Abstract
Chitinases of pathogens have been proposed as potential targets of vaccines or specific inhibitors. We studied the genomic organization, transcript levels, developmental expression, and biological function of chitinases in the rodent filarial nematode Acanthocheilonema viteae, a model organism for human-pathogenic filarial worms. Characterization of nine genomic clones from an A. viteae phage library and Southern blot experiments revealed the existence of three different chitinase genes, two of which could theoretically yield functional transcripts. The deduced proteins of these genes had the common modular organization of family 18 chitinases. Northern blot experiments and rapid amplification of cDNA ends-PCR with adult worms and larval stages showed that only one gene is expressed, with high variation in transcript levels, as determined by real-time PCR. Chitinase transcript levels were lowest in the late male stage 4 larva (L4) and peaked in the stage 3 larva (L3), which was corroborated by Western blotting. RNA interference (RNAi) experiments showed that treatment of L3 and adult female worms with double-stranded RNA of chitinase inhibited molting of L3 worms and hatching of microfilariae. RNAi also led to the death of 50% of female worms, revealing the essential role of chitinase in the life cycle of filarial nematodes.
Filarial nematodes are the causative agents of filariases, tropical diseases that afflict about 160 million people worldwide (27). Currently, there are neither safe and efficient drugs nor vaccines available to eliminate or prevent these infections, which makes the development of new control strategies a priority. Chitin, one of the most abundant polysaccharides in nature, is known to be present in the eggshell (7, 14, 26) and the microfilarial sheath (9) of nematodes and is an integral part of their pharynx (24, 30). Because chitin has not been found in vertebrates, enzymes associated with chitin metabolism might lend themselves as targets for the development of antihelminthic drugs and vaccines.
Chitin is metabolized consecutively by two types of glycoside hydrolases, as follows: chitinase breaks down the β-1,4-glycosidic bonds of chitin to release N-acetylglucosamine dimers, which are then acted upon by N-acetylglucosaminidase. Enzymes of the glycosyltransferase family are responsible for chitin synthesis.
Chitinases, in particular, have been proposed to have a role in remodeling processes during the molting of filariae and in the hatching of larvae from the eggshell (3, 5). However, the existence of large families of chitinases in the free-living nematode Caenorhabditis elegans suggests that these enzymes also fulfill other functions (17). The genome of this nematode contains 42 predicted glycoside hydrolase genes, comprising chitinase and N-acetylglucosaminidase genes (Carbohydrate-Active Enzymes server at http://afmb.cnrs-mrs.fr/CAZY). This raises questions regarding the large number of chitinases in nematodes and what particular roles these enzymes play in the worm's life cycle.
Filarial chitinases have been considered as vaccine candidates and targets of the host immune response, in particular against third-stage larvae (L3) and microfilarial stages (reviewed in reference 2). For example, passive immunization of jirds infected with Brugia malayi with an antichitinase monoclonal antibody significantly decreased the number of blood microfilariae. Furthermore, DNA vaccination with a chitinase gene inhibited the development of Onchocerca volvulus L3.
To understand the role of chitinases in filarial nematodes, we studied the structure of chitinase genes in Acanthocheilonema viteae, a rodent filarial worm used as a model to study human filarial infections. We determined the number of chitinase genes in the genome, studied their pattern of expression during the life cycle, and analyzed their function by performing RNA interference (RNAi) experiments.
MATERIALS AND METHODS
Maintenance of A. viteae.
The life cycle of A. viteae was maintained essentially as described previously (13).
Adult A. viteae worms were isolated from the subcutaneous and intramuscular tissues, the inguinal and subscapular regions, and periodically, the thoracic chamber of infected Meriones unguiculatus jirds. The female and male worms were collected separately in complete RPMI (RPMI 1640 medium supplemented with 1 unit/ml penicillin and streptomycin, 2 mM l-glutamate, and 10% fetal calf serum).
L3 of A. viteae were isolated from infected Ornithodoros moubata ticks. The ticks were cut medially, rinsed briefly in a petri dish with RPMI 1640 to remove blood meal and loose tissue, and incubated in RPMI medium for 1 h.
Postinvasive L3 and early L4 were obtained from jirds after 5 and 10 days as described previously (16).
Identification of genomic A. viteae chitinase genes.
An A. viteae genomic library (provided by Jörg Hirzmann, University of Giessen, Germany) was constructed in λ Dash II, using genomic DNAs from adult worms. The genomic DNAs were partially digested with MboI, size selected for inserts of between 9 and 23 kb, and cloned into BamHI sites. The library was amplified once. A digoxigenin (DIG)-labeled A. viteae chitinase probe of 1,115 nucleotides (nt) was produced by PCR amplification with a DIG PCR labeling system (Roche Diagnostics), using a cDNA template of A. viteae L3 chitinase (3) and forward (5′-CGGGATCCCTACGTTCGCGGATGTTAC) and reverse (5′-ATCCTCGAGTGTTTGCTCACTTTCAAGCCC) primers. Approximately 4 × 104 independent PFU of the above library was screened on duplicate nitrocellulose filters. Prehybridization was carried at 45°C for 6 h, followed by hybridization at 45°C for 12 h in hybridization solution (Roche Diagnostics). High-stringency posthybridization washes were carried out in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.5% sodium dodecyl sulfate (SDS) (25°C, 5 min), 2× SSC-0.1% SDS (65°C, 15 min, twice), and 0.1× SSC-0.1% SDS (65°C, 1 h, two changes). Membranes were incubated with anti-DIG alkaline phosphatase-conjugated antibody (Roche Diagnostics) as recommended. Blots were developed using disodium 3-(4-methoxyspiro[1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.1(3,7)]decan]-4-yl) phenyl phosphate (CSPD) and then exposed to X-ray film. Following four rounds of hybridization, clonal plaques were used for the purification and subsequent analysis/manipulation of phage DNA. Phage DNA was isolated and purified using standard methods (19).
