Abstract
Borrelia burgdorferi, the causative agent of Lyme disease, undergoes rapid adaptive gene expression in response to environmental signals encountered during different stages of its life cycle in the arthropod vector or the mammalian host. Among all the plasmid-encoded genes of B. burgdorferi, several linear plasmid 54 (lp54)-encoded open reading frames (ORFs) exhibit the greatest differential expression in response to mammalian host-specific temperature, pH, and other uncharacterized signals. These ORFs include members of the paralogous gene family 54 (pgf 54), such as BBA64, BBA65, and BBA66, present on lp54. In an attempt to correlate transcriptional up-regulation of these pgf 54 members to their role in infectivity, we inactivated BBA64 and characterized the phenotype of this mutant both in vitro and in vivo. There were no major differences in the protein profiles between the BBA64 mutant and the control strains, while immunoblot analysis indicated that inactivation of BBA64 resulted in increased levels of BBA65. Moreover, there was no significant difference in the ability of the BBA64 mutant to infect C3H/HeN mice compared to that of its parental or complemented control strains as determined by culturing of viable spirochetes from infected tissues. However, enumeration of spirochetes using quantitative real-time PCR revealed tissue-specific differences, suggesting a minimal role for BBA64 in the survival of B. burgdorferi in select tissues. Infectivity analysis of the BBA64 mutant suggests that B. burgdorferi may utilize multiple determinants to establish infection in mammalian hosts.
Lyme disease is the most prevalent arthropod-borne infection in the United States and remains a significant public health issue in certain geographic loci (3, 46). It is a multiphasic disorder with clinical symptoms involving the cutaneous, musculoskeletal, cardiovascular, and nervous systems (62). Borrelia burgdorferi, the causative agent of Lyme disease, is transmitted to several vertebrate hosts, including humans, by the bite of infected Ixodes ticks. When ticks consume a blood meal from mammalian hosts, there is a rapid alteration of gene expression in B. burgdorferi, facilitating adaptation of the spirochetes to the highly disparate environmental conditions that exist between the tick vector and the vertebrate host (4, 9, 10, 15, 29, 30-32, 44, 53, 57-59, 63, 67). This adaptive gene expression may aid in the efficient trafficking of the spirochetes from the tick vector to the mammalian host and subsequently facilitate dissemination and colonization of various host tissues (16, 22).
Whole-genome transcriptional analyses using B. burgdorferi, propagated under in vitro growth conditions that mimic either the tick vector or mammalian host environment, have revealed preferential expression of plasmid-encoded genes (4, 44, 53, 66). Among the several linear and circular plasmids present in B. burgdorferi, linear plasmid 54 (lp54) encodes the largest number of open reading frames (ORFs) that exhibit differential gene expression in response to these changing environmental signals. Moreover, lp54 encodes adhesins, such as outer surface protein A (OspA; BBA15) (15, 17, 18, 48, 70) and decorin binding proteins A and B (DbpA and DbpB; BBA24/BBA25) (23, 24), as well as pH-regulated (9, 10) and temperature-regulated proteins, such as the members of the paralogous gene family 54 (pgf 54) (22). Members of pgf 54 that are clustered on the right terminal region of lp54 are a part of the lp54-specific “transcriptome” observed in response to exposure of B. burgdorferi to multiple mammalian host-specific signals (2, 11, 21, 44, 45, 53, 66). Among these pgf 54 members, BBA64, BBA65, BBA66, and BBA73 have been shown to be transcriptionally elevated in response to mammalian host-specific pH (7.0) or temperature (35°C) present in fed ticks (12, 44, 53). This is in contrast to a lack of expression of BBA64 and BBA66 at pH 8.0 and 23°C, mimicking the conditions associated with unfed ticks (66). A recent study demonstrated the presence of antibodies against BBA64 and BBA66 in the serum of mice experimentally infected with B. burgdorferi and in serum samples of patients diagnosed with early, disseminated Lyme disease (42). Moreover, the temporal expression of various members of the pgf 54 in an infected mammalian host was also attributed to the involvement of multiple regulatory pathways (12, 22). Taken together, these observations suggest that BBA64 may play a role in B. burgdorferi infection either independently or in association with other members of the pgf 54.
Transcriptional up-regulation of an ORF in B. burgdorferi grown under mammalian host-specific conditions and/or the presence of serum antibodies against an antigenic determinant in infected hosts are two commonly used correlates for establishing the significance of an individual ORF in the infectious process (4, 5, 16, 44, 53). However, a more direct approach would be to generate strains with mutations in one or more differentially expressed ORFs and determine their infectivities with the appropriate animal models. This would help establish a correlation between signal-dependent transcriptional regulation of an ORF and its role in the infectious process. The purpose of the present study was to determine whether the inactivation of BBA64 leads to attenuation of infectivity with the murine model of Lyme disease.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A clonal derivative of B. burgdorferi sensu stricto strain B31, ML23, lacking linear plasmid 25 (lp25), was exclusively used for generation of mutants (mts) (36). Another clonal derivative, MSK5, which contains all plasmids, was also used in infectivity experiments (35, 36). All B. burgdorferi cultures used for transformation were grown in 1% CO2 at 32°C in Barbour-Stoenner-Kelly II (BSK-II) liquid medium supplemented with 6% normal rabbit serum (Pel-Freez Biologicals, Rogers, AR). We propagated various B. burgdorferi strains to a density of 1 × 108 to 2 × 108/ml of spirochetes in BSK-II growth medium (40-ml cultures) that mimicked tick midgut conditions before (pH 7.6 and 23°C) and after (pH 6.8 and 37°C) a blood meal or shifted from pH 7.6/23°C to pH 6.8/37°C (67). A combination of the above conditions was also employed to dissect the roles of pH and temperature as either discrete or overlapping signals in the regulation of expression of the pgf 54 members. Escherichia coli TOP10 cells were used for all cloning steps and for transformation of PCR products cloned into the pCR2.1-TOPO vector (Invitrogen Corp., Carlsbad, CA).
In vitro mutagenesis.
A 4.5-kb region of lp54 extending from the 3′ end of BBA64 to the 5′ end of BBA68 was amplified using primers BBA64R and BBA68F (see Table 2). The amplicon was ligated into the pCR2.1-TOPO cloning vector, and a clone, designated pMM2, containing a region of lp54 between the nucleotide coordinates 42563 and 47080 was used for further manipulation (Table 1). An in vitro mutagenesis strategy was employed to insertionally inactivate BBA64 using the GPS-Mutagenesis system (New England Biolabs, Ipswich, MA) as described previously (60). Briefly, the reaction was assembled using pMM2 as the “target” and pML102 containing a customized “donor” transposon (PflgB-Strr flanked by Tn7 ends in the pGPS3 vector) in the presence of Tn7-specific transposase (TnpABC) for 1 h at 37°C. The “donor” plasmid was inactivated using the PI-SceI enzyme, and the mutagenized target plasmids were transformed into E. coli TOP10 cells (Invitrogen) and selected on LB agar plates supplemented with kanamycin (50 μg/ml), ampicillin (100 μg/ml), and spectinomycin (100 μg/ml). Plasmids with potential transposon “hits” resulting in insertional inactivation of BBA64 were identified through restriction enzyme digestion analysis, and the site of insertion was determined by sequence analysis. A plasmid, designated pMM4, containing a transposon insertion at position 154 relative to the 5′ end of BBA64 was chosen and used for the generation of the BBA64 mt.
TABLE 2.
Oligonucleotides used in this study
| Primer designation | Sequence (5′→3′)a |
|---|---|
| BBA64F | ACGCCATATGGACAGCAATGAAAGCAAA |
| BBA64R | ACGCCTCGAGCTGAATTGGAGCAAGAAT |
| BBA65F | ACGCCATATGGATCTAAACAACAAAGAC |
| BBA68F | ACGCCATATGAGCAAAATCGATCCTAAA |
| Tn7N | ACTTTATTGTCATAGTTTAGATCTATTTTG |
| Tn7S | TCCCCACCTTTACCTCAAAAATTCCTAATA |
| actF | TCACCCACACTGTGCCCATC (Ref 47) |
| actR | GGATGCCACAGGATTCCAT (Ref 47) |
| flagellinF | TCTTTTCTCTGGTGAGGGAGC |
| flagellinR | CCAAGAAGGAAGGCTGGAAAA |
| LE/b-actinF | CAAGTCATCACTATTGGCAACGA |
| LE/b-actinR | CCAAGAAGGAAGGCTGGAAAA |
| E22F | ACGCGGTACCTTTTTATATTGTGAGCCGGTT |
| E22R | ACGCGGTACCTCTATGTATCCCCTTGTTCA |
Restriction sites underlined.
