Abstract
Complement is a key arm of the innate immune defenses against the pathogenic neisseriae. We previously identified lipooligosaccharide on Neisseria meningitidis as an acceptor for complement C4b. Little is known about other neisserial targets for complement proteins C3 and C4, which covalently attach to bacterial surfaces and initiate opsonization and killing. In this study we demonstrate that Neisseria gonorrhoeae porin (Por) 1B selectively binds C4b via amide linkages and C3b via ester linkages. Using strains expressing hybrid Por1A/1B molecules, a region spanned by loops 4 and 5 of Por1B was identified as the preferred binding site for C4b. We also identified the opacity protein (Opa), a major adhesin of pathogenic neisseriae, as a target for C4b and C3b on both N. meningitidis and N. gonorrhoeae. Using N. gonorrhoeae variants that predominantly expressed individual Opa proteins, we found that all Opa proteins tested (A, B, C, D, E, F, and I) bound C4b and C3b via amide and ester linkages, respectively. Amide linkages with Por1B and Opa were confirmed using serum containing only the C4A isoform, which exclusively forms amide linkages with targets. While monomers and heterodimers of C4Ab were detected on bacterial targets, C4Bb appeared to preferentially participate in heterodimer (C5 convertase) formation. Our data provide another explanation for the enhanced serum sensitivity of Por1B-bearing gonococci. The binding of C3b and C4b to Opa provides a rationale for the recovery of predominantly “transparent” (Opa-negative) neisserial isolates from persons with invasive disease, where the bacteria encounter high levels of complement.
Complement is an important arm of the innate immune system that combats neisserial infections. Persons deficient in terminal complement components are predisposed to recurrent neisserial infections (19, 60). The complement cascade comprises the classical, lectin, and alternative pathways. All three pathways converge at the level of C3b deposition. C3b is an integral component of both the classical and alternative pathway C5 convertases (C4bC3b,C2a and C3bC3b,Bb, respectively), which cleave C5 to initiate membrane attack complex (C5b-9) formation. Prior work has shown that antibody (Ab)-dependent classical pathway activation is essential to initiate direct complement-dependent neisserial killing (31). Binding of complement-fixing immunoglobulin to the bacterial surface results in binding of the activated C1 complex to appropriately spaced Fc domains. Activated C1s in the complex first cleaves C4 to C4b, which covalently binds, either via an ester or an amide linkage, to the bacterial surface and then cleaves C2 that binds to C4b, leading to the formation of the C4b,C2a complex, which is the C3 convertase of the classical pathway. Binding of a C3b molecule to or close to the classical pathway C3 convertase imparts C5 convertase activity to the enzyme complex (reviewed in reference 75). Similarly, C3b covalently bound to the bacterium can bind factor B to form the C3bB complex. Cleavage of factor B in this complex by the enzyme factor D leads to formation of the alternative pathway C3 convertase, which can further cleave more C3 molecules to C3b. Binding of a second C3b molecule to the C3 convertase results in C5 convertase activity by forming C3bC3b,Bb.
The early events in complement activation, in particular, targets for C4b and C3b, on neisseriae have not been well defined. Edwards et al. have shown that gonococcal lipooligosaccharide (LOS) serves as a site for C3 deposition (15), and we have shown that meningococcal LOS is a target for C4b and that the location of phosphoethanolamine residues on heptose II determines the nature of the C4b linkage and modulates serum resistance of bacteria (49). In this study we characterize additional ligands for C4b and C3b on Neisseria gonorrhoeae and Neisseria meningitidis. This investigation provides a better understanding of how complement is activated on the neisserial surface and provides further insights into the pathogenesis of these organisms.
MATERIALS AND METHODS
Bacterial strains.
Neisserial strains were routinely cultured on chocolate agar plates supplemented with IsoVitaleX equivalent at 37°C in an atmosphere of 5% carbon dioxide. N. gonorrhoeae strains MS11 (41), F62 (63), FA1090 (76) (all Por1B), and UU1 (77), 15253 (78), and FA19 (46) (all Por1A) have been described previously. Opa phase variants of strain FA1090 that express predominately a single Opa protein (A, B, C, D, E, or F), an Opa-negative strain (FA1090 OpaA-K), in which all opa genes have been genetically inactivated, and an OpaI-expressing strain and an OpaB+ phase-locked strain (the latter two strains were constructed in the FA1090 OpaA-K background) were all provided by Janne Cannon (University of North Carolina, Chapel Hill). Methods for the selection of specific Opa phase variants have been described previously and were used to independently validate the Opa expression of these strains (35). Gonococcal strains bearing FA19/MS11 hybrid Por molecules (9) were provided by P. F. Sparling and C. Elkins (both of the University of North Carolina, Chapel Hill).
N. meningitidis strains MC58 (72) and H44/76 (25) have been described previously. MC58 siaD lst was constructed by insertional inactivation of both the polysialyltransferase (siaD) gene and the LOS sialyltransferase (lst) gene as described previously (39, 49). These mutations resulted in the loss of expression of capsular polysaccharide and in the inability to sialylate LOS. Serogroup A meningococcal strain Z2087 (24) and its Opa-negative isogenic mutant were also provided by Janne Cannon.
Sera and complement reagents.
Normal human serum (NHS), isolated from seven healthy human volunteers with no prior history of immunization with the meningococcal vaccine, was pooled and stored at −80°C. NHS contains two isoforms of C4 called C4A and C4B. Serum containing only C4A or C4B (C4B and C4A deficient, respectively) has been previously described (64) and was provided by Seppo Meri (Haartman Institute, Helsinki, Finland). These sera were obtained from individuals without known deficiencies of other complement components.
Antibodies.
Polyclonal sheep anti-human C4 (Biodesign, Saco, ME) was used in Western blotting experiments as described previously (49). To selectively detect C3b bound to bacteria in Western blot assays, monoclonal Ab (MAb) 755, which is directed against the C-terminal end (amino acids 1499 to 1519 of C3) of the α-chain of C3, was used (37, 74). Similarly, iC3b-target complexes were localized using MAb G-3E (30). Anti-Por1B MAb 5.51 is specific for the fifth loop of MS11 Por1B (17), and anti-Opa MAb 4B12 recognizes nearly all neisserial Opa proteins.
To produce polyclonal anti-Opa antibody, Opa was purified from N. meningitidis MC58 as previously described (4) and five female, 6- to 8-week-old C57B/6J mice (Jackson Laboratories, Bar Harbor, ME) were each immunized subcutaneously with 50 μg of purified Opa emulsified in complete Freund's adjuvant. Mice were given booster injections at week 4. Antisera were collected at weeks 4 and 7 by tail bleed. Anti-Opa antibodies were measured by enzyme-linked immunosorbent assay, and specificity of the sera was assessed by Western blotting against whole-cell lysates (see Fig. S1 in the supplemental material).
Detection of C4b and C3b targets.
Western blotting to localize C4- and C3-target complexes was carried out as described previously (49). Briefly, 3 × 108 bacteria suspended in Hanks balanced salt solution (HBSS) containing 0.15 mM CaCl2 and 1 mM MgCl2 (HBSS2+) were incubated with NHS (concentration specified for each experiment) in a final reaction volume of 500 μl for 30 min at 37°C. Bacteria were washed twice in HBSS2+ and divided into two aliquots that were treated with either buffer alone (to detect C4b bound to targets via ester and amide linkages) or 1 M methylamine, pH 11 (to disrupt ester bonds), for 1 h at 37°C, in a final reaction volume of 40 μl. Direct determination of the specific amide-bound C4b or C3b/iC3b is not possible, because it cannot be separated intact from acceptor surfaces without altering its primary structure.