Genomic inserts (between 10 and 14 kb) were released from lambda arms by NotI digestion and subcloned into pBluescript II KS(+) in Escherichia coli XL1-Blue, and recombinant plasmid DNA was used for restriction analysis. Sequencing (Agowa custom DNA sequencing service) was done using pBluescript recombinants directly or PCR fragments generated from the original insert by a PeqLab mid-range PCR system (PeqLab Biotechnology).
Genomic DNA isolation and Southern hybridization.
Genomic DNAs from adult A. viteae worms were isolated as described previously (16). An A. viteae chitinase-specific fragment (nt 502 to 1,200) was amplified by PCR and labeled with [α-32P]dCTP, using a random primer DNA labeling system (Life Technologies). Prehybridization was done for 1 h at 65°C in 6× SSC, 5× Denhardt's reagent, 0.5% SDS, and 100 μg/ml salmon sperm DNA; hybridization was done overnight. After the final wash, membranes were exposed to a phosphorimager plate for 3 h.
Isolation of total RNA, Northern blotting, and rapid amplification of cDNA ends (RACE).
Adult nematodes, L2, L3, blood microfilariae, and uterine microfilariae isolated from adult female A. viteae worms were homogenized in 100 μl of TRIzol RNA isolation reagent (Life Technologies), and RNAs were isolated following the recommended procedures of the manufacturer.
For Northern blot hybridization, total RNA (10 μg/lane) was separated by agarose gel electrophoresis under denaturing conditions and blotted onto Hybond N+ membranes. Production of DIG-labeled RNA probes and detection of hybridized samples by chemiluminescence were performed using a DIG Northern starter kit (Roche Diagnostics) following the recommendations of the supplier. Nucleic acid sequences corresponding to the glycosyl hydrolase domain of A. viteae chitinase gene I and exon M of gene III were generated by PCR and subcloned into pGEM-T Easy (Promega). Plasmid DNA was used as a template, and SP6 polymerase was used to generate DIG-labeled RNA probes.
For RACE-PCR, first-strand cDNA was synthesized from 1 μg total RNA, using poly(T) CDS-primer A (Smart RACE cDNA amplification kit; BD Biosciences Clontech) with PowerScript reverse transcriptase (BD Biosciences Clontech) following the manufacturer's instructions. The following gene-specific forward primers were designed from a conserved region of chitinase genomic sequence: exon J, 5′-GCGGTGCCTTTATGTGGACTCTTG; and nested primer, 5′-AAGTTGTGGCAAAGGTCCATA.
PAGE and Western blotting.
Adult worms, L3, L4, and blood and uterine microfilariae were homogenized and resuspended in Tris-buffered saline (TBS) containing a cocktail of protease inhibitors (Roche Applied Science). Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) by standard techniques under denaturing conditions (50 mM dithiothreitol), transferred to nitrocellulose membranes, and blocked for 1 h with 5% nonfat milk powder in TBS with 0.2% Tween 20, followed by incubation for 2 h with an anti-chitinase mouse serum generated against the recombinant N-terminal part of A. viteae chitinase (amino acids 18 to 391) (1:2,000 in blocking buffer). Following three washes of 5 min each with large volumes of wash buffer (TBS, 0.2% Tween 20), membranes were incubated for 1 h with horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G (1:3,500 in blocking buffer; Amersham Biosciences). After three washes, detection was done using the enhanced chemiluminescence substrate Luminol (ECL Western blotting detection reagents; Amersham Biosciences) following the manufacturer's instructions.
RNAi.
A 500-bp fragment of the A. viteae chitinase gene (GenBank accession no. U14638; bp 964 to 1464) was used as a template for the synthesis of double-stranded RNA (dsRNA) for A. viteae chitinase (AvChi dsRNA), using a MegaScript RNAi kit (Ambion) according to the manufacturer's instructions. An amplicon of the bacterial maltose binding protein (Mal) was used for the synthesis of Mal dsRNA, which served as a negative control for the RNAi experiments.