TABLE 1.
Plasmids and B. burgdorferi strains used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Plasmids | ||
| pCR2.1-TOPO | PCR cloning vector | Invitrogen |
| pBBE22 | Borrelial shuttle vector confers Kanr | 50 |
| pML102 | Customized donor plasmid for in vitro transposition | 61 |
| pMM2 | 4.5 kb of lp54 spanning BBA64 to BBA68 in pCR2.1-TOPO | This study |
| pMM4 | pMM2 with 1.6-kb insertion of PflgBaadA at 154 bp from 5′ end of BBA64 | This study |
| pBBE22/bba64+ | Borrelial shuttle vector with BBA64-BBA65 | This study |
| β-Actin/pCR2.1 | 347 bp of β-actin in pCR2.1-TOPO | This study; 47 |
| Strains | ||
| ML23 | B31, lp25−, noninfectious | 36 |
| MSK5 | B31 isolate with all infection-associated plasmids | 36 |
| MM4 | B31, lp25−, BBA64−, Strr | This study |
| ML23/pBBE22 (wt) | ML23 with pBBE22 (BBE22+), Kanr | This study |
| MM4/pBBE22 (mt) | MM4 with pBBE22 (BBA64− BBE22+), Strr Kanr | This study |
| MM4/pBBE22-bba64+ (ct) | MM4 with pBBE22/BBA64, (BBE22+ BBA64+), Strr Kanr | This study |
Generation of BBA64 mt in ML23.
A clonal derivative of B. burgdorferi strain B31 lacking lp25, ML23, was electrotransformed with pMM4 using a procedure described previously (35, 36, 55, 56, 60, 61). After electroporation, the transformants were incubated for 24 h at 32°C in BSK-II growth medium without antibiotics and plated on BSK-II agarose overlays containing 50 μg/ml of streptomycin. The plates were then incubated at 32°C in 1% CO2 for 14 to 18 days or until individual colonies were visible in the overlays. Colonies were isolated aseptically into BSK-II growth medium with 50 μg/ml of streptomycin and grown at 32°C until the cultures reached a density of 5 × 107 spirochetes per ml. One milliliter of these cultures was used to extract total genomic DNA, and the presence of the PflgB-aadA cassette within BBA64 was confirmed using primers specific to the ends of Tn7 (Tn7N and Tn7S), as well as forward and reverse primers specific to BBA64 (Table 2). Two recombinants were identified in which the BBA64 amplicon size was increased by 1.6 kb due to the presence of a PflgB-aadA marker compared to that for the wild-type (wt) parental control strains (data not shown). One of the mt clones, designated MM4, was further characterized by Southern blot analysis.
Southern blot analysis.
To further confirm the inactivation of BBA64, total genomic DNA was extracted from MM4 and from the isogenic parental control strain, ML23 (60, 61). The DNA was digested with different restriction enzymes, separated on a 1% agarose gel, transferred onto a nylon membrane (Amersham Hybond -N+; GE Healthcare, Buckinghamshire, United Kingdom), and hybridized either with the aadA gene (Strr marker) or with the PCR-amplified, full-length BBA64 gene, which was labeled using the Enhanced Chemiluminescence labeling and detection system (GE Healthcare). The membranes were hybridized with the labeled probes overnight at 42°C and developed as per the manufacturer's instructions.
Complementation of BBA64 mt.
In order to restore a functional copy of BBA64 in the mt, a region of lp54 corresponding to the DNA coordinates 42563 and 44409 with engineered restriction enzyme sites was amplified using primers BBA64R and BBA65F (Table 2) and cloned into the pCR2.1-TOPO cloning vector. The insert was excised using BamHI and PstI and ligated into the borrelial shuttle vector pBBE22, containing the minimal region of lp25 needed to restore the infectivity of ML23 in the murine model of Lyme disease (50, 61). The BBA64 mt (MM4) was transformed by electroporation with either pBBE22/bba64 or pBBE22 alone to generate both a complemented (ct) strain and a BBA64-negative mt containing the minimal region of infectivity (Table 1). The transformants were selected on BSK-II agar overlays supplemented with 50 μg/ml of streptomycin and 200 μg/ml of kanamycin. The presence of pBBE22/bba64 and pBBE22 was verified using primers specific to the BBE22 region (Table 2; see Fig. 3), and levels of the BBA64 protein in the parental, mt, and ct strains were verified by immunoblot analysis (data not shown).
FIG. 3.
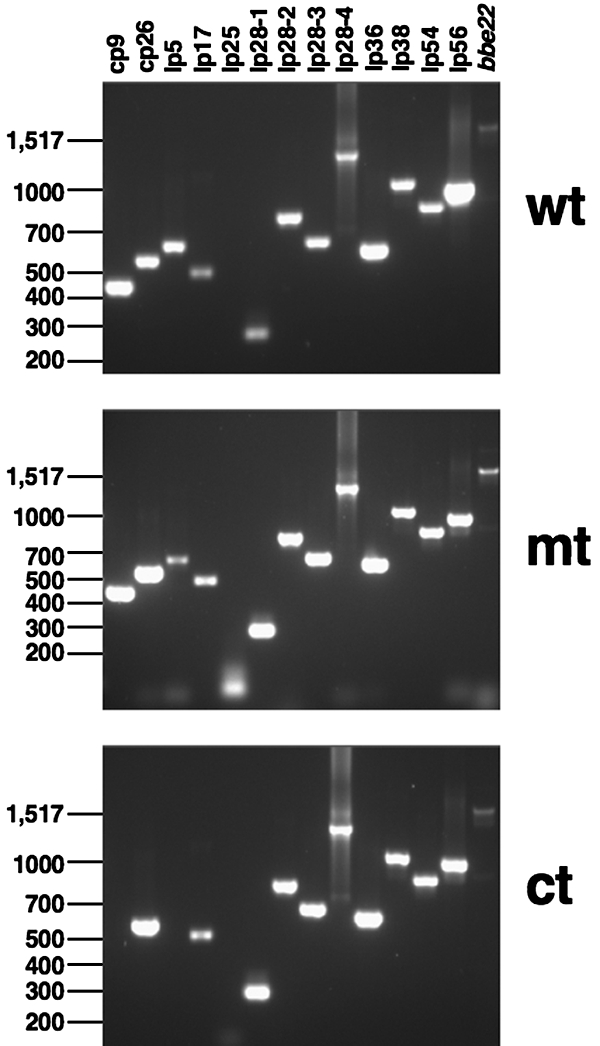
Plasmid profiles of the parental (wt; ML23/pBBE22), BBA64 mt (MM4/pBBE22), and ct (MM4/pBBE22-bba64+) strains. PCR analysis was performed as described in Materials and Methods using primers pairs listed in reference 36. The amplicons were resolved by electrophoresis in a 1.5% agarose gel. The primer pair used to generate the amplicon in the lane designated bbe22 is listed in Table 2. Molecular size markers (in base pairs) are indicated on the left.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblot analysis.
B. burgdorferi whole-cell lysates were prepared and separated by SDS-12.5% polyacrylamide gel electrophoresis as described previously (59). The separated proteins were either visualized by Coomassie brilliant blue staining or transferred onto a polyvinylidene difluoride membrane (Amersham Hybond-P; GE Healthcare, Buckinghamshire, United Kingdom) and subjected to immunoblot analysis. The membranes were probed with rat anti-BBA64, anti-BBA66 serum (5), or with mouse anti-BBA65 (V. L. Sexton and J. Seshu, unpublished data). Immunoblots developed with mouse anti-P66 serum served as a control for levels of loading of proteins from different strains (M. D. Esteve-Gassent and J. Seshu, unpublished data). The blots were developed following incubation with appropriate dilutions of horseradish peroxidase-conjugated antirat or antimouse secondary antibodies using ECL Western blotting reagents (GE Healthcare, Buckinghamshire, United Kingdom).