Gonococcal strains FA1090, MS11, 15253, and FA19 bind the complement regulatory protein C4b-binding protein (C4BP) (51), which results in degradation of C4b bound to the organism. To minimize C4b cleavage, NHS was supplemented with fAb104 (fAb fragment of anti-C4BP antibody 104), which blocks C4BP binding to gonococci and inhibits the function of C4BP in solution, thus preventing C4BP from serving as a cofactor in the factor I-mediated cleavage of C4b to its C4c and C4d fragments (51). Blocking C4b degradation enables identification of each C4b target linked to a single C4 fragment, thereby allowing a more accurate estimate of the molecular masses of C4b target molecules. In some experiments, we used the K-76 salt of monoacetic acid (K-76COONa; kind gift of Guang Wang, Otsuka Pharmaceuticals, Rockville, MD) as a factor I inhibitor (27, 28) to prevent the cleavage of C4b and C3b to C4d and iC3b, respectively. Two volumes of a 4-mg/ml solution of K-76COONa (9.09 mM) in phosphate-buffered saline (PBS) was mixed with 1 volume of serum (final concentration of K-76, 2.6 mg/ml) for 1 h on ice prior to use in Western blotting experiments (26).
Samples were solubilized with lithium dodecyl sulfate sample buffer (NuPAGE LDS sample buffer; Invitrogen) containing 2-mercaptoethanol (2.5% final concentration) at 37°C for 30 min. Proteins were separated on NuPAGE Novex 4 to 12% bis-Tris gradient gels using NuPAGE 3-morpholinopropanesulfonic acid running buffer (Invitrogen) (40 mV for 15 h at 4°C). Western blotting was performed as described previously (70). C4b was detected using anti-C4 at a dilution of 1:1,000 in PBS containing 0.05% Tween 20 and disclosed as previously described (49). Similarly, C3b and iC3b were detected using MAb 755 (1 μg/ml) and MAb G-3E (undiluted tissue culture supernatants containing ∼20 μg/ml of Ab), respectively. Gonococcal Por1B and Opa proteins were detected using anti-Por1B MAb 5.51 (1 μg/ml) and mouse anti-Opa MAb 4B12 (1:1,000 or 1 μg/ml) diluted in PBS-Tween.
Relative molecular weights of C4b- and C3b-target complexes were calculated based on a logarithmic curve fit of the migration of known standards. In all cases the R2 value of the regression equation exceeded 0.97. Discrepancies between observed and predicted molecular weights may be explained by incomplete denaturation. In this system, the C4b- and C3b-target complexes remain covalently linked (i.e., they are not fully denatured) and thus their migration will be affected by both the molecular mass and the conformation of the complex.
RESULTS
C4b targets on N. gonorrhoeae.
Activation of the classical pathway ultimately results in the 87-kDa C4bα′ fragment of C4 being linked, via either an amide or ester bond, to a target molecule on the bacterial cell surface. These C4b-target complexes can be detected by Western blot analysis using anti-C4 Ab and their masses crudely estimated based on migration velocities. The nature of the C4b-target bond can be inferred from methylamine cleavage of ester bonds. We examined C4b targets on five strains of N. gonorrhoeae (three representing Por1A and two representing Por1B serotypes) following incubation with 20% NHS. Four of these strains (FA1090, MS11, 15253, and FA19) bind the complement regulatory protein C4BP (51), which serves as a cofactor in factor I-mediated cleavage of C4b to its C4c and C4d fragments. We used fAb104 (51) to block C4BP binding and subsequent C4b degradation. This resulted in each C4b target being linked to a single C4 fragment (C4bα′), thereby enabling a more accurate estimation of the molecular masses of C4b-binding molecules.
Using these techniques, three gonococcal C4b-target complexes with calculated masses of 88 kDa, ∼100 kDa, and 130 kDa were detected (Fig. 1). After accounting for the mass of C4bα′ (87 kDa), these data implicate gonococcal C4b targets of approximately 1 kDa, 13 kDa, and 43 kDa. Of note, C4b-target complexes likely exhibit anomalous migration in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, because proteins migrate relative to their molecular mass only when they are fully denatured. Following covalent attachment of C4b to microbial targets, we intentionally avoided heating of the samples to prevent complete denaturation of the complexes and potential loss of epitopes needed for recognition by antibodies in Western blot experiments; therefore, tertiary and quaternary structure of these complexes may have impacted migration and calculation of true molecular masses. None of the anti-C4-reactive bands was seen in control samples lacking NHS (data not shown; see the anti-C4 blots in Fig. 2 and 4, below, as examples).
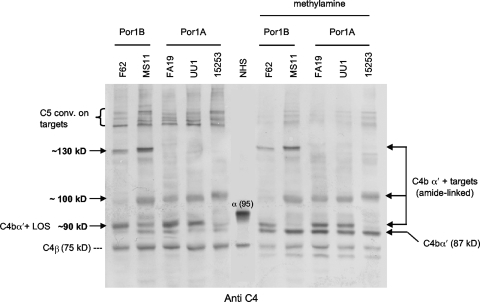
FIG. 1.
C4b binding to five strains (two Por1B and three Por1A) of N. gonorrhoeae. Bacteria were incubated with NHS (final concentration, 20% [vol/vol]) containing fAb104, which blocked binding of C4BP to bacteria and prevented degradation of C4b. Western blots were probed with polyclonal anti-human C4 and show C4-target complexes at ∼90 kDa, ∼100 kDa, and ∼130 kDa (the latter only on the two Por1B strains). Higher-molecular-mass (∼200-kDa) complexes were also seen (indicated by the left bracket) and represent C4b-containing heterodimers (C5 convertases) linked to these bacterial targets. Methylamine treatment, which releases ester-bound, but not amide-linked, C4b α′ chains (87 kDa), did not dissociate the ∼100-kDa and ∼130-kDa C4b-target complexes. The LOS-C4b complexes on strains F62, FA19, and UU1 (but not MS11 and 15253) were predominantly methylamine resistant. The position of the C4 α chain in the lane marked NHS is indicated by α (95).
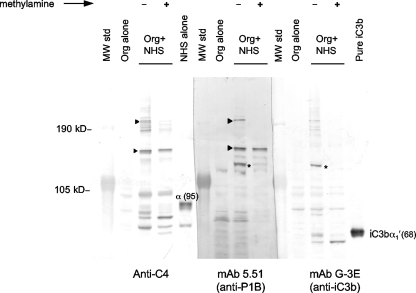
FIG. 2.
Por1B binds C4b and iC3b. Strain MS11 (Por1B) was incubated with NHS treated with fAb104, and samples were processed for Western blotting as described in the legend for Fig. 1. Organisms incubated with only HBSS2+ (Org alone) were used as negative controls. Parallel samples transferred to the same membrane were incubated with anti-C4, anti-Por1B MAb 5.51, or anti-iC3b MAb G3-E. Arrowheads indicate bands that migrated in parallel and reacted with both the anti-Por1B MAb 5.51 and the anti-C4 antibody. Similarly, asterisks indicate bands that migrated in parallel and reacted with both the anti-Por1B MAb 5.51 and the anti-iC3b antibody. The location of the 95-kDa C4 α chain [α (95)] and the 68-kDa iC3b α1′ chain [iC3bα1′(68)] are indicated in the lanes containing NHS alone and pure iC3b, respectively.