RNAi in A. viteae adults was carried out essentially as described previously (1), using a microvolume dialysis chamber system for culture of the worms. Briefly, groups of 5 adult female or 10 male worms were incubated in 500-μl microcentrifuge tubes containing 300 μl of culture medium (RPMI 1640, 100 units/ml penicillin and streptomycin, 2 mM l-glutamine) and sterile dsRNA, either AvChi or Mal dsRNA, at an end concentration of 750 pmol/ml. Tubes were covered with a single layer of dialysis membrane (molecular size cutoff, 15 kDa) and sealed by the original lock without the domed cap. This was removed and replace by the dialysis membrane. Each tube was immersed individually in a 50-ml Falcon tube containing 30 ml prewarmed culture medium supplemented with 10% fetal calf serum. Samples were incubated for 16 h at 37°C under 5% CO2. After 16 h, the nematodes were transferred to Falcon tubes with 10 ml of complete culture medium and cultured for an additional 16 h, 24 h, 36 h, or 48 h.
For RNAi experiments with L3 of A. viteae, the larvae were harvested from the tick host and washed three times in culture medium, and 100 L3 were soaked for 16 h at 37°C in a total volume of 100 μl containing either AvChi dsRNA or Mal dsRNA at a final concentration of 750 pmol/ml. The L3 were either frozen for RNA extraction or used to infect jirds (100 L3 per animal). At 4 days postinfection, the L3 were recovered by incubating minced tissues of the infected jirds in warm complete culture medium. The obtained late L3 were washed and cultured for another 78 h to observe molting to L4. The larvae were considered to have molted if they shed their cuticle into the medium.
Quantitative real-time PCR.
Real-time PCR was used to analyze the expression levels of chitinase in the different stages of A. viteae and also to quantify the loss of chitinase transcripts following RNAi. Real-time PCR was performed with a model 7300 real-time PCR system (Applied Biosystems), using TaqMan reagents (Applied Biosystems). Tropomyosin of A. viteae (AvTropo; GenBank accession no. AAB53807) was used as an endogenous control. To ensure that the assessment of transcription levels was not affected by contamination of the cDNA preparation with genomic DNA, the primers and probes were designed for the exon-exon junctions for both AvChi and AvTropo, as follows: AvChit-Forw, 5′-GGAGAGAGAAACATCCGGAACTG; AvChit-Rev, 5′-CTTTCTCGGCCCAATAATTTGCT; AvChit-Probe, TCTGTAATACCGAATTTGC; AvTropo-Forw, 5′-GGAAAAAGCAACTCATACAGCTGATG; AvTropo-Rev, TGTTAGCACGTTCTTCATCTTGGAA; and AvTropo-Probe, CCGACCGCGTTCGCA. The PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Initial experiments were done to check the efficiencies of the individual primer pairs, as recommended by the manufacturer (Applied Biosystems). The slopes of the standard curves for the chitinase and tropomyosin primer pairs were −3.31 and −3.29, respectively. Primer efficiencies (E%) for chitinase and tropomyosin were calculated from the slopes of the standard curves (E = 10−(1/slope) − 1) and found to be 100%, showing similar and good efficiencies.
Expression of chitinase transcript levels relative to the endogenous tropomyosin control transcript level was determined using the 2−ΔΔCT method as described previously (12). Values represent the relative amounts of chitinase transcripts normalized to the levels of the endogenous reference tropomyosin and the relative calibrator chitinase from the late male L3/L4, because it has the lowest level of chitinase expression of all stages.
Bioinformatic and statistical analyses.
Partial genomic sequences were aligned and assembled to contiguous genomic sequences, using MacVector 7.0 (Accelrys). The positions of splice sites and exon/intron structures and boundaries were predicted using NetGene2 (http://www.cbs.dtu.dk/services/NetGene2) and annotated by using the Artemis program. Sequences immediately 5′ of the start ATG (nt −1500 to +1) were analyzed for putative transcription factor binding sites and regulatory elements by using the MatInspector Professional program (Genomatix software).
Data are presented as mean values with standard deviations. Statistical significance was tested by the Wilcoxon test.
Nucleotide sequence accession numbers.
The sequences of A. viteae genomic chitinases have been submitted to GenBank (www.ncbi.nih.gov/Genbank) and assigned accession numbers EF052872 for gene I and EF052873 for genes II and III. A. viteae chitinase cDNA has been submitted to GenBank under accession number U14638.
RESULTS
The A. viteae genome contains three chitinase genes.
Screening of 4 × 104 recombinant phage of an A. viteae genomic library by plaque hybridization yielded a total of nine independent clones with inserts of 10 to 14 kb. Restriction analysis indicated three independent groups of inserts. Two representative clones of each group were sequenced by primer walking and revealed the presence of a total of three chitinase genes (Fig. 1). Two genes were clustered, separated only by a 3.4-kb intergenic region; the third gene was separated from this cluster and was not a directly adjoining sequence. Analysis revealed a highly similar structure for the chitinase genes, with few notable differences. Genes I and III have all the features of a regularly transcribed gene that could encode a functional protein, with a start ATG followed by 12 or 13 exons separated by 11 or 12 intron sequences, respectively. Both sequences have a regular stop codon and a consensus polyadenylation signal. Generally, the splice donor and acceptor sites follow the common GT/AG rule (21), with the exception of introns E and Z of gene III and intron K of gene I, which follow the GC/AG rule (23) and offer the possibility of alternative splicing.