PCR analysis of plasmid profiles of strains used in infectivity.
Total DNA was extracted from liquid cultures (10 ml) of each strain when the density of the spirochetes reached 1 × 108 cells/ml. The spirochetes were pelleted at 4,000 × g for 20 min at 4°C and washed twice with phosphate-buffered saline (pH 7.4), and total genomic DNA was extracted and used as a template as described previously (61). Primer sets used to amplify specific target regions in different linear and circular plasmids have been described previously (36). The amplicons were separated on a 1.5% agarose gel and visualized by staining with ethidium bromide. A primer set (E22F/E22R) (Table 2) was also used to amplify the BBE22 region of lp25 present in the borrelial shuttle vector pBBE22.
Infectivity studies.
All animal procedures were done in accordance with the approved animal use protocol from the Institutional Animal Care and Use Committee of The University of Texas at San Antonio. Groups (n = 3) of 6-week-old female C3H/HeN mice (Charles River Laboratories, Wilmington, MA) were infected at doses of 102, 103, 104 and 105 spirochetes per mouse intradermally with the following strains of B. burgdorferi: wt (ML23/pBBE22); mt (MM4/pBBE22), and ct (MM4/pBBE22-bba64+). We also infected a group of three mice with MSK5 at 103 spirochetes per mouse to serve as a positive control for infection (35, 36). Groups of mice (n = 3) were also infected with 103 and 105 spirochetes/mice with ML23 to serve as a negative control for infection (35, 36). On day 21 postinfection, the spleen, left tibiotarsal joint, left inguinal lymph node, heart, and bladder and a piece of abdominal skin were removed aseptically from infected mice and the tissues were processed to facilitate isolation of spirochetes in BSK-II growth medium (35, 61). All the cultures were blind passed after 5 days into fresh BSK-II growth medium to minimize the toxicity associated with the degradation of host tissues and to facilitate the growth of spirochetes. The cultures were scored for growth of B. burgdorferi after 2 to 3 weeks using dark-field microscopy.
Quantitative real-time PCR analysis.
We further enumerated the spirochetes by real-time PCR analysis using primers specific to a constitutively expressed borrelial gene, flaB, and normalized the total DNA extracted from different tissues to the number of copies of mouse β-actin (47, 61). A portion of skin, spleen, right inguinal lymph node, and right tibiotarsal joint was collected aseptically, and total DNA was extracted using the High Pure PCR template preparation kit (Roche Applied Bioscience, Piscataway, NJ). The manufacturer's suggested protocol for extracting nucleic acids from the tail of the mouse was adapted to obtain total genomic DNA from different infected tissues. Briefly, the tissue samples were homogenized in 200 μl of lysis buffer containing proteinase K (final concentration, 2 mg/ml) and collagenase (final concentration, 1 mg/ml; Sigma Chemicals, St. Louis, MO). After incubation at 56°C overnight in a water bath, total genomic DNA was extracted and quantified using a Synergy HT Multi-Detection microplate reader (BioTek Instruments, Inc., Winooski, VT). Primers were designed (Table 2) for the borrelial flaB gene (flagellin-F and flagellin-R) and mouse β-actin (LE/β-actin/F and LE/β-actin/R) using Primer Express software (Applied Biosystems, Foster City, CA) (61). The mouse β-actin target was amplified by PCR using the actF and actR primers (Table 2) to generate a 347-bp amplicon (47) that was cloned into pCR2.1-TOPO vector (β-actin/pCR2.1). Total genomic DNA from 109 spirochetes was extracted using the High Pure PCR template preparation kit (Roche Applied Bioscience) and used to generate a standard curve to obtain genome equivalents with the flagellin-F and flagellin-R primers. Known amounts of plasmid DNA containing β-actin or the genomic equivalents from the spirochetes were used as standards to determine the total numbers of spirochetes in different mouse tissues. Total genomic DNA isolated from different infected mouse tissues was subjected to quantitative real-time PCR using SYBR green PCR master mix with a final concentration of 0.3 μM of oligonucleotides using the ABI Prism 7300 system (Applied Biosystems). The spirochete burden was expressed as the number of borrelial flaB copies per 106 mouse β-actin copies. The data were analyzed using a one-way analysis-of-variance test with Bonferroni's multiple-comparison test. Statistical significance was accepted when P values were less than 0.05.
Histopathological examination of joint tissue.
Groups (n = 3) of 6-week-old female C3H/HeN mice were infected with 103 spirochetes per mouse intradermally with the wt (ML23/pBBE22) and BBA64 mt (MM4/pBBE22) strains of B. burgdorferi. At 62 days postinfection, the spleen, left tibiotarsal joint, left inguinal lymph node, heart, and bladder and a piece of abdominal skin were removed aseptically from infected mice and the tissues were processed to facilitate isolation of spirochetes in BSK-II growth medium as previously described. The right tibiotarsal joints from each of the mice were examined for histopathological changes. The joints were formalin fixed, decalcified, sectioned, and stained with hematoxylin and eosin using standard procedures. The specimens were qualitatively examined to determine if histopathological lesions were present in the joints after long-term infection. Due to the limitation on the number of specimens after the long-term infection, we did not evaluate the histopathology using the scoring system as a measure of inflammation.
RESULTS
Construction of the BBA64 mt.
We inactivated the BBA64 gene using a customized transposon containing a streptomycin resistance marker under the control of a borrelial promoter (PflgB-Strr), employing an in vitro mutagenesis strategy as described previously (60, 61). A plasmid obtained using this approach, designated pMM4, with insertional inactivation of BBA64 at position 154 relative to its 5′ end, was used to transform a noninfectious, lp25-negative (lp25−) clonal isolate of B. burgdorferi strain B31 (ML23). We employed a two-step approach, where we first used ML23 to generate the BBA64::Strr mt, exploiting the lack of a restriction/modification system (BBE02) carried on lp25 to obtain higher transformation frequencies (33) and overcoming the lack of infectivity in the second step by the use of a borrelial shuttle vector (pBBE22) that encoded the minimal region of lp25, thereby restoring infectivity (50).
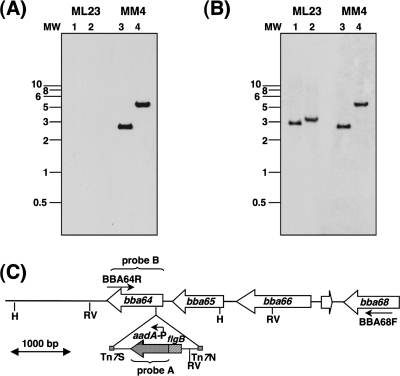
Following the transformation of ML23 with pMM4, streptomycin-resistant colonies were screened using a PCR-based approach with primers specific to BBA64. Two positive clones were identified based on the observation that the BBA64 amplicon size was increased by 1.6 kb compared to that of the wt parental control strain due to the presence of the PflgB-aadA marker (data not shown). Southern blot analysis of one of the positive clones, designated MM4, showed that there was hybridization of the aadA probe to DNA fragments of 2.8 kb and 5.1 kb from MM4 (Fig. 1A, lanes 3 and 4, respectively) while there was no hybridization with the DNA from the parental strain (ML23) digested with EcoRV or HinDIII (Fig. 1A, lanes 1 and 2, respectively). The sizes of these DNA fragments were consistent with expected sizes based on the restriction enzyme profile of the region of BBA64 in lp54 and the presence of these sites on the customized transposon. We further confirmed that the DNA fragments of 2.9 kb (EcoRV fragment) and 3.5 kb (HinDIII fragment) from the isogenic parental control (ML23) hybridized to labeled BBA64 used as a probe (Fig. 1B, lanes 1 and 2, respectively) and that there was a 1.6-kb increase in the corresponding size of the HinDIII fragment in MM4 (Fig. 1B, lane 4). This increase in the size of the HinDIII fragment is due to the presence of the aadA gene (Strr). A similar increase in the EcoRV fragment was not observed, since the additional EcoRV site (Fig. 1B, lane 3) within the transposon renders the corresponding fragment from MM4 smaller than that in the parental strain (Fig. 1C). These observations confirmed the inactivation of BBA64 and the clonality of the mt MM4. The comparable growth characteristics of MM4 and the parental strain suggested that BBA64 is dispensable for in vitro growth.