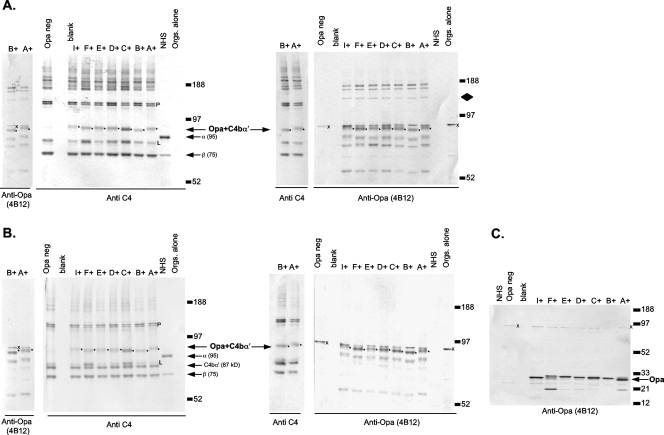
FIG. 4.
Gonococcal Opa binds to C4b. (A and B) FA1090 derivatives that express predominantly an individual Opa protein (OpaA, -B, -C, -D, -E, -F, or -I), FA1090 that expresses OpaB, and an FA1090 Opa-negative mutant were incubated with NHS containing fAb104 (blocks C4BP binding to gonococci) and processed with (B) or without (A) methylamine as described in the legend for Fig. 1. Control lanes contained NHS and OpaA-expressing FA1090 and were processed as above but not treated with serum (Orgs. alone). Parallel samples, on the same blot, were probed with either anti-C4 or anti-Opa monoclonal Ab 4B12. Anti-C4- and anti-Opa-reactive bands that migrated at the same velocity are indicated as Opa+C4bα′ and marked with asterisks to the right of the bands. C4-Opa complexes were methylamine resistant (asterisks), suggesting amide linkages. The control Opa-negative mutant does not show C4-Opa complexes at ∼100 kDa. The faint 140-kDa Opa-reactive band (marked by a diamond in the top right blot that is absent in the Opa-negative mutant and in organisms not treated with serum), which does not localize with any band in the corresponding anti-C4 blot and that also becomes less intense with methylamine treatment, represents C3b bound to Opa (see Fig. 5, below). The location of the 95-kDa C4 α chain is indicated in each NHS lane as α (95). The relative molecular weights of the molecular weight standards are indicated. The C4b α′ (87 kDa) released from C5 convertases and ester-linked targets is indicated on the blots treated with methylamine. (C) Specificity of MAb 4B12 for Opa proteins in whole-cell lysates. The position of the Opa protein in each strain is noted by an arrow. A ∼96-kDa neisserial protein marked with an x also reacts with MAb 4B12. This protein is also present in the Opa-negative mutant and in samples incubated with or without serum. Porin-C4b and LOS-C4b complexes are marked with a P and L, respectively.
C4b formed complexes with a low-Mr (∼1-kDa) surface molecule in all strains tested (Fig. 1, C4bα′+LOS). We demonstrated previously that similar complexes in N. meningitidis (which has a LOS structurally similar to N. gonorrhoeae) are C4bα′-chain linked to phosphoethanolamine residues on LOS via amide bonds or C4bα′-chain linked to hydroxyl groups on LOS via ester bonds (49). The C4b-LOS bonds on strains F62, FA19, and UU1 were predominantly methylamine resistant, indicating amide-C4b-LOS bonds (Fig. 1). In contrast, the linkages on strains MS11 and 15253 were completely disrupted by methylamine treatment, indicating the presence of only ester linkages between C4b and LOS.
The two remaining gonococcal C4b targets have not been described previously. The ∼130-kDa C4bα′-target complex was observed on strains F62 and MS11, whose porin molecules are of the Por1B serovar, but the complex was absent on Por1A strains FA19, UU1, and 15253. Porin, the most abundant protein in the gonococcal outer membrane, has a predicted molecular mass of ∼35 kDa (Por1A) or ∼37 kDa (Por1B) and forms trimers in the outer membrane. The fifth exposed loop of Por1B is approximately 15 amino acids longer than the corresponding loop in Por1A, hence, the smaller molecular mass of Por1A. We hypothesized that C4bα′ was complexed with Por1B. In addition, all gonococcal strains, with the exception of F62, showed a C4b-containing band that migrated at approximately 100 kDa and appeared to be polymorphic (Fig. 1). None of the C4b-containing complexes at 100 kDa was affected by methylamine treatment, suggesting that these molecules formed amide bonds with C4b.
Gonococcal Por1B is a target for C4b and iC3b.
To determine if Por1B was indeed present in the 130-kDa species, samples prepared from Por1B strain MS11 were evaluated in parallel lanes of the same Western blot probed with either anti-C4 Ab or anti-Por1B MAb (MAb 5.51). As seen in Fig. 2, MAb 5.51-reactive bands migrated in parallel with anti-C4-reactive bands at ∼130 kDa and ∼200 kDa, indicating that Por1B was bound to C4b in these complexes. The higher-molecular-weight (MW) band likely represents C4-containing heterodimers (C5 convertases) assembled on Por1B. The C4bα′-Por1B band was resistant to methylamine treatment, indicating an amide linkage. The C5 convertases containing amide-linked C4bα′-Por1B were reduced by methylamine treatment, as expected, due to the presence of an internal ester bond between C3b and C4bα′ in these structures (69).
An approximately 110-kDa MAb 5.51-reactive band, which did not migrate in parallel with any C4-reactive band, was also noted (Fig. 2). This band was diminished in samples treated with methylamine, suggesting it contained Por1B linked to another serum component via an ester bond. C3 is the most abundant complement protein in human serum. Activation of C3 results in deposition of C3b (106 kDa) on bacterial surface molecules, an important step that leads to the eradication of microbes. Deposition of C3b, like C4b, occurs by reduction of an internal thioester that results in an ester or amide bond with the target. C3b prefers hydroxylated targets and is most often found linked to targets by an ester bond. Much of C3b deposited on Neisseria is converted to iC3b (68 kDa), which results from cleavage of C3b by factor H (cofactor) and factor I (enzyme) (16, 34, 44, 74), and we postulated that the ∼110-kDa band could be iC3b ester linked to Por1B. Complexes of iC3b with target molecules can be detected by Western blotting using anti-iC3b Ab (MAb G-3E). As seen in Fig. 2, the ∼110-kDa MAb 5.51-reactive band migrated in parallel with an iC3b-reactive band, indicating that Por1B also bound C3b that was subsequently converted to iC3b. Treatment with methylamine released the ester-linked α1′ (68-kDa) fragment of iC3b, which appears as C3bα1′ in the methylamine-treated lane probed with MAb G-3E (anti-iC3b). The released Por1B is not visible because it has migrated off the gel.
The central region of Por1B contains the binding site for C4b.