FIG. 1.
Schematic diagram of Acanthocheilonema viteae chitinase gene sequences. Identified genes with lettered exons are shown with filled boxes. Blank boxes represent introns, while intergenic regions are shown as lines.
In comparison to genes I and III, gene II is incomplete and does not contain the first exon with the start ATG. The lack of the first exon with the start codon in the region upstream of exon B was confirmed by sequencing of two additional independent clones. There is also no alternative ATG codon located in the adjacent region up- or downstream of exon B that could serve as a start codon.
This suggests that this sequence represents a pseudogene without a regular translation product.
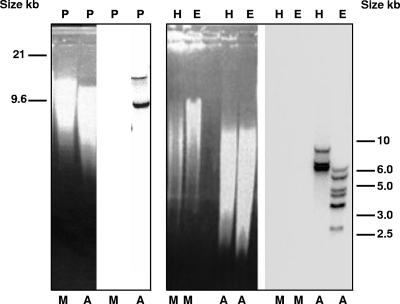
To confirm the number of chitinase genes in the A. viteae genome, we performed a Southern hybridization experiment. A 700-bp DNA probe was generated from an A. viteae chitinase cDNA (3) and hybridized under stringent conditions to genomic DNA digested with PvuII, HindIII, or EcoRI (Fig. 2). It was apparent that there were differences in the intensities of hybridization of different bands. For the PvuII digestion (Fig. 2, lanes P), which yielded two hybridized bands, the band of approximately 15 kb was recognized by fewer probe molecules than the fragment of 9.6 kb. The presence of more binding sites for the hybridization probe on the 9.6-kb fragment compared to the 15-kb fragment would be consistent with the localization of genes I and III on the 9.6-kb restriction fragment. This arrangement is likely, as there are no PvuII restriction sites in the corresponding genomic sequences containing these two chitinase genes. The hybridization patterns for the HindIII- and EcoRI-digested samples also correlated with the fragment sizes predicted from the known nucleic acid sequences. There are two HindIII sites in or around each chitinase gene, which will produce hybridizing restriction fragments of 7.7, 5.6, and 5.5 kb, while there are two EcoRI sites within each gene, which will lead to two hybridizing fragments per gene, resulting in a total of six fragments for the three genes.
FIG. 2.
Southern blot analysis of Acanthocheilonema viteae genomic DNA. DNAs from Meriones jirds (M) and A. viteae (A) (20 μg per lane) were digested with PvuII (P), HindIII (H), and EcoRI (E). Digested DNAs in ethidium bromide-stained gels are shown on the left, while hybridization results for both samples are shown on the right.
Thus, the results of the Southern blot experiments support the presence of three genes and the arrangement of these genes in the A. viteae genome.
Structure of the A. viteae chitinase genes.
A comparison of the deduced amino acid sequences of the exons showed a high similarity of 77 to 97% identity among the three genes. There is an apparent correlation of certain exons to functional protein domains. For example, the deduced amino acid sequence of exon A, present in genes I and III but missing in gene II, represents a typical signal peptide sequence with a potential cleavage site.
The enzymatically active glycosyl hydrolase domain is encoded by exons B to J. The active center is encoded by exon E, with the conserved amino acid sequence FDGFDLDWEYP, in which the essential glutamate residue is involved in the catalytic mechanism (22, 25). This region showed the highest degree of similarity, with 82 to 96% identical amino acids among the three genes. A comparative phylogenetic analysis of the glycosyl hydrolase domain revealed that A. viteae chitinase genes I and II are more closely related to each other than either is to A. viteae chitinase gene III (data not shown) and suggested that gene II is most likely a duplication of gene I.
The glycosyl hydrolase domain is followed by a highly diverse linker region that varies in all three A. viteae chitinase genes (Fig. 1). In gene I, exons K and L encode a serine-threonine-rich region. These exons are represented in gene III by one large related exon, called exon Z, which is also serine-threonine rich but is about six times longer. Exon Z contains, among others, a slightly modified motif of exon L that is repeated 19 times. Studies of other chitinases suggest that the potential glycosylation sites of this region are activated and that glycosylation leads to protection from proteases (4, 5). Gene II lacks exons encoding this linker region. The 3′ ends of all genes comprise exon M, which encodes a chitin-binding type 2 domain, responsible for substrate binding (21). The deduced amino acid sequence of exon M showed only a low similarity of 45 to 62% between the deduced gene products.
In general, the intron sequences showed only minor similarity between the different chitinase genes, with the exception of introns D to J of the glycosyl hydrolase domain region of genes I and II. On average, 97% of the nucleotides of these introns were identical. Interestingly, the amino acid sequences of the corresponding exons also showed a high degree of similarity (98% identical amino acids).
Expression of chitinase transcripts in different life stages of A. viteae.
To study the expression of chitinase genes in different stages of A. viteae, total RNAs were isolated from L3 and adult male and female worms and used for Northern blot analysis. For hybridization, we used an RNA probe corresponding to the glycosyl hydrolase domain, which shows >90% identity among the three genes, as well as an RNA probe specific for the sequence of exon M of gene III. Hybridization of the probe for the glycosyl hydrolase domain with RNAs from L3 and female worms revealed a band of about 1.6 kb for both stages. The probe for exon M of gene III did not hybridize with any band of RNA from L3 and females (data not shown), suggesting that gene III was not transcribed.
