of Escherichia coli. Mol Gen Genet. 1975 Nov 24;141(2):97–111. doi: 10.1007/BF00267677. [DOI] [PubMed] [Google Scholar]
- Reeh S., Pedersen S., Friesen J. D. Biosynthetic regulation of individual proteins in relA+ and relA strains of Escherichia coli during amino acid starvation. Mol Gen Genet. 1976 Dec 22;149(3):279–289. doi: 10.1007/BF00268529. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- West S. C., Emmerson P. T. Induction of protein synthesis in Escherichia coli following UV- or gamma-irradiation, mitomycin C treatment or tif Expression. Mol Gen Genet. 1977 Feb 28;151(1):57–67. doi: 10.1007/BF00446913. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Yura T., Suzuki T., Iino T. Ribonucleic acid polymerase mutant of Escherichia coli defective in flagella formation. J Bacteriol. 1977 Oct;132(1):254–261. doi: 10.1128/jb.132.1.254-261.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]