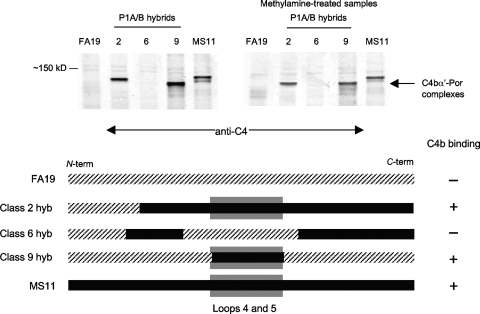
Each porin polypeptide contains eight surface-exposed loops. Although the primary amino acid sequences of the Por1A and Por1B polypeptides are nearly identical in the membrane-spanning regions, the sequences are variable in the surface-exposed domains. To define the region in Por1B that acted as the binding site for C4b, we assessed C4b deposition in gonococcal Por1A/B hybrid strains that were created with strains FA19 (Por1A) and MS11 (Por1B). As seen in Fig. 3, only strains that bore a Por molecule containing Por1B loops 4 and 5 bound C4b. These data suggest that the primary binding site for C4b on Por1B is a region spanned by Por1B loops 4 and 5 and that sequence variation in the exposed portions of these loops in the Por1A molecule may abrogate binding of C4b.
FIG. 3.
C4b binds to Por1B within the region spanned by loops 4 and 5. C4b binding to strains that express hybrid Por1A/1B molecules (derived from Por1A strain FA19 and Por1B strain MS11) was examined following incubation with NHS and fAb104 as described in the legend for Fig. 1. A schematic representing the Por molecule of each hybrid strain and their ability to bind to C4b are shown beneath the blots. The hybrid class designation is as described by Carbonetti et al. (10). Por1A (FA19) sequence is indicated by the hatched line, and the Por1B (MS11) sequence is indicated by the solid black line. C4b-Por complexes were seen only in strains that possess Por1B loops 4 and 5 (indicated by a shaded gray box in the schematic).
Opa is a target for C4b and C3b.
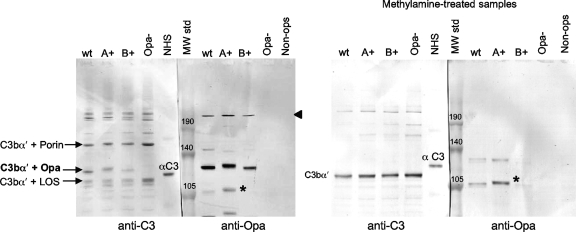
The neisserial opacity proteins (Opa) are a polymorphic family of phase-variable outer membrane proteins with molecular masses of approximately 28 kDa that are associated with colony opacity. Gonococci may carry as many as 11 distinct opa genes (opaA to opaK) that are independently switched on and off at a frequency of 10−2 to 10−3 per cell generation. Based on the allelic variation of the 100-kDa C4b-target complex, we hypothesized that this C4b target was Opa. To test this hypothesis, we analyzed C4b deposition on variants of FA1090 that expressed predominantly a particular Opa (Opa A, B, C, D, E, F, or I) or no Opa at all. fAb104 was used to block C4BP activity on the bacterial surface. Parallel Western blots were probed with the anti-C4 Ab and anti-Opa MAb 4B12. Representative binding of C4b to the FA1090 Opa variants is shown in Fig. 4. The polymorphic 100-kDa complex was seen on all Opa-expressing strains and was recognized by both anti-C4 and anti-Opa Abs (Fig. 4). The size variations seen in the Opa-C4b complexes with the anti-C4 antibody are also mirrored with the anti-Opa antibody. In further support of Opa as a C4b target, the Opa-negative mutant did not show a complex that reacted with either Ab at this location (Fig. 4). All Opa-C4b complexes were resistant to methylamine treatment, suggesting that C4b formed amide bonds with Opa (Fig. 4). Together, these data corroborate C4b-Opa interactions on strain FA1090. As seen above with Por1B interactions with C4b and iC3b, anti-C4 and anti-Opa coreactive bands were also seen at ∼200 kDa; these likely represent C4-containing heterodimers (C5 convertases) bound to Opa and were dissociated by methylamine.
An Opa-containing fragment with an apparent molecular mass of ∼140 kDa was also evident (Fig. 4). This band did not migrate in parallel with any C4-reactive band and was diminished in samples treated with methylamine, suggesting it contained Opa linked to a serum component via an ester bond. Although we did not block factor I activity, the apparent molecular mass of this complex suggested that it could represent residual C3bα′ (106 kDa) linked to Opa (∼28 kDa).
Although prior work has shown that most of the C3b deposited on Neisseria is converted over 30 to 60 min to iC3b (16, 34, 44, 74), we postulated that C3b bound to certain bacterial targets may be resistant to cleavage to iC3b. C3bα′-Opa complexes with a predicted molecular mass of ∼134 kDa would not have been detected by the anti-iC3b MAb (G-3E) that we have used thus far. To address this possibility, we blocked C3b conversion to iC3b using the factor I inhibitor K-76COONa and detected C3b using MAb 755. Confirmation of a C3b-Opa complex is provided in Fig. 5. Data with wild-type strain FA1090, its Opa A+ and Opa B+ variants, and the Opa-negative control are shown. Parallel migration of the ∼140-kDa target with anti-Opa and anti-C3 MAb 755 is indicated in Fig. 5 as C3bα′+Opa. The Opa-negative mutant lacks a C3bα′-containing complex at this location. The additional C3bα′-containing complexes seen migrating at ∼150 kDa and ∼110 kDa represent the C3bα′ chain (∼106 kDa) bound to Por1B (∼37 kDa) and LOS (∼4 kDa) targets. Methylamine treatment (right blot) resulted in release of almost all C3b bound to bacterial targets and the appearance of the released 106-kDa C3bα′ chain. The methylamine-resistant Opa-containing bands around 105 kDa seen on the Opa-expressing strains represent C4b bound to Opa as described above.
FIG. 5.
Opa binds C3b via ester linkages. Samples were incubated with NHS containing fAb104 and K-76COONa (the monoacetic salt of K-76, which inhibits factor I activity), with anti-C3 MAb 755, and polyclonal anti-Opa antibody. Shown are representative data with the OpaA+, OpaB+, and Opa-negative derivatives of strain FA1090. Opa-C3b complexes are indicated by the arrow labeled C3bα′ + Opa. The susceptibility of the linkage to methylamine indicates an ester C3b-Opa linkage. The position of the α-chain of C3 (115 kDa) in the NHS lane is indicated as αC3; the released C3b α′ chain (∼106 kDa) in methylamine-treated samples probed with MAb 755 is indicated as C3b α′. The location of the Opa-C4b complexes (shown above in Fig. 4) at ∼105 kDa is indicated by an asterisk.
Binding of isoforms of C4 to gonococci.
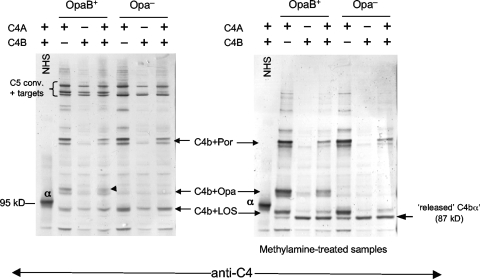
Two isoforms of C4, called C4A and C4B, which differ in their biological properties, are found in normal human serum. C4A forms amide linkages with its targets, while C4B forms mainly ester linkages. We used sera that contained only C4A or C4B to determine how each of these isoforms of C4 binds to gonococci. FA1090 OpaB+ and the Opa-negative mutant were used to illustrate these differences. As seen in Fig. 6, serum that contained only C4A showed monomers of C4Ab bound to LOS, Opa, and Por1B on the OpaB+ strain. In addition, heterodimers assembled on these three distinct targets were apparent. In contrast, with serum containing only C4B, bands representing monomeric C4Bb on these targets were very faint; however, the C4Bb-containing C5 convertases were readily visualized, highlighting the propensity for C4Bb to preferentially form C5 convertases. Methylamine treatment of these C4Bb-containing convertases resulted in release of free C4Bbα′ but not in the appearance of amide-linked targets. This suggests that C4Bb was ester linked to LOS, Opa, and Por1B in these complexes. When both isoforms were present, a pattern that was intermediate and representative of both C4 isoforms was seen. Similar results were seen with the Opa-negative mutant, except that the Opa-C4 monomers as well as the heterodimers assembled on Opa (seen on the Opa-positive strain) were absent.