To reach a higher sensitivity of detection, total RNAs from uterine and blood microfilariae, L2, L3, L4, and adult male and female worms were reverse transcribed and used for RACE. We used a forward primer that was conserved among all three chitinase sequences upstream of the variable serine-threonine-rich linker region. It was expected to yield products of 0.6 and 2.0 kb for genes I and III, respectively, while amplification of any transcripts of gene II would have yielded a 0.3-kb product. PCR amplification produced a major band of 0.6 kb for all life cycle stages (data not shown), which was confirmed by nested PCR and by sequencing to be a transcript of gene I. There were some faint bands visible in the PCR samples for males, females, microfilariae, and L3 stages, respectively, but nested PCR experiments confirmed that these were nonspecific amplification products.
To quantify chitinase gene I transcripts in different life stages of A. viteae, real-time PCR experiments were performed (Fig. 3). In comparison to the chitinase transcript amount of late male L4, the total RNAs of uterine and blood microfilariae contained 15.2 and 12.7 times more transcripts, respectively. L2 contained 92 times and L3 contained 150 times larger amounts of chitinase transcripts. During the development to L4, chitinase transcript levels continuously decreased to a very small amount. At the late L4 stage (24 days postinfection), when male and female worms can be differentiated on the basis of their morphology, male L4 had the lowest chitinase transcript levels, while transcripts in female L4 were 2.5 times higher. This difference in transcript levels between males and females remained constant, although the levels increased for mature adult worms.
FIG. 3.
Quantification of chitinase transcripts in different Acanthocheilonema viteae stages by real-time PCR. The ordinate depicts the log10 values of relative chitinase expression, calculated by the 2−ΔΔCT method. The values represent the relative amounts of chitinase normalized to the endogenous reference (tropomyosin) and relative to the calibrator (chitinase) from the late male L4, since it had the lowest level of chitinase expression of all life stages.
To characterize potential chitinase translation products, equal amounts of total protein homogenates of different parasite stages were analyzed by immunoblotting, using a mouse serum against the N terminus of A. viteae chitinase for detection (Fig. 4). The N terminus represents the most conserved part, including the glycosyl hydrolase domain. Therefore, an appropriate serum should recognize all potential versions of A. viteae chitinases genes. For L3, bands of 68 kDa and a high molecular mass of about 200 kDa were immunostained, while for microfilariae only the 68-kDa protein was detected. In contrast, early L4 contained only the high-molecular-mass protein of about 200 kDa. No chitinase protein bands were detectable in male and female worms. However, using uterine contents of female worms, the 68-kDa band and a faint band of about 200 kDa were detectable.
FIG. 4.
Western blot analysis of expression of chitinase protein in different stages of Acanthocheilonema viteae. Under reducing conditions (50 mM dithiothreitol), 12 μg of A. viteae male (M) and female (F) worms, blood microfilariae (Mf), uterine microfilariae (ut Mf), and L3 and L4 homogenates of 42 larvae were resolved by 12% SDS-PAGE and transferred to nitrocellulose. Blots were probed with mouse anti-A. viteae chitinase serum.
Suppression of chitinase transcripts by RNAi inhibits microfilarial development and molting of L3.
To determine the function of chitinase in male and female worms as well as in L3 of A. viteae, we performed RNAi experiments using dsRNA for chitinase, with dsRNA for the maltose binding protein of E. coli (Mal) as a control. Soaking of male worms with dsRNA for A. viteae chitinase did not cause any changes in phenotype compared to the control males treated with Mal dsRNA. In contrast, soaking of female worms resulted in the death of 50% of the parasites during the 16-h incubation period, whereas exposure to Mal dsRNA did not reduce the viability of worms (Table 1). Analysis of chitinase RNA levels by real-time PCR revealed a 20% lower level of chitinase in female worms treated with chitinase dsRNA than that in control worms treated with Mal dsRNA. However, chitinase RNA levels in uterine contents of female worms 24 h after dsRNA treatment were reduced nearly 85% in comparison to those in the uterine contents of control worms (Table 1). Soaking of the female worms in dsRNA for A. viteae chitinase also resulted in a 65% reduction of the release of microfilariae during the 48-h observation period in comparison to that for control worms (Fig. 5). In addition, there was a clear morphological difference in that about 50% of microfilariae from AvChi dsRNA-treated females were still surrounded by the eggshell, while control worms nearly exclusively gave birth to hatched microfilariae.
TABLE 1.
Phenotypic changes and decrease in chitinase transcripts after chitinase RNAi treatment of different A. viteae stagesa
| Parasite stage | Phenotypic change(s) | Influence of RNAi on chitinase transcript level |
|---|---|---|
| L3 | 87% inhibition of molting to L4 | 93% decrease |
| Adult female | 50% mortality, 57 to 68% decrease in release of microfilariae, 42 to 58% inhibition of hatching of microfilariae | 18 to 20% decrease in chitinase transcripts of adult female worms, 85% decrease in chitinase transcripts of uterine contents |
| Adult male | No changes | No decrease |
Values depict averages for three independent experiments.