FIG. 1.
Southern blotting confirms the mutation in the BBA64 gene of lp54 in ML23 (noninfectious, lp25−, B. burgdorferi strain B31). (A and B) Total genomic DNA from the ML23 parent strain (lanes 1 and 2) and MM4 (BBA64 mt; lanes 3 and 4) was digested with EcoRV (lanes 1 and 3) and HinDIII (lanes 2 and 4) and probed with aadA (Strr marker; probe A) (A) or BBA64 (PCR amplified using BBA64F and BBA64R primers; probe B) (B). Numbers on the left of each panel indicate the sizes of the markers in kb. (C) Schematic of the BBA64 region of lp54 with the transposition site showing the customized transposon insertion (PflgB-Strr). The numbers indicate the gene designation within the BBA64 region of lp54. Hatched boxes indicate the respective locations of the Tn7 repeats. Arrows indicate the orientations of the BBA64R and BBA68F primers used to amplify the region of BBA64 on lp54. H, HinDIII; RV, EcoRV.
Complementation of MM4.
Since MM4 (BBA64::Strr mt) was generated in a noninfectious, lp25− strain of B. burgdorferi, it was essential to restore the minimal region of lp25 required for infectivity. This was achieved by transformation of both ML23 and MM4 with pBBE22 carrying the region of lp25 that would restore a functional copy of BBE22 or pncA in trans (50, 54, 61). In addition, MM4 was also transformed with pBBE22 carrying a functional copy of BBA64 (a region of lp54 corresponding to the DNA coordinates 42563 and 44409) to obtain a genetically complemented strain. Transformants selected in the presence of kanamycin (conferred by pBBE22) were screened by PCR using primers specific to BBE22 or BBA64 and were found to carry pBBE22 (Table 2). At this stage, it was critical to determine if all the transformed strains expressed VlsE, an lp28-1-encoded lipoprotein that exhibits antigenic variation facilitating persistence of B. burgdorferi in the murine model of Lyme disease (35, 71-73). Immunoblot analysis of wt (ML23/pBBE22), BBA64 mt (MM4/pBBE22), and ct (MM4/pBBE22-bba64+) strains using anti-VlsE serum revealed similar levels of expression of VlsE (data not shown), indicating that all these strains carried the two critical determinants of infectivity, namely, pncA and vlsE. Thus, by adopting a two-step transformation strategy, we successfully generated a BBA64 mt along with its parental and ct strains that have the requisite genetic background to facilitate infectivity analysis using the murine model of Lyme disease.
Plasmid profile of BBA64 mt.
In addition to evaluating the expression of VlsE present on lp28-1, we also determined the plasmid profile of the BBA64 mt and the control strains. As shown in Fig. 3, the plasmid profile of wt and mt strains was identical whereas the ct strain does not contain lp5 and cp9. All three strains retain lp28-1 (which encodes VlsE), needed for persistence. While all three strains do not show an amplicon specific to lp25 (using primers specific for BBE16), primers specific to the E22 region of lp25 (Table 2) did amplify a 2-kb fragment in all three strains. This is due to the transformation of all three strains with the borrelial shuttle vector pBBE22, which restores the minimal region of lp25 required for infectivity. Previous studies (35, 51) have demonstrated little or no significant loss of infectivity with isolates of B. burgdorferi lacking lp5 or cp9, and hence we proceeded with the infectivity analysis using the ct strain lacking the aforementioned plasmids.
Expression of BBA64, BBA65, and BBA66 proteins in the wt, mt, and ct strains.
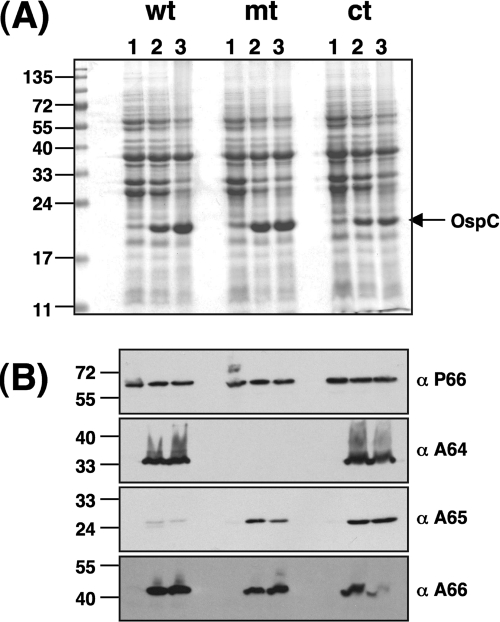
To test the contribution of the BBA64 gene to the infectivity of B. burgdorferi for the mammalian host, it was necessary to confirm if different environmental signals induced comparable levels of expression of pgf 54 members in the strains to be used in the infectivity analysis (12, 22, 43). Immunoblot analysis using monospecific rat anti-BBA64 or anti-BBA66 (5) or monospecific mouse anti-BBA65 antibodies was used to detect the levels of these proteins. As shown in Fig. 2A, there were no overt differences in the protein profiles between the BBA64 mt and the parental or ct strain. Previous studies have shown a significant increase in the levels of outer surface protein C (OspC) when B. burgdorferi is exposed to conditions such as increased temperature, reduced pH, and other undefined signals that mimic the mammalian host (10, 67). Hence, the levels of OspC serve as an indicator of the mammalian host-adapted state of B. burgdorferi as well as the functionality of critical regulatory pathways, such as the Rrp2-RpoN-RpoS pathway. This pathway has been shown to control expression of lipoproteins important for pathogenesis, such as OspC and DbpA (6-8, 20, 28, 68, 69). As expected, there was a significant increase in the levels of OspC in B. burgdorferi cultured at pH 6.8/37°C (Fig. 2A, lane 2, for all strains) or when shifted from pH 7.6/23°C to pH 6.8/37°C (Fig. 2A, lane 3, for all strains) compared to levels in cultures grown at pH 7.6/37°C (Fig. 2A, lane 1, for all strains). This observation is consistent with previous reports demonstrating that a change in environmental conditions that mimic tick feeding (increased temperature and lowered pH) results in increased levels of OspC (9, 10, 67) and that ospC expression was similar between the mt and the control strains.
FIG. 2.
Expression of the BBA64, BBA65, and BBA66 proteins in response to mammalian host-specific temperature or pH. wt (ML23/pBBE22), mt (MM4/pBBE22), and ct (MM4/pBBE22-bba64+) borrelial strains were shifted from pH 7.6/23°C to pH 7.6/37°C (lanes 1), from pH 6.8/23°C to pH 6.8/37°C (lanes 2), and from pH 7.6/23°C to pH 6.8/37°C (lanes 3). (A) Total protein samples were separated on an SDS-12.5% polyacrylamide gel and stained with Coomassie brilliant blue. The arrow corresponds to OspC, and the numbers to the left indicate the molecular masses of protein markers in kDA. (B) Immunoblot analysis using monospecific serum against the P66, BBA64, BBA65, or BBA66 protein. The blots were developed using the Enhanced Chemiluminescence system. The numbers to the left of each panel indicate the molecular masses of proteins markers in kDa. α-P66, -A64, -A65, and -A66, anti-P66, -A64, -A65, and -A66.