FIG. 6.
Binding of C4 isoforms to N. gonorrhoeae. Sera containing only the C4A isoform (preferentially forms amide linkages) or only the C4B isoform (preferentially forms ester linkages) were incubated with OpaB+ and Opa− variants of strain FA1090, and the nature of the C4b linkage to targets was determined by Western blotting as described in the legend for Fig. 1. fAb104 was added to sera to minimize binding of C4BP and diminish C4b degradation to C4d. C4A bound to LOS, Opa, and Por; no release of C4b α′-chain was seen with methylamine treatment, indicating that C4Ab bound to these three targets via amide linkages. Note that the binding of C4Ab to Por and Opa appears as “doublets.” In contrast, C4Bb bound to targets was detected predominantly as part of high-MW C5 convertases assembled via the three major targets; a minimal amount of “monomeric” C4Bb bound to targets. Bound C4Bb was completely released by methylamine, suggesting internal ester linkages within the convertases. Incubation of strain FA1090 with NHS and fAb104 (indicated as lanes containing both C4A+ and C4B+) showed a pattern that was intermediate or representative of both the C4A- and C4B-only containing sera.
Methylamine treatment confirmed the amide nature of the C4Ab-target complexes. Intensification of C4b+ target(s) with methylamine treatment compared to non-methylamine-treated specimens, best seen in lanes 2 and 5 of the methylamine-treated samples (right blot), resulted from release of the C3 fragment that is ester linked to the C5 convertase, which also contained C4b-target complexes. The released C4b target(s) then migrated together with preexisting monomeric C4Ab-target complexes, thereby resulting in an increased intensity of the C4Ab-target adduct. A faint band corresponding to released 87-kDa α′-chain was seen (in lanes 2 and 5 of the right blot, for example), suggesting a predominance of amide linkages. In contrast, bacteria incubated with C4B-containing serum showed a prominent released 87-kDa α′ chain upon methylamine treatment, also confirming the presence of ester linkages between C4Bb and bacterial targets.
C4 binding sites on meningococci.
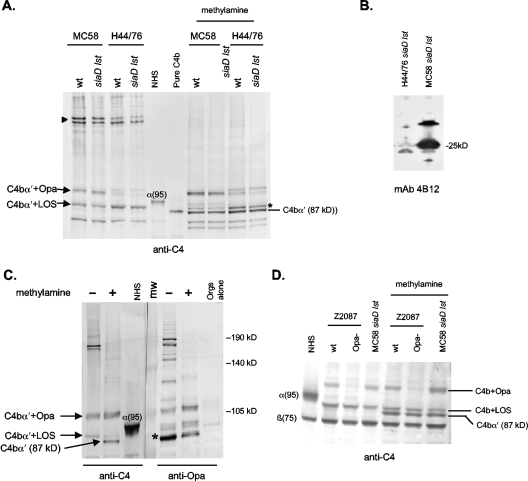
We previously described LOS as a target for C4b on meningococci (49). Our studies presented above have indicated that in gonococci LOS, Opa, and Por1B are all targets for C4b. Meningococci express Opa and Por proteins that are similar to the gonococcal counterparts and, thus, we wished to further examine C4b targets in meningococci. We examined C4 binding sites on two serogroup B meningococcal strains, MC58 and H44/76, and their unencapsulated mutants that lack LOS sialic acid (siaD lst mutants). Because capsular polysaccharide and LOS sialic acid regulate complement (55, 73), the wild-type strains were incubated with 50% NHS, while the siaD lst mutants were incubated with 10% NHS. We did not use fAb104 in these experiments, because binding of C4BP to meningococci in physiologic buffers is very weak (33) and preliminary experiments showed that the majority of C4b bound to meningococci remained unprocessed. As seen in Fig. 7A, the targets for C4b were similar on the wild-type strains and the siaD lst mutants, suggesting that neither serogroup B capsular polysaccharide nor LOS sialic acid altered the site of C4b binding when bacteria were incubated with NHS. Two C4b-containing complexes were seen with MC58. One, migrating at approximately 91 kDa, is the previously identified complex containing C4b and LOS (49), and the other, akin to the observations above with gonococci, was a complex that migrated at ∼105 kDa. The ∼105-kDa complex was fainter on H44/76, while the LOS-C4b band appeared more intense. Methylamine treatment did not release the ∼20-kDa target from C4b but did disrupt the LOS-C4b linkage on MC58. Methylamine-resistant C4b-LOS complexes were seen on H44/76 and its siaD lst mutant (Fig. 7A), suggesting the presence of amide linkages between the two molecules. Phosphoethanolamine residues have been shown to mediate amide linkages of C4b to LOS. Two C4-reactive bands are also present at ∼200 kDa (Fig. 7A), and these likely represent C5 convertases assembled on LOS and the ∼20-kDa target.
FIG. 7.
C4b targets on N. meningitidis. Wild-type strains MC58 and H44/76 (encapsulated and LOS sialylated) and their siaD lst derivative mutants (unencapsulated; LOS not sialylated) were incubated with NHS (final concentration, 50% [vol/vol]) for wild-type strains and 10% [vol/vol] for siaD lst mutants); C4b targets were determined as described in the legend for Fig. 1. (A) C4b bound to a low-MW target (complex at ∼90 kDa) on both strains, which has been identified previously as LOS (49). Methylamine-resistant C4b-LOS complexes seen in H44/76 are marked with an asterisk. A second complex at ∼105 kDa (shown to be Opa in panel C) was more prominent on MC58. C5 convertases assembled via these two targets were seen at ∼200 kDa (marked by the arrowhead). Both the 105-kDa C4b α′-chain-containing complex and the LOS-C4b α′ complex on H44/76 resisted methylamine treatment, indicating the presence of amide linkages. (B) Opa expression on MC58 and H44/76. Western blots of bacterial lysates (normalized in amounts electrophoresed and transferred) were probed with anti-Opa MAb 4B12; strain MC58 shows greater Opa expression. (C) The ∼105-kDa C4b-target complex on MC58 siaD lst contains Opa. MC58 siaD lst was incubated with NHS, and samples were processed as described previously. Samples run in parallel were probed with either anti-C4 or with anti-Opa MAb 4B12. The half of the blot probed with anti-Opa MAb 4B12 revealed an Opa-containing band that migrated in parallel with the ∼105-kDa C4b α′-containing complex. This band resisted methylamine treatment, indicating an amide linkage. The asterisk indicates a methylamine-sensitive band containing Opa bound to the 68-kDa iC3bα1′ chain (data not shown). (D) Deleting Opa results in loss of the ∼105-kDa C4b α′-containing complex. Strain Z2087 and its Opa-negative isogenic mutant were examined for C4b binding as described above. Strain MC58 siaD lst was used as a positive control. The Opa-negative mutant of Z2087 did not show a 105-kDa adduct, confirming Opa as a target for C4b.