FIG. 5.
Reduction of release of microfilariae from female worms following RNAi of Acanthocheilonema viteae chitinase gene I. AvChi dsRNA, a total of 30 adult worms (six groups of five worms each) were treated with chitinase dsRNA; Mal dsRNA, 30 adult worms (six groups of five worms each) were treated with dsRNA for the control maltose binding protein. Asterisks represent significant differences between AvChi dsRNA- and Mal dsRNA-treated groups (P < 0.01).
To determine the possible function of chitinase during molting, L3 were incubated for 16 h in A. viteae chitinase dsRNA and then used to infect Meriones jirds. The larvae were allowed to develop for 4 days and then isolated and observed for an additional 44 h. Only 8% of them molted to L4, in comparison to 60% of L3 that had been treated with control Mal dsRNA. This approximates an 87% decrease in molting by RNAi treatment. Determination of chitinase transcript levels in L3 after RNAi treatment by quantitative real-time PCR showed a 93% decrease in comparison to those in controls treated with Mal dsRNA (Table 1).
DISCUSSION
Although chitinases of several filarial nematodes have been described at the molecular level (3, 8, 18, 28), relatively little is known about the function of these proteins during the life cycle of the parasites. Several questions have yet to be answered, including the following. (i) Are the chitinases described for L3 and microfilariae encoded by the same gene? (ii) Do other chitinases exist in other stages? (iii) If this is indeed a gene family, what role do the other enzymes play? It is important to answer these questions, as chitinases represent important vaccine and drug targets. In this context, the existence of several paralogues which could replace each other would require other strategies of intervention compared to the presence of a single protein. To address these issues, we studied the organization of chitinase genes and their pattern of expression.
According to our data, A. viteae contains three different chitinase genes but expresses only one. This finding was surprising, as two genes showed all the necessary features to form functional transcripts. Southern blot analysis of B. malayi (6, 8) and Wucheria bancrofti (18) DNAs showed that at least two chitinase genes are present in the genomes of these filariae. However, the hybridization patterns of Southern blots and different intensities of bands suggested, as in our experiments, that the chitinase genes are arranged in a clustered form. For this reason, it is likely that more than two copies of chitinase genes also exist in Brugia and Wucheria filariae. In addition, searching for chitinase-like genes in the preliminary B. malayi genome database (www.tigr.org/parasiteProjects.shtml) revealed four different contigs containing chitinase genes. The analysis of the B. malayi database suggests that two of these genes are clustered, being separated from each other by an intergenic region of 6 kb. Interestingly, evaluation of B. malayi expressed sequence tag data suggests that only one chitinase gene of the four genes is expressed.
Using two independent approaches, we demonstrated transcripts for only chitinase gene I in A. viteae. The mRNA of this gene is present in all stages of the life cycle, albeit with great differences regarding transcript levels. Despite our detailed analysis and sensitive examination of all stages, we cannot absolutely exclude the existence of an additional transcript that could be expressed for a short window of time, e.g., during prolonged maturation within the vertebrate host. Tightly regulated expression has been demonstrated for another enzyme involved in chitin metabolism, the chitin synthase of C. elegans. In this case, two genes exist, only one of which is expressed in a period of 4 hours preceding the molt to L4 (24). However, if the chitinase encoded by gene III was expressed and escaped our analysis, it is likely that the expression would be transient.
Chitinase transcripts were found in all stages, but detectable proteins were identified only in microfilariae, early L4, and L3. In Western blots of L3, two protein bands, with apparent molecular masses of about 200 kDa and 68 kDa, were visible, but for microfilariae, only the 68-kDa protein was recognized, and for L4, only the 200-kDa protein was recognized. No chitinase protein was detected in male or female worms, but the uterus contents of female worms, which are enriched in developing microfilariae, contained detectable amounts of chitinase. In addition, our Western blot experiments corroborated that L3 had the largest amounts of chitinase protein in comparison to microfilariae and L4.
Previous analyses by Western blotting with different stages of A. viteae also identified two chitinase protein bands, of 205 and 68 kDa, and the authors presented evidence that the 205-kDa band of L3 was an oligomer formed from 68-kDa monomers (3).
The high levels of chitinase transcript and protein in L3 raise the question of the function of chitinase in this particular stage. Our RNAi studies showed that this protein is critical in the process of molting, as knockdown prevented about 87% of L3 from molting. However, chitin has so far not been described as a component of the cuticle of nematodes, and even for well-studied nematodes, such as C. elegans, it has been described only as a component of the pharynx (24, 30). Immunoelectron microscopy analysis detected chitinase in the pharyngeal glands of L3 of A. viteae (3) and O. volvulus (29).