To delineate the role of temperature and pH as signals for the induction of the BBA64, BBA65, and BBA66 proteins, we performed immunoblot analysis using the different borrelial strains that were propagated initially at pH 7.6/23°C and then shifted at low densities to growth media at pH 7.6/37°C (Fig. 2, lane 1) or pH 6.8/37°C (Fig. 2, lane 3). Samples propagated initially at pH 6.8/23°C with a shift to pH 6.8/37°C (Fig. 2, lane 2) were also analyzed. There was no detectable expression of the BBA64, BBA65 and BBA66 proteins in any of the strains with propagation at pH 7.6/37°C (Fig. 2B, α-Α64, α-Α65, and α-Α66, lane 1; all strains). When the wt strain was shifted from pH 6.8/23°C to pH 6.8/37°C, there was a significant increase in the levels of the BBA64, BBA65, and BBA66 proteins (Fig. 2B, lane 2). A similar increase was also noted for the ct strain under identical growth conditions (Fig. 2B, lane 2). When the pH of the growth media was reduced to 6.8 from 7.6 with a concomitant increase in temperature from 23°C to 37°C, there was increased expression of each of the analyzed lipoproteins (Fig. 2B, lane 3, wt and ct). There was no expression of the BBA64 protein when the BBA64 mt was cultivated under conditions described for its parental control, confirming the complete loss of expression of the BBA64 protein in the mt (Fig. 2B, α-A64, lanes 1, 2 and 3; mt). We noted an increased expression of the BBA65 protein in the BBA64 mt compared to results for the wt strain when the spirochetes were grown at pH 6.8/37°C (Fig. 2B, α-A65, lane 2; mt) or shifted from pH 7.6/23°C to 6.8/37°C (Fig. 2B, α-A65, lane 3; mt). The BBA64 mt, however, did not exhibit an increase in the levels of BBA66 (Fig. 2B, α-A66, lanes 2 and 3; mt) compared to those for the parental control under similar growth conditions. There were no significant differences in the levels of expression of BBA66 between the parental control and the ct samples (Fig. 2B, α-A66, lanes 2 and 3). Immunoblot analysis using mouse anti-P66 serum established that the levels of P66 were independent of pH and temperature and that there were comparable levels of P66 between the BBA64 mt and its parental and ct strains (Fig. 2B, α-P66, lanes 1, 2, and 3; all strains). These observations were consistent with those of a previous study where it was demonstrated that the levels of P66 were not affected by either temperature, pH, or a combination of these factors (14). The above analysis also suggests that the loss of expression of BBA64 did not affect other critical determinants which are known to play a role in the infectious process, albeit this analysis was limited to OspC and P66.
We further analyzed if pH, independently of temperature, has an overriding effect on the expression of the BBA64, BBA65, and BBA66 proteins. All three strains (wt, mt, and ct) were first grown at pH 7.6/23°C and then inoculated into growth media at pH 6.8/37°C, pH 7.6/23°C, or pH 6.8/23°C at low densities to facilitate adaptation to the latter environmental conditions. As previously described, the levels of OspC were found to be enhanced only in borrelial samples from cultures at pH 6.8/37°C for all three strains (Fig. 2, lane 3). There was no expression of BBA65 and BBA66 at pH 7.6/23°C or at pH 6.8/23°C, indicating that temperature and pH have a combined effect on the expression of these members of pgf 54 (data not shown).
Infectivity analysis of BBA64 mt in C3H/HeN mice.
Recent studies have demonstrated that several members of the pgf 54 present on lp54 are upregulated under mammalian host-specific conditions (4, 44, 53, 66). However, the individual or collective contribution of these genes to infectivity has not been studied. We have shown that all three strains have the requisite genetic background and regulatory pathways (Fig. 2 and 3) to allow direct analysis of the role of BBA64 in the initial colonization and dissemination of B. burgdorferi using the murine model of Lyme disease (61). As shown in Table 3, there were no significant differences when the spirochetes were propagated in BSK-II medium from different tissues in mice infected with either the BBA64 mt strain or its parental or ct strain, indicating that loss of the BBA64 gene alone does not result in the attenuation of B. burgdorferi infectivity in the murine host.
TABLE 3.
The BBA64 locus is dispensable for infection of C3H/HeN mice
| Strain and dose | No. of cultures positive/no. tested
|
No. of mice infected/no. tested | ||||||
|---|---|---|---|---|---|---|---|---|
| Skin | Spleen | Joint | Lymph node | Heart | Bladder | All sites | ||
| MSK5 | ||||||||
| 103 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| ML23 | ||||||||
| 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/18 | 0/3 |
| 105 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/18 | 0/3 |
| wt (ML23/pBBE22) | ||||||||
| 102 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| 103 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 17/18 | 3/3 |
| 104 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| 105 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| mt (MM4/pBBE22) | ||||||||
| 102 | 0/3 | 2/3 | 2/3 | 2/3 | 2/3 | 2/3 | 10/18 | 2/3 |
| 103 | 3/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 17/18 | 3/3 |
| 104 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| 105 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| ct (MM4/pBBE22-bba64+) | ||||||||
| 102 | 3/3 | 2/3 | 3/3 | 3/3 | 2/3 | 3/3 | 16/18 | 3/3 |
| 103 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| 104 | 2/3 | 2/3 | 2/3 | 2/3 | 2/3 | 2/3 | 12/18 | 2/3 |
| 105 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
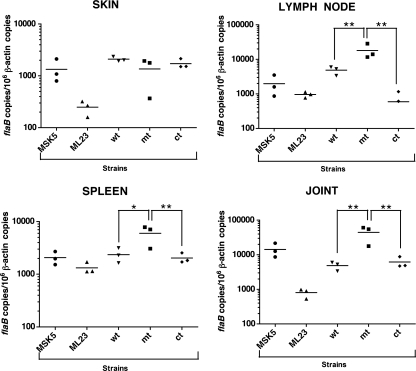
Even though there is no significant difference among the wt, mt, and ct strains in their ability to disseminate and colonize different tissues, we further enumerated the spirochetes by real-time PCR analysis to see if there are any subtle differences in the numbers of spirochetes in different tissues (61). As shown in Fig. 4 (representative data from mice infected with 103 spirochetes/strain/mouse), there were no significant differences (P > 0.05) in the levels of spirochetal burden in the skin from mice infected with either the wt, mt, or ct strain. This was true for mice infected with different doses of spirochetes (102 to 105; P > 0.05; data not shown). However, there were significantly higher numbers of spirochetes in lymph nodes (P < 0.01), spleen (P < 0.05), and joints (P < 0.01) from mice infected with the BBA64 mt strain than from mice infected with the wt or ct strain (103 spirochetes/mouse) (Fig. 4). Mice infected with higher doses (104 and 105 spirochetes/mouse) of the BBA64 mt did not exhibit significant differences in the number of spirochetes in the spleen and joints (P > 0.05) from those for the control strains, while the opposite was true with lower doses (102 and 103 spirochetes/mouse; P < 0.05). The lymph nodes, however, had a significantly higher spirochetal burden for mice infected with the BBA64 mt at all the tested doses (102 to 105 spirochetes/mouse) than for the mice infected with the wt or ct strain at the same levels. Quantification of spirochetes in target tissues indicated that while the loss of BBA64 did not have an effect on the total numbers of spirochetes in the skin (independent of the size of the inocula), there were significantly higher numbers of BBA64-negative spirochetes in the lymph nodes (at all inocula) than was the case for the control strains. These results indicate that there are subtle tissue-specific differences in the distribution of the BBA64 mt even though these differences were not apparent in terms of propagation of spirochetes from infected tissues in BSK-II growth medium.
FIG. 4.
Quantitative real-time PCR analysis of the spirochetal burden in mice infected with the BBA64 mt. Groups (n = 3) of 6-week-old C3H/HeN female mice were infected intradermally with B. burgdorferi strains—wt (ML23/pBBE22), mt (MM4/pBBE22), or ct (MM4/pBBE22-bba64+)—at doses ranging logarithmically from 102 to 105 spirochetes per mouse. A similar group (n = 3) of mice was infected with MSK (103) or ML23 (103 and 105) to serve as the positive or negative control, respectively, for infection. Total genomic DNA was isolated from tissues (skin, spleen, lymph node, and joint) using the High Pure PCR template preparation kit, and quantitative real-time PCR was performed. Results for the infection dose of 103/mouse are shown. Numbers of borrelial flaB copies were normalized against total mouse β-actin copies. Only two out of three samples of lymph nodes from mice infected with the ct strain had detectable levels of spirochetal DNA. The asterisks indicate levels of significance as follows: **, P < 0.01; *, P < 0.05.