We hypothesized that the ∼20-kDa target was Opa and that strain MC58 expressed more Opa than strain H44/76, accounting for greater intensity of this complex. Consistent with this hypothesis, Western blot assays of whole-cell lysates of MC58 and H44/76 with anti-Opa MAb 4B12 confirmed greater Opa expression on MC58 (Fig. 7B). Coomassie blue staining of outer membrane preparations validated equal sample loading and corroborated Western blot analysis results (data not shown). Parallel migration of the ∼105-kDa anti-C4-reactive band with the anti-Opa MAb bands confirmed the presence of Opa as a target for C4b on MC58 (Fig. 7C). Band intensity was not affected by methylamine treatment, indicating amide linkages between Opa and C4b. In a separate experiment, the prominent ∼90-kDa anti-Opa-reactive band (Fig. 7C) that decreased in intensity with methylamine treatment was shown to be Opa bound to the 68-kDa iC3bα1′ chain (data not shown). As additional evidence for Opa-C4b interactions in N. meningitidis, we analyzed C4b deposition in serogroup A strain Z2087 and its isogenic mutant that lacks Opa expression. As seen in Fig. 7D, Opa deletion resulted in a corresponding loss of the 105-kDa complex. MC58 siaD lst was included as a positive control. Collectively, these data provide strong evidence that meningococcal Opa also binds C4b via amide linkages.
MC58 and H44/76 both express PorB3 molecules which bear sequence homology to gonococcal Por1A. We observed no C4b-containing complexes at molecular masses that would implicate this meningococcal porin as a C4b target. Previously published data using meningococcal strain Y2220, which expresses a PorB2 molecule (the meningococcal equivalent of gonococcal Por1B) (49), in addition to our unpublished data from using two other PorB2-expressing strains called C2120 and W171, showed no evidence of PorB2-C4b interactions (data not shown). Based on these observations, it appears that meningococcal porins, in contrast to gonococcal Por 1B porins, are not targets for C4b deposition.
DISCUSSION
The complement system recognizes invading pathogens, such as pathogenic Neisseria, and facilitates killing directly or by enhancing opsonophagocytosis using a process of targeted complement activation on the microbial surface. Two complement molecules that play a key role in activation are C4 and C3. Both of these molecules possess internal thioester bonds which, when activated, are highly labile and can bind to proximate hydroxyl or amide groups that serve as electron donors (14, 29). While activation of complement on a microbial surface may be beneficial in combating infection, unimpeded activation can cause excessive inflammation and result in detrimental effects on host tissues. Therefore, activation of the complement system is tightly controlled by several fluid-phase and membrane-bound regulatory molecules (reviewed in reference 75). The intrinsic nature of a complement binding surface plays an important role in determining whether activation will be favored over regulation. For example, factor H (inhibitor) has a greater affinity than factor B (activator) for C3b on normal sheep erythrocytes, rendering this surface a nonactivator of complement; desialylation results in enhanced affinity of factor B for C3b and results in complement activation (18, 36).
Activation of the complement system can result in rapid death of an invading organism and, therefore, it is not surprising that microbes, particularly uniquely human pathogens like neisseriae, have evolved a broad array of strategies to limit activation of human complement on their surfaces (59). Capsular polysaccharides may limit C3 binding to bacteria or may shift C3 deposition to less-effective sites on the bacterial membrane (45), thereby limiting membrane attack complex insertion. Another mechanism to evade complement activation is to bind complement regulatory proteins such as factor H and C4BP (reviewed in reference 38). Evasion of complement is critical for neisseriae to colonize humans and cause disease, and these bacteria employ several, often redundant, mechanisms to escape killing by complement. Both N. gonorrhoeae and N. meningitidis bind factor H and C4BP, which results in complement regulation and enhanced serum resistance (33, 39, 48, 50-53, 55). In addition, the ability to modulate binding of C4b and C3b may further impact the net amount of complement that can be activated.
Neisserial LOS has previously been identified as a target for both C3b and C4b (15, 49). In this study we have established that two additional membrane structures, gonococcal Por1B and Opa, also serve as ligands for C3 and C4 binding. These targets were detected using multiple serum pools, and immunoblotting was used to demonstrate that the NHS was not biased to anti-Por, anti-Opa, or anti-LOS antibodies (data not shown). Por1A-bearing gonococci have been associated with a serum-resistant phenotype that persists after subpassage and an ability to cause disseminated disease, while gonococcal strains expressing Por1B usually are serum sensitive after passage (unsialylated) and more commonly cause a local genital infection (8, 23, 58). The difference in serum resistance between Por1A and Por1B isolates results from preferential binding of factor H and C4BP to Por1A strains (51, 54). In this study we have described preferential binding of C4b and C3b to Por1B. This may contribute further to the enhanced serum sensitivity of Por1B strains relative to their Por1A counterparts. The site of C4b binding was localized to the region spanned by loops 4 and 5 of Por1B. Loop 5 in Por 1B of N. gonorrhoeae may contain the binding site(s) for C4b because of the presence of unique sequences that either diverge or are absent in the corresponding regions of Por1A (13, 24); the predicted surface-exposed region of loop 5 of Por1A contains only ∼9 amino acids, while the corresponding region of Por1B has ∼25 amino acids. Binding of C4b was restricted to gonococcal Por1B and was not observed in the meningococcal homologue of gonococcal Por1B, PorB2. The amino acid sequence of the fifth loop of the meningococcal PorB2 is not conserved with the gonococcal Por1B loop 5 sequence (∼12% identity).
The interaction of complement C3 with porin molecules has been described in other organisms. The facultative intracellular pathogen Legionella pneumophila uses surface-bound C3 fragments to gain entry into mononuclear cells via CR1- and CR3-mediated phagocytosis. The major outer membrane protein, a porin molecule, is the only C3-fixing molecule that has been found on the surface of L. pneumophila (2). Further, Alberti et al. have shown that porins are the most important site of C3b deposition in serum-sensitive strains of Klebsiella pneumoniae and have further proposed that differences in the amount of C3b bound dictate serum sensitivity versus serum resistance. In serum-resistant K. pneumoniae, C3b binding to porin is inhibited by smooth lipopolysaccharide and total C3b deposition is lower than on serum-sensitive strains (1).
We also found that Opa proteins bound C4b and C3b. Opa proteins play a key role in mediating adhesion and invasion of bacteria into epithelial cells (10, 11, 40). An earlier report indicated that gonococcal colonies identified in women around menses (when menstrual blood contributes to a higher level of complement locally) and examined after a single agar subpassage are mostly of the transparent phenotype (i.e., more likely to be Opa negative), while colonies identified at other times in the cycle tend to be opaque (68). Women are uniquely susceptible to disseminated gonococcal infection during menses (57), and serum-resistant strains isolated from blood are also more likely to produce transparent colonies (43). Similarly, meningococci recovered from the blood or cerebrospinal fluid commonly grow as transparent colonies, while those recovered from the nasopharynges of asymptomatic carriers are usually opaque (67). Thus, it appears that neisseria that encounter high levels of complement (as occurs during menses or during bloodstream infection) tend to turn Opa expression “off.” It is possible that loss of a target for C4b and C3b may confer an advantage in combating complement.