Because it is unlikely that remodeling of the pharynx alone requires large amounts of chitinase, we suppose that the nematode cuticle contains chitin-like polysaccharides or glycosylated proteins that might be targets of chitinases. Several nematode molecules, such as the secreted phosphorylcholine-containing glycoprotein ES-62 (11), contain 1,4-beta-linked N-acetylglucosamines. Further studies should address whether chitinase could have a role in remodeling such components of the cuticle or other compartments of nematodes during development. Ultrastructural work showed that the chitinase of O. volvulus L3 is stored within granules of cells of the esophageal glands. During postinfective development, degranulation and secretion occurred within 48 h of in vitro culture (29). Thereafter, chitinase was found mostly in the cuticle of O. volvulus L3. The route of transportation from the esophageal glands to the surfaces of L3 was not ascertained in the above-mentioned study, but it was suggested that material from the esophageal glands can be transported to the cuticle via a system of lacunae, suggesting transport of chitinase from the glands via a pseudocoelom to the cuticle (15).
This form of release is compatible with a role of chitinase in remodeling of the L4 cuticle and casting of the L3 cuticle but also with a concomitant release into the host tissues, as observed in vitro (3). Whether the release of chitinase in substantial amounts into the host tissue would influence the host has yet to be addressed.
Chitin has been described as a component of the eggshells of nematodes (7, 14, 26) and the sheaths of microfilariae of some filarial species (9). The microfilarial sheath represents a modified and extended eggshell, which in some filarial species, e.g., A. viteae, is cast within the uterus, such that unsheathed microfilariae are born. Furthermore, eggshells of the filarial worms are permeable for dsRNA, as successful RNAi experiments demonstrated (1). Earlier ultrastructural studies revealed that immature uterine microfilariae of A. viteae bear chitinase within their eggshells, while the protein appears on the surface of the cuticle when maturation is completed (3). This localization is compatible with the observed phenotype of microfilariae born from RNAi-treated female worms.
Treatment of female A. viteae worms with chitinase dsRNA resulted in the release of microfilariae that were mostly unhatched and still surrounded by the eggshell. This observation proves that chitinase is relevant for casting of the eggshell within the uterus. The high mortality of female worms, which was in stark contrast to the vitality of male worms, suggests that disturbance of chitin metabolism in female worms has gender-specific fatal consequences beyond the inhibition of eggshell casting. A simple explanation would be that unhatched microfilariae cannot be born and congest the female reproductive system, but we also cannot exclude the possibility that chitinase plays an additional hitherto unknown role in the metabolism of female worms.
In conclusion, our studies show that A. viteae chitinase has a vital function in at least two stages of the life cycle of the parasite. Inhibition of chitinase in both of these stages, i.e., L3 and female worms, leads to substantial mortality. The fact that only one of the two intact genes is transcribed to a detectable degree suggests that possible intervention measures could be targeted to one protein.
Further studies have to show whether parasitic nematodes of other taxa have similar chitin biology, in which case the inhibition of chitinases by chemical compounds or vaccines could be a valuable approach to control a wide spectrum of nematode infections.
Acknowledgments
This work was supported by grants from the German Research Foundation (Lu325/8-1, Lu325/8-2, and Lu325/10-1). Babila Tachu was the recipient of a scholarship from the German Academic Exchange Service for his Ph.D. work.
We thank Juliet Fuhrman, Francine Perler, and Frank Seeber for critical readings and helpful comments in preparation of the manuscript.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Aboobaker, A. A., and M. L. Blaxter. 2003. Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. Mol. Biochem. Parasitol. 12941-51. [DOI] [PubMed] [Google Scholar]
- 2.Adam, R., B. Drabner, and R. Lucius. 2001. Chitinase of filarial nematodes, p. 195-210. In M. Kennedy and W. Harnett (ed.), Parasitic nematodes: molecular biology, biochemistry and immunology. CAB International, Wallingford, United Kingdom.