There was no growth of spirochetes in tissues isolated from mice infected with ML23 and cultured in BSK-II growth medium (Table 3), but we were able to detect spirochete-specific DNA in all the tissues tested. This may be due to the presence of residual DNA from spirochetes that were not completely cleared even though they were not capable of growth and replication in the BSK-II growth medium (35). It has previously been shown that ML23 (lp25−) is capable of dissemination to various tissues in C3H/HeN mice but cannot be propagated in BSK-II growth medium from these tissues after 2 to 4 days postinfection (35). Borrelial flaB was not present in tissues isolated from uninfected mice (data not shown).
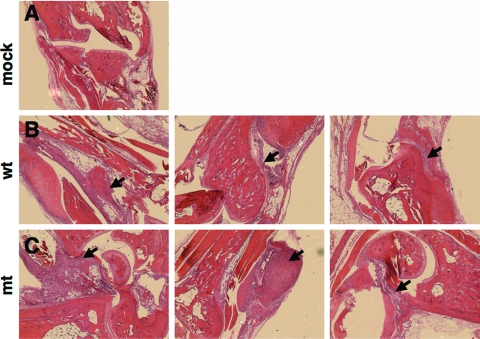
We extended the infectivity analysis to 62 days postinfection to determine if long-term infections would facilitate determination of differences between wt and mt strains using a limited number of mice (n = 3) and a single inoculum dose (103 spirochetes/animal). As shown in Table 4, there were no significant differences in the ability to propagate wt or mt spirochetes from different tissues at 62 days postinfection. However, histological examination of the joint tissues indicated a greater degree of inflammatory cellular infiltration in the joints of mice infected with the mt strain than for those infected with the wt (Fig. 5). Consistent with results of previous studies, the degree of inflammatory cellular infiltration was greater in mice infected with the wt than was the case for joints from uninfected control mice (mock; Fig. 5). The inflammatory infiltrates consisted predominantly of mononuclear cells. Since the long-term infection was carried out with a limited set of mice and a single dose of infection, we were unable to conduct quantitative scoring to measure inflammation induced in a dose-dependent manner. Nonetheless, there were increased levels of tissue inflammation in joints from mice infected with the BBA64 mt strain compared to results with the wt strain at 62 days postinfection, which may be related to an increased number of spirochetes present in the joint tissues of mice infected with the mt compared to that for the wt controls at 21 days postinfection as determined by quantitative real-time PCR (Fig. 4).
TABLE 4.
The BBA64 locus is dispensable for long-term infection of C3H/HeN mice
| Strain, dose | No. of cultures positive/no. tested
|
No. of mice infected/no. tested | ||||||
|---|---|---|---|---|---|---|---|---|
| Skin | Spleen | Joint | Lymph node | Heart | Bladder | All sites | ||
| wt (ML23/pBBE22), 103 | 3/3 | 1/3 | 3/3 | 3/3 | 3/3 | 3/3 | 16/18 | 3/3 |
| mt (MM4/pBBE22), 103 | 3/3 | 1/3 | 3/3 | 3/3 | 3/3 | 3/3 | 16/18 | 3/3 |
FIG. 5.
Histological examination of joints from C3H/HeN mice infected intradermally with 103 spirochetes per mouse. The tibiotarsal joints were collected 62 days postinfection, processed as described in Materials and Methods, and stained with hematoxylin and eosin. The joint-tissue samples are from mice inoculated with phosphate-buffered saline (mock), the wt (ML23/pBBE22; n = 3), or the mt (MM4/pBBE22; n = 3). Note the presence of increased levels of infiltration in joint tissue of mice (samples 1 and 2) inoculated with the mt compared to that of mice inoculated with the wt, as indicated by arrows.
In summary, in vivo analyses of the BBA64 mt indicated that there were no significant differences in the ability to propagate spirochetes in the growth medium from various tissues of mice infected with the BBA64::Strr mt or the control strains. Quantitative real-time PCR revealed that there were differences with various levels of significance in the numbers of spirochetes in different tissues. The foregoing analysis demonstrates that loss of BBA64 does not result in any significant changes in the protein profile of B. burgdorferi, nor does it alter infectivity in the murine model of Lyme disease.
DISCUSSION
B. burgdorferi alters its gene expression to adapt to the highly disparate environmental conditions that it encounters in the tick vector and the mammalian host. Several whole-genome transcriptional profile analyses revealed that much of the above differential gene expression was associated with the plasmid-encoded genes present in lp54 (4, 44, 53, 66). Lp54 encodes several determinants with known functions, such as OspA/B, DbpA/B, and CRASP-1 (23-27, 34, 40), as well as several members of pgf 54 which are transcriptionally upregulated under mammalian host-specific conditions albeit with no defined functions (4, 5, 11, 21, 22, 53, 66). We therefore hypothesized that the loss of expression of genes that may facilitate the initial stages of adaptation of the spirochetes to the mammalian-host environment may result in the attenuation of the virulence of B. burgdorferi.
Contrary to our expectation, inactivation of BBA64 did not exhibit any significant difference either in the protein profile (Fig. 2) or in infectivity with the murine model of Lyme disease (Table 3). Subtle differences were observed in the expression of BBA65, another member of pgf 54, along with differences in the number of mt spirochetes in select tissues (Fig. 4). There was also no significant difference in infectivity between wt and mt strains following a long-term infection (62 days postinfection), while there was an increased inflammatory response in joints of mice infected with the BBA64 mt (Fig. 5). This analysis has helped to establish that even though BBA64 is transcriptionally upregulated, its inactivation does not have a significant bearing on infectivity of B. burgdorferi in the murine model of Lyme disease.
Due to the difficulties associated with genetic manipulation of infectious strains of B. burgdorferi (54), we employed a two-step transformation strategy to inactivate BBA64 and confirmed that all the strains retained plasmids critical for infectivity. The first step was to inactivate BBA64 in a noninfectious lp25− strain of B. burgdorferi (ML23) (35). The second step was to restore the minimal region of infectivity using a borrelial shuttle vector (50, 61). By this approach, we limited the effects of restriction/modification systems present on select linear plasmids (like lp25-encoded BBE02), which resulted in reduced transformation efficiencies (37). We were able to successfully generate a BBA64 mt (Fig. 1) and subsequently restore the minimal region of infectivity to facilitate analysis of the role of BBA64 in mammalian host infectivity (Table 3). This strategy has been used previously to determine the effect of loss of the fibronectin binding protein BBK32 in the murine model of Lyme disease and hence is a viable strategy to obtain mts that can be tested for infectivity (61). A limitation inherent in this strategy is the inability to analyze the phenotype of the mts in terms of acquisition, survivability, or transmission by ticks because the complementation step restores only the minimal region of lp25 (BBE22) required for infectivity of the mammalian host but not the region required for long-term survival in ticks and for completion of the life cycle (BBE16) (52, 64, 65). Nonetheless, generation of mts using this approach facilitated analysis of the effect of loss of one or more genes in the infectivity of B. burgdorferi for the mammalian host.
Since the differential expression of pgf 54 family members in response to mammalian host-specific signals is crucial to the initial stages of infectivity, it was important to determine if the regulation of expression of a subset of these members is similar in the wt parental strain, the BBA64 mt, and the ct strain. As shown in Fig. 2, the expression of the BBA64 protein in the wt parental strain (ML23/pBBE22) was consistent with results in previous studies (9). There was increased expression of the BBA64 protein when the wt strain was grown at a low pH (6.8) and higher temperature (37°C), while there was no detectable expression of the BBA64 protein at a higher pH (7.6) and lower temperature (23°C). These studies clearly indicated the significance of the coordinate effects of pH and temperature in the up-regulation of BBA64 and that the gene regulatory circuits are intact and functional in wt, mt, and ct strains.