A prior report by Bos et al. (7) indicated that Opa expression by gonococcal strain MS11 enhances serum resistance. We were unable to detect differences in serum resistance between FA1090 and its Opa variants that were used in this study. All the strains were resistant (>50% survival) to even 40% NHS; FA1090 is resistant to serum by virtue of its ability to bind C4BP (51). In contrast, the MS11 variant used by Bos et al. (7) was sensitive to serum. LOS analysis by silver staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels showed that FA1090 and each of its Opa mutants expressed more than one LOS species and that there was wide variation in LOS expression among the strains (data not shown). LOS structure also impacts complement binding and serum resistance (20, 21, 49, 65, 66), and therefore it was not possible to assess the effects of Opa expression on complement-dependent killing independent of other potential serum resistance-modifying variables on the strains we used. It is possible that Opa expression may have different effects on serum resistance in different strains, and LOS-Opa interactions may also play a role in modulating the effects of Opa (4, 47).
Human C4 is subject to great genetic diversity stemming from a complex pattern of genetic differences in gene size, gene number, and nucleotide polymorphisms (12, 80). In humans, deficiency in some aspect of C4 expression, often without consequences, such as increased susceptibility to infections, represents the most common form of complement deficiency (6). Individuals generally have between two and eight C4 genes (diploid chromosome), with each locus encoding either a C4A or C4B molecule (5, 79). In addition more than 41 C4 variants can be detected by differences in electrophoretic mobility (42). Partial C4 isoform deficiencies are relatively common, with the prevalence of complete C4A or C4B deficiencies being reported at a combined frequency of 2% (6, 22). Functionally, C4A and C4B are defined by variance of four critical amino acids located at residues 1101 to 1106. These differences endow C4A with an ability to react with amino group-containing antigens, a longer half-life, and an important role in immune clearance and opsonization. C4B preferentially forms ester bonds with hydroxylated targets, has a short half-life, and is important in accelerating classical pathway activation and membrane attack complex formation (14, 32, 56, 62). C4A deficiency has been associated with susceptibility to autoimmune diseases, while C4B deficiency may be more associated with vulnerability to bacterial and viral infections (3, 6, 61, 71). Differential complement activation, resulting from partial or complete C4 deficiency, may impact the susceptibility of a person to disease. For example, an individual that possesses only the C4B isoform (forms predominantly ester linkages) may have an impaired ability to activate complement on a surface where there are relatively limited numbers of available hydroxyl groups that can act as electron donors. Similarly, if available targets on the bacterial surface dictate the nature of the C4-target linkages, and therefore the isoform of C4 that binds to the bacterium, then these targets may impact the immune response. It is noteworthy that LOS and Opa are phase variable, which could allow the bacteria to further modulate complement activation.
A closer examination of the Por1B-C4b complex reveals a double band in several instances. The slower-migrating band is more prominent in strain MS11 (Fig. 1, 2, and 3), while both bands are of similar intensity in strain FA1090 (Fig. 4 and 6). The smaller of the two C4b-Por1B complexes in serum-sensitive MS11 appears to be sensitive to methylamine and thus may be C4Bb, which preferentially forms ester bonds, linked to Por1B. C4Bb-containing complexes are a component of the active C5 convertase, which is more likely to lead to the formation of membrane attack complexes (14, 32, 56, 62). In contrast, both of the C4b-Por1B complexes in serum-resistant strain FA1090 (which also binds the complement regulatory protein C4b-binding protein) resist methylamine treatment and thus may be C4Ab complexes, which are less likely to form membrane attack complexes.
We did not detect C4b or C3b binding to other more conserved and constitutively expressed membrane molecules, such as Rmp or lipoprotein H.8 (data not shown).
In summary, we have defined neisserial Opa and gonococcal Por1B, in addition to LOS, as targets of binding for complement components C4b and C3b. The ability of pathogenic Neisseria to infect humans depends on effective evasion of complement; resistance to complement-mediated killing is determined in part by the qualitative aspects of complement deposition and activation. A better understanding of neisserial structures that are targets for complement deposition and their role in complement resistance will enhance our understanding of susceptibility to neisserial disease and may contribute to rational vaccine design.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health AI32725 (P.A.R.) and AI054544 (S.R.).
We thank Janne Cannon (University of North Carolina, Chapel Hill) for providing us with the Opa variants of strain FA1090, Seppo Meri (Haartman Institute, Helsinki, Finland) for C4A- and C4B-deficient sera, and Guang Wang (Otsuka Pharmaceuticals) for K-76COONa.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 5 November 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alberti, S., D. Alvarez, S. Merino, M. T. Casado, F. Vivanco, J. M. Tomas, and V. J. Benedi. 1996. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect. Immun. 644726-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger-Kawahara, C., and M. A. Horwitz. 1990. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J. Exp. Med. 1721201-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishof, N. A., T. R. Welch, and L. S. Beischel. 1990. C4B deficiency: a risk factor for bacteremia with encapsulated organisms. J. Infect. Dis. 162248-250. [DOI] [PubMed] [Google Scholar]
- 4.Blake, M. S., and E. C. Gotschlich. 1984. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J. Exp. Med. 159452-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchong, C. A., E. K. Chung, K. L. Rupert, Y. Yang, Z. Yang, B. Zhou, J. M. Moulds, and C. Y. Yu. 2001. Genetic, structural and functional diversities of human complement components C4A and C4B and their mouse homologues, Slp and C4. Int. Immunopharmacol. 1365-392. [DOI] [PubMed] [Google Scholar]
- 6.Blanchong, C. A., B. Zhou, K. L. Rupert, E. K. Chung, K. N. Jones, J. F. Sotos, W. B. Zipf, R. M. Rennebohm, and C. Yung Yu. 2000. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J. Exp. Med. 1912183-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos, M. P., D. Hogan, and R. J. Belland. 1997. Selection of Opa+ Neisseria gonorrhoeae by limited availability of normal human serum. Infect. Immun. 65645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan, T. M., and J. F. Hildebrandt. 1981. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect. Immun. 32985-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbonetti, N., V. Simnad, C. Elkins, and P. F. Sparling. 1990. Construction of isogenic gonococci with variable porin structure: effects on susceptibility to human serum and antibiotics. Mol. Microbiol. 41009-1018. [DOI] [PubMed] [Google Scholar]