- 3.Adam, R., B. Kaltmann, W. Rudin, T. Friedrich, T. Marti, and R. Lucius. 1996. Identification of chitinase as the immunodominant filarial antigen recognized by sera of vaccinated rodents. J. Biol. Chem. 2711441-1447. [DOI] [PubMed] [Google Scholar]
- 4.Arakane, Y., Q. Zhu, M. Matsumiya, S. Muthukrishnan, and K. J. Kramer. 2003. Properties of catalytic, linker and chitin-binding domains of insect chitinase. Insect Biochem. Mol. Biol. 33631-648. [DOI] [PubMed] [Google Scholar]
- 5.Arnold, K., L. J. Brydon, L. H. Chappell, and G. W. Gooday. 1993. Chitinolytic activities in Heligmosomoides polygyrus and their role in egg hatching. Mol. Biochem. Parasitol. 58317-323. [DOI] [PubMed] [Google Scholar]
- 6.Arnold, K., A. Venegas, C. Houseweart, and J. A. Fuhrman. 1996. Discrete transcripts encode multiple chitinase isoforms in brugian microfilariae. Mol. Biochem. Parasitol. 80149-158. [DOI] [PubMed] [Google Scholar]
- 7.Brydon, L. J., G. W. Gooday, L. H. Chappell, and T. P. King. 1987. Chitin in egg shells of Onchocerca gibsoni and Onchocerca volvulus. Mol. Biochem. Parasitol. 25267-272. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman, J. A., W. S. Lane, R. F. Smith, W. F. Piessens, and F. B. Perler. 1992. Transmission-blocking antibodies recognize microfilarial chitinase in brugian lymphatic filariasis. Proc. Natl. Acad. Sci. USA 891548-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuhrman, J. A., and W. F. Piessens. 1985. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol. Biochem. Parasitol. 1793-104. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Houston, K. M., and W. Harnett. 2004. Structure and synthesis of nematode phosphorylcholine-containing glycoconjugates. Parasitology 129655-661. [DOI] [PubMed] [Google Scholar]
- 12.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 13.Lucius, R., and G. Textor. 1995. Acanthocheilonema viteae: rational design of the life cycle to increase production of parasite material using less experimental animals. Appl. Parasitol. 3622-33. [PubMed] [Google Scholar]
- 14.Mansfield, L. S., H. R. Gamble, and R. H. Fetterer. 1992. Characterization of the eggshell of Haemonchus contortus. I. Structural components. Comp. Biochem. Physiol. B 103681-686. [DOI] [PubMed] [Google Scholar]
- 15.McKerrow, J. H., T. Huima, and S. Lustigman. 1999. Do filarid nematodes have a vascular system? Parasitol. Today 15123. [DOI] [PubMed] [Google Scholar]
- 16.Pogonka, T., U. Oberlander, T. Marti, and R. Lucius. 1999. Acanthocheilonema viteae: characterization of a molt-associated excretory/secretory 18-kDa protein. Exp. Parasitol. 9373-81. [DOI] [PubMed] [Google Scholar]
- 17.Popovici, C., R. Roubin, F. Coulier, P. Pontarotti, and D. Birnbaum. 1999. The family of Caenorhabditis elegans tyrosine kinase receptors: similarities and differences with mammalian receptors. Genome Res. 91026-1039. [DOI] [PubMed] [Google Scholar]
- 18.Raghavan, N., D. O. Freedman, P. C. Fitzgerald, T. R. Unnasch, E. A. Ottesen, and T. B. Nutman. 1994. Cloning and characterization of a potentially protective chitinase-like recombinant antigen from Wuchereria bancrofti. Infect. Immun. 621901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, New York.
- 20.Senapathy, P., M. B. Shapiro, and N. L. Harris. 1990. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 183252-278. [DOI] [PubMed] [Google Scholar]
- 21.Suetake, T., S. Tsuda, S. Kawabata, K. Miura, S. Iwanaga, K. Hikichi, K. Nitta, and K. Kawano. 2000. Chitin-binding proteins in invertebrates and plants comprise a common chitin-binding structural motif. J. Biol. Chem. 27517929-17932. [DOI] [PubMed] [Google Scholar]
- 22.Synstad, B., S. Gaseidnes, D. M. Van Aalten, G. Vriend, J. E. Nielsen, and V. G. Eijsink. 2004. Mutational and computational analysis of the role of conserved residues in the active site of a family 18 chitinase. Eur. J. Biochem. 271253-262. [DOI] [PubMed] [Google Scholar]
- 23.Thanaraj, T. A., and F. Clark. 2001. Human GC-AG alternative intron isoforms with weak donor sites show enhanced consensus at acceptor exon positions. Nucleic Acids Res. 292581-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veronico, P., L. J. Gray, J. T. Jones, P. Bazzicalupo, S. Arbucci, M. R. Cortese, M. Di Vito, and C. De Giorgi. 2001. Nematode chitin synthases: gene structure, expression and function in Caenorhabditis elegans and the plant parasitic nematode Meloidogyne artiellia. Mol. Genet. Genomics 26628-34. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe, T., K. Kobori, K. Miyashita, T. Fujii, H. Sakai, M. Uchida, and H. Tanaka. 1993. Identification of glutamic acid 204 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J. Biol. Chem. 26818567-18572. [PubMed] [Google Scholar]
- 26.Wharton, D. 1980. Nematode egg-shells. Parasitology 81447-463. [DOI] [PubMed] [Google Scholar]
- 27.WHO/TDR. 2005. Tropical disease research: progress 2003-2004. Seventeenth programme report of TDR. World Health Organization, Geneva, Switzerland.
- 28.Wu, Y., R. Adam, S. A. Williams, and A. E. Bianco. 1996. Chitinase genes expressed by infective larvae of the filarial nematodes, Acanthocheilonema viteae and Onchocerca volvulus. Mol. Biochem. Parasitol. 75207-219. [DOI] [PubMed] [Google Scholar]
- 29.Wu, Y., G. Egerton, A. P. Underwood, S. Sakuda, and A. E. Bianco. 2001. Expression and secretion of a larval-specific chitinase (family 18 glycosyl hydrolase) by the infective stages of the parasitic nematode, Onchocerca volvulus. J. Biol. Chem. 27642557-42564. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y., J. M. Foster, L. S. Nelson, D. Ma, and C. K. Carlow. 2005. The chitin synthase genes chs-1 and chs-2 are essential for C. elegans development and responsible for chitin deposition in the eggshell and pharynx, respectively. Dev. Biol. 285330-339. [DOI] [PubMed] [Google Scholar]