There was no evidence of expression of BBA64 in immunoblot analysis of the BBA64 mt, confirming the loss of expression of this determinant, while complementation of the mt with a functional copy of BBA64 resulted in the restoration of expression when it was propagated under mammalian host-specific conditions (Fig. 2). There were no overt differences in the protein profile of the mt and control strains. However, the levels of expression of BBA65 were found to be higher by immunoblot analysis for the mt than for the parental wt strain, suggesting that the loss of BBA64 may result in altered expression of other pgf 54 members. Contrary to our expectation, we also observed increased levels of expression of BBA65 in the ct strain. This may be due to a lack of effect of providing BBA64 in trans to restore the BBA65 protein to wt levels. Alternatively, it is also possible that the lack of cp9 and lp5 in the ct strain may have yet-to-be-determined effects, albeit subtle, on the expression of these members of pgf 54. The loss of BBA64 did not have an effect on the levels of expression of another member of pgf 54, namely, BBA66.
The process of generating mts may inadvertently result in selection of mutations in distal genes or loss of plasmids with significant effects on the infectivity of B. burgdorferi (36, 41, 51, 64). There were no overt differences in the protein profile and the level of OspC was significantly higher under mammalian host-specific conditions in all three strains, indicating that both the regulatory mechanisms and the expression of other critical determinants of virulence were similar and intact in the mt and control strains (1, 58, 63, 67). Moreover, we also analyzed the plasmid profile of the parental, mt, and ct strains and found that all the plasmids critical for infectivity (lp25 and lp28-1) were present in all three strains. The two plasmids that were missing in the ct strain, namely, cp9 and lp5, have been shown to be dispensable for infectivity of B. burgdorferi in the murine model of Lyme disease (36, 51). Moreover, the ct strain did not show any significant difference in levels of infectivity from the wt strain, indicating that the lack of cp9 or lp5 did not have a significant bearing on the outcome of infectivity.
Recently, Gilmore et al. described the kinetics of expression of the BBA64, BBA65, and BBA66 genes during persistent infection in mice up to 100 days and found that the expression of BBA64 was reduced at 20 days postinfection (22). While the expression of BBA64 remained low, the transcriptional levels of both BBA65 and BBA66 were found to be elevated during the entire course of infection, suggesting a role for multiple mechanisms or regulators being involved in their expression. Analysis of the upstream region of BBA66 revealed a σ70 consensus sequence very similar in organization to that of BBA64 (12, 30, 31), suggesting a direct or indirect role of the RpoN-RpoS regulatory pathway in the expression of BBA64, BBA65, and BBA66. These observation were further corroborated by the loss of expression of BBA66 in the absence of either σN (RpoN) or σS (RpoS) (12). The RpoN-RpoS regulatory pathway has been characterized as influencing the expression of several lipoproteins, such as OspC and DbpA (20, 28, 68, 69). More recently, BBA64 was found to be upregulated by greater than 18-fold in the wt strain compared to results for the rpoS mt when these strains were propagated under in vitro growth conditions (8). Furthermore, there was greater than 1,600-fold differential expression of BBA64 in the wt strain compared to results for the rpoS mt when the strains were grown under host-adapted conditions (8). A highly significant, concomitant transcriptional up-regulation was also observed with BBA65, BBA66, and BBA73, indicating that these pgf 54 members are regulated in an rpoS-dependent manner and in response to mammalian host-adapted conditions (8). It is conceivable from the above analysis that up-regulation of multiple pgf 54 members would facilitate the full spectrum of infectivity. While whole-genome transcriptional profiles facilitate identification of a subset of genes that may play a role in a defined environmental niche, phenotypic analysis of mts in one or more of these genes facilitates a more direct evaluation of their roles in mediating adaptation to those conditions. For example, if these upregulated ORFs encode lipoproteins, it is important that the surface expression of these proteins coincide temporally in microenvironments that facilitate migration of spirochetes from the midgut to the salivary glands or participate in other stages of infection, such as mediating attachment to host matrices or evasion of innate immunity (13, 39). Even though BBA64 has been shown to be significantly upregulated under mammalian host-adapted conditions, its inactivation did not alter the dissemination and colonization of the mammalian host (Tables 3 and 4). Therefore, it is critical to evaluate the link between transcriptional levels of borrelial genes and their role in infectivity using the murine model of Lyme disease (8).
Even though BBA64 is dispensable for infectivity, enumeration of spirochetes in infected tissues, on the other hand, revealed differences, which indicates that the expression of BBA64 temporally or in specific microenvironments may play a role in facilitating the full spectrum of infectivity in the murine model of Lyme disease. There were no significant differences in the numbers of spirochetes in the skin of mice infected with the mt or the control strain (Fig. 4). However, there were increased numbers of spirochetes present in the spleen, joints, and lymph nodes of mice infected with the BBA64 mt compared to results for the control strains (Fig. 4). While the exact mechanisms responsible for these observations are not known, it is interesting to speculate that there is a minimal role for BBA64 for survival of B. burgdorferi in certain select tissues. But at the same time, these differences in the spirochetal burden in different tissues as determined by quantitative real-time PCR may not translate into a significant difference in infectivity, since this assay does not distinguish between live and dead spirochetes. It is also plausible that the lack of antibodies directed against the BBA64 protein, as would be expected for mice infected with the BBA64-negative mt, may also lead to reduced neutralization of the spirochetes and thereby facilitate increased colonization in these tissues. However, long-term infections (62 days) using the murine model of Lyme disease also did not reveal any significant differences between the mt and wt even though this analysis was done using a single infectious dose. This is consistent with the observation that there are no apparent mechanisms of antigenic variation associated with the expression of the members of pgf 54 (12). There was an increased level of inflammation in joint tissues associated with the mt strain, and this correlates with the ability to detect increased numbers of mt spirochetes in the joints.
The lack of attenuation of infectivity of the BBA64 mt can be explained by compensatory mechanisms that are responsible for the colonization and dissemination of B. burgdorferi (19). Since BBA64 is one of 12 members of the pgf 54, it is possible that one or more paralogs of this gene family are upregulated to compensate for the loss of BBA64 (22). This scenario is consistent with the observation that BBA65 is elevated in the BBA64 mt at pH 6.8/37°C. While it is not possible to directly attribute the lack of attenuation of the BBA64 mt to the up-regulation of BBA65, it is plausible that compensatory gene expression over a broad array of pgf 54 members may alleviate the effect of inactivation or loss of expression of an individual paralog. Alternatively, the sequence similarity of these members could result in determinants that functionally compensate for the loss of one or more members of pgf 54. In a recent study, temporal expression of BBA64, BBA65, and BBA66 monitored by quantitative real-time PCR indicated that while BBA64 expression was considerably reduced in mouse tissues from that observed during in vitro growth, there was increased expression of BBA65 and BBA66 throughout the course of infection and up to 100 days postinfection (22). This suggested a preferential role for BBA64 in the vector-to-mammalian-host transmission and/or in the early stages of infection.
Our study indicates that there is no significant reduction in the infectivity of the BBA64 mt from that of its isogenic parental or ct strain following short-term (21 days) or long-term (62 days) infection based on cultivation of spirochetes from infected tissues. However, this does not preclude the possibility of a contribution of BBA64 to the trafficking of spirochetes from the tick midgut to the salivary glands or subsequent transmission to mammalian hosts. The transcriptional down-regulation of BBA64 in the mammalian host (22) may indicate the lack of a role for this protein in the course of infection. This may therefore translate into a lack of attenuation of infectivity following short- or long-term infection. Inactivation of proteins in B. burgdorferi that serve as adhesins, such as BBK32 (38, 61) and Bgp (49), has resulted in either partial or no attenuation of infectivity, suggesting the role of multiple borrelial determinants in initiation and maintenance of infection. Hence, it is interesting to speculate that infectivity analysis of a BBA64/BBA65/BBA66-negative triple mt will help further characterize the role of a subset of pgf 54 family members in the colonization and dissemination of B. burgdorferi within the mammalian host. Analysis of the in vivo phenotype of strains with mutations in ORFs that are significantly upregulated under mammalian host-specific conditions will contribute to understanding their role in infectivity and possibly in determining their function(s).
Acknowledgments
We are grateful to Darrin R. Akins for the anti-BBA64 and anti-BBA66 sera used in this study. We thank Jonathan T. Skare for providing B. burgdorferi strains ML23 and MSK5 and the plasmid pML102. We also thank Robert D. Gilmore for anti-OspC monoclonal antibodies. We are grateful to Steven J. Norris for the plasmid pBBE22. We also thank M. Neal Guentzel, Ashlesh K. Murthy, and Stephen P. Saville for critical reading of the manuscript and for helpful comments.
This work was supported by Public Health Service grant AI-065953 from NIAID, a Faculty Research Award from UTSA (both to J.S.), and an Undergraduate Research Fellowship supported by NIGMS MBRS-RISE grant GM-60655 (to V.L.S.).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 5 November 2007.
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 1012240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita, J., S. Samanta, B. Revilla, K. Suk, S. Das, S. W. Barthold, and E. Fikrig. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 681222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour, A. G., and D. Fish. 1993. The biological and social phenomenon of Lyme disease. Science 2601610-1616. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 713371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, C. S., S. R. Vuppala, A. M. Jett, and D. R. Akins. 2006. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 74296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caimano, M. J., C. H. Eggers, C. A. Gonzalez, and J. D. Radolf. 2005. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J. Bacteriol. 1877845-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caimano, M. J., C. H. Eggers, K. R. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 726433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caimano, M. J., R. Iyer, C. H. Eggers, C. Gonzalez, E. A. Morton, M. A. Gilbert, I. Schwartz, and J. D. Radolf. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 651193-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 686677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 673181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35490-516. [DOI] [PubMed] [Google Scholar]
- 12.Clifton, D. R., C. L. Nolder, J. L. Hughes, A. J. Nowalk, and J. A. Carroll. 2006. Regulation and expression of bba66 encoding an immunogenic infection-associated lipoprotein in Borrelia burgdorferi. Mol. Microbiol. 61243-258. [DOI] [PubMed] [Google Scholar]
- 13.Coburn, J., J. R. Fischer, and J. M. Leong. 2005. Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol. Microbiol. 571182-1195. [DOI] [PubMed] [Google Scholar]
- 14.Cugini, C., M. Medrano, T. G. Schwan, and J. Coburn. 2003. Regulation of expression of the Borrelia burgdorferi β3-chain integrin ligand, P66, in ticks and in culture. Infect. Immun. 711001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Silva, A. M., and E. Fikrig. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Investig. 99377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Silva, A. M., and E. Fikrig. 1997. Borrelia burgdorferi genes selectively expressed in ticks and mammals. Parasitol. Today 13267-270. [DOI] [PubMed] [Google Scholar]
- 17.de Silva, A. M., D. Fish, T. R. Burkot, Y. Zhang, and E. Fikrig. 1997. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect. Immun. 653146-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fikrig, E., S. W. Barthold, N. Marcantonio, K. Deponte, F. S. Kantor, and R. A. Flavell. 1992. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect. Immun. 60657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fikrig, E., and S. Narasimhan. 2006. Borrelia burgdorferi—traveling incognito? Microbes Infect. 81390-1399. [DOI] [PubMed] [Google Scholar]
- 20.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 1025162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fuji, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore, R. D., Jr., R. R. Howison, V. L. Schmit, A. J. Nowalk, D. R. Clifton, C. Nolder, J. L. Hughes, and J. A. Carroll. 2007. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice. Infect. Immun. 752753-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Hook. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30711-723. [DOI] [PubMed] [Google Scholar]
- 24.Guo, B. P., S. J. Norris, L. C. Rosenberg, and M. Hook. 1995. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect. Immun. 633467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 662674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 684759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson, M. S., D. R. Cassatt, B. P. Guo, N. K. Patel, M. P. McCarthy, D. W. Dorward, and M. Hook. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 662143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 9812724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyde, J. A., J. P. Trzeciakowski, and J. T. Skare. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indest, K. J., and M. T. Philipp. 2000. DNA-binding proteins possibly involved in regulation of the post-logarithmic-phase expression of lipoprotein P35 in Borrelia burgdorferi. J. Bacteriol. 182522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indest, K. J., R. Ramamoorthy, and M. T. Philipp. 2000. Transcriptional regulation in spirochetes. J. Mol. Microbiol. Biotechnol. 2473-481. [PubMed] [Google Scholar]
- 32.Indest, K. J., R. Ramamoorthy, M. Sole, R. D. Gilmore, B. J. Johnson, and M. T. Philipp. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect. Immun. 651165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 727147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. M. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 2792421-2429. [DOI] [PubMed] [Google Scholar]
- 35.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 714608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 704798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, X., X. Liu, D. S. Beck, F. S. Kantor, and E. Fikrig. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect. Immun. 743305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDowell, J. V., K. M. Hovis, H. Zhang, E. Tran, J. Lankford, and R. T. Marconi. 2006. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 743030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect. Immun. 693670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowalk, A. J., R. D. Gilmore, Jr., and J. A. Carroll. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 743864-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowalk, A. J., C. Nolder, D. R. Clifton, and J. A. Carroll. 2006. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 62121-2134. [DOI] [PubMed] [Google Scholar]
- 44.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 711689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojaimi, C., V. Mulay, D. Liveris, R. Iyer, and I. Schwartz. 2005. Comparative transcriptional profiling of Borrelia burgdorferi clinical isolates differing in capacities for hematogenous dissemination. Infect. Immun. 736791-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orloski, K. A., E. B. Hayes, G. L. Campbell, and D. T. Dennis. 2000. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill. Summ. 491-11. [PubMed] [Google Scholar]
- 47.Pahl, A., U. Kuhlbrandt, K. Brune, M. Rollinghoff, and A. Gessner. 1999. Quantitative detection of Borrelia burgdorferi by real-time PCR. J. Clin. Microbiol. 371958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal, U., X. Li, T. Wang, R. R. Montgomery, N. Ramamoorthi, A. M. Desilva, F. Bao, X. Yang, M. Pypaert, D. Pradhan, F. S. Kantor, S. Telford, J. F. Anderson, and E. Fikrig. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119457-468. [DOI] [PubMed] [Google Scholar]
- 49.Parveen, N., K. A. Cornell, J. L. Bono, C. Chamberland, P. Rosa, and J. M. Leong. 2006. Bgp, a secreted glycosaminoglycan-binding protein of Borrelia burgdorferi strain N40, displays nucleosidase activity and is not essential for infection of immunodeficient mice. Infect. Immun. 743016-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48753-764. [DOI] [PubMed] [Google Scholar]
- 51.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 9713865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Revel, A. T., J. S. Blevins, C. Almazan, L. Neil, K. M. Kocan, J. de la Fuente, K. E. Hagman, and M. V. Norgard. 2005. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc. Natl. Acad. Sci. USA 1026972-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 991562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosa, P. A., K. Tilly, and P. E. Stewart. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 3129-143. [DOI] [PubMed] [Google Scholar]
- 55.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samuels, D. S., K. E. Mach, and C. F. Garon. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 1766045-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 922909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seshu, J., J. A. Boylan, F. C. Gherardini, and J. T. Skare. 2004. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect. Immun. 721580-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seshu, J., J. A. Boylan, J. A. Hyde, K. L. Swingle, F. C. Gherardini, and J. T. Skare. 2004. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol. Microbiol. 541352-1363. [DOI] [PubMed] [Google Scholar]
- 61.Seshu, J., M. D. Esteve-Gassent, M. Labandeira-Rey, J. H. Kim, J. P. Trzeciakowski, M. Hook, and J. T. Skare. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 591591-1601. [DOI] [PubMed] [Google Scholar]
- 62.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 1131093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 634535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strother, K. O., A. Broadwater, and A. De Silva. 2005. Plasmid requirements for infection of ticks by Borrelia burgdorferi. Vector Borne Zoonotic Dis. 5237-345. [DOI] [PubMed] [Google Scholar]
- 65.Strother, K. O., and A. de Silva. 2005. Role of Borrelia burgdorferi linear plasmid 25 in infection of Ixodes scapularis ticks. J. Bacteriol. 1875776-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 725419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 371470-1479. [DOI] [PubMed] [Google Scholar]
- 68.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 10011001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, X. F., M. C. Lybecker, U. Pal, S. M. Alani, J. Blevins, A. T. Revel, D. S. Samuels, and M. V. Norgard. 2005. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 1874822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89275-285. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, J. R., and S. J. Norris. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect. Immun. 663698-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, J. R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 663689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]