- 10.Chen, T., R. J. Belland, J. Wilson, and J. Swanson. 1995. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J. Exp. Med. 182511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, T., F. Grunert, A. Medina-Marino, and E. C. Gotschlich. 1997. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 1851557-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawkins, R., C. Leelayuwat, S. Gaudieri, G. Tay, J. Hui, S. Cattley, P. Martinez, and J. Kulski. 1999. Genomics of the major histocompatibility complex: haplotypes, duplication, retroviruses and disease. Immunol. Rev. 167275-304. [DOI] [PubMed] [Google Scholar]
- 13.Derrick, J. P., R. Urwin, J. Suker, I. M. Feavers, and M. C. Maiden. 1999. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 672406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodds, A. W., X. D. Ren, A. C. Willis, and S. K. Law. 1996. The reaction mechanism of the internal thioester in the human complement component C4. Nature 379177-179. [DOI] [PubMed] [Google Scholar]
- 15.Edwards, J. L., and M. A. Apicella. 2002. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell Microbiol. 4585-598. [DOI] [PubMed] [Google Scholar]
- 16.Edwards, J. L., E. J. Brown, S. Uk-Nham, J. G. Cannon, M. S. Blake, and M. A. Apicella. 2002. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol. 4571-584. [DOI] [PubMed] [Google Scholar]
- 17.Elkins, C., N. H. Carbonetti, V. A. Varela, D. Stirewalt, D. G. Klapper, and P. F. Sparling. 1992. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol. Microbiol. 62617-2628. [DOI] [PubMed] [Google Scholar]
- 18.Fearon, D. T. 1978. Regulation by membrane sialic acid of β1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc. Natl. Acad. Sci. USA. 751971-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fijen, C. A., E. J. Kuijper, M. T. te Bulte, M. R. Daha, and J. Dankert. 1999. Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clin. Infect. Dis. 2898-105. [DOI] [PubMed] [Google Scholar]
- 20.Griffiss, J. M., G. A. Jarvis, J. P. O'Brien, M. M. Eads, and H. Schneider. 1991. Lysis of Neisseria gonorrhoeae initiated by binding of normal human IgM to a hexosamine-containing lipooligosaccharide epitope(s) is augmented by strain-specific, properdin-binding-dependent alternative complement pathway activation. J. Immunol. 147298-305. [PubMed] [Google Scholar]
- 21.Guymon, L. F., M. Esser, and W. M. Shafer. 1982. Pyocin-resistant lipopolysaccharide mutans of Neisseria gonorrhoeae: alterations in sensitivity to normal human serum and polymyxin B. Infect. Immun. 36541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauptmann, G., G. Tappeiner, and J. A. Schifferli. 1988. Inherited deficiency of the fourth component of human complement. Immunodefic. Rev. 13-22. [PubMed] [Google Scholar]
- 23.Hildebrandt, J., L. Mayer, S. Wang, and T. Buchanan. 1978. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect. Immun. 20267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs, M. M., T. M. Alcorn, R. H. Davis, W. Fischer, J. C. Thomas, I. Martin, C. Ison, P. F. Sparling, and M. S. Cohen. 1999. Molecular typing of Neisseria gonorrhoeae causing repeated infections: evolution of porin during passage within a community. J. Infect. Dis. 179371-381. [DOI] [PubMed] [Google Scholar]
- 25.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong, K., T. Kinoshita, and K. Inoue. 1981. Simple methods for preparing EAC1,4b,2a,3b and EAC4b,3b with human or guinea pig complement components using an anticomplementary agent, K-76 monocarboxylic acid. J. Immunol. 127109-114. [PubMed] [Google Scholar]
- 27.Hong, K., T. Kinoshita, H. Kitajima, and K. Inoue. 1981. Inhibitory effect of K-76 monocarboxylic acid, an anticomplementary agent, on the C3b inactivator system. J. Immunol. 127104-108. [PubMed] [Google Scholar]
- 28.Hong, K., T. Kinoshita, W. Miyazaki, T. Izawa, and K. Inoue. 1979. An anticomplementary agent, K-76 monocarboxylic acid: its site and mechanism of inhibition of the complement activation cascade. J. Immunol. 1222418-2423. [PubMed] [Google Scholar]
- 29.Hostetter, M. K., M. L. Thomas, F. S. Rosen, and B. F. Tack. 1982. Binding of C3b proceeds by a transesterification reaction at the thiolester site. Nature 29872-75. [DOI] [PubMed] [Google Scholar]
- 30.Iida, K., K. Mitomo, T. Fujita, and N. Tamura. 1987. Characterization of three monoclonal antibodies against C3 with selective specificities. Immunology 62413-417. [PMC free article] [PubMed] [Google Scholar]
- 31.Ingwer, I., B. H. Petersen, and G. Brooks. 1978. Serum bactericidal action and activation of the classic and alternate complement pathways by Neisseria gonorrhoeae. J. Lab. Clin. Med. 92211-220. [PubMed] [Google Scholar]
- 32.Isenman, D. E., and J. R. Young. 1984. The molecular basis for the difference in immune hemolysis activity of the Chido and Rodgers isotypes of human complement component C4. J. Immunol. 1323019-3027. [PubMed] [Google Scholar]
- 33.Jarva, H., S. Ram, U. Vogel, A. M. Blom, and S. Meri. 2005. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J. Immunol. 1746299-6307. [DOI] [PubMed] [Google Scholar]
- 34.Jarvis, G. A. 1994. Analysis of C3 deposition and degradation on Neisseria meningitidis and Neisseria gonorrhoeae. Infect. Immun. 621755-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerse, A. E., M. S. Cohen, P. M. Drown, L. G. Whicker, S. F. Isbey, H. S. Seifert, and J. G. Cannon. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 179911-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazatchkine, M. D., D. T. Fearon, and K. F. Austen. 1979. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J. Immunol. 12275-81. [PubMed] [Google Scholar]
- 37.Klos, A., V. Ihrig, M. Messner, J. Grabbe, and D. Bitter-Suermann. 1988. Detection of native human complement components C3 and C5 and their primary activation peptides C3a and C5a (anaphylatoxic peptides) by ELISAs with monoclonal antibodies. J. Immunol. Methods 111241-252. [DOI] [PubMed] [Google Scholar]
- 38.Kraiczy, P., and R. Wurzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 4331-44. [DOI] [PubMed] [Google Scholar]
- 39.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makino, S., J. P. van Putten, and T. F. Meyer. 1991. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 101307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maness, M. J., and P. F. Sparling. 1973. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J. Infect. Dis. 128321-330. [DOI] [PubMed] [Google Scholar]
- 42.Mauff, G., B. Luther, P. M. Schneider, C. Rittner, B. Stradmann-Bellinghausen, R. Dawkins, and J. M. Moulds. 1998. Reference typing report for complement component C4. Exp. Clin. Immunogenet. 15249-260. [DOI] [PubMed] [Google Scholar]
- 43.McCutchan, J. A., S. Levine, and A. I. Braude. 1976. Influence of colony type on susceptibility of gonococci to killing by human serum. J. Immunol. 1161652-1655. [PubMed] [Google Scholar]
- 44.McQuillen, D. P., S. Gulati, S. Ram, A. K. Turner, D. B. Jani, T. C. Heeren, and P. A. Rice. 1999. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J. Infect. Dis. 179124-135. [DOI] [PubMed] [Google Scholar]
- 45.Merino, S., S. Camprubi, S. Alberti, V. J. Benedi, and J. M. Tomas. 1992. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect. Immun. 602529-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer, T. F., N. Mlawer, and M. So. 1982. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell 3045-52. [DOI] [PubMed] [Google Scholar]
- 47.Moore, J., S. E. Bailey, Z. Benmechernene, C. Tzitzilonis, N. J. Griffiths, M. Virji, and J. P. Derrick. 2005. Recognition of saccharides by the OpcA, OpaD, and OpaB outer membrane proteins from Neisseria meningitidis. J. Biol. Chem. 28031489-31497. [DOI] [PubMed] [Google Scholar]
- 48.Ram, S., R. Boden, D. C. Stein, A. D. Cox, B. O'Reilly, M. A. Apicella, S. Gulati, A. M. Blom, and P. A. Rice. 2002. C4b-binding protein interactions with Neisseria gonorrhoeae porin is influenced by hexose substitutions on the heptose 1 chain of lipooligosaccharide, p. 1336-1337. In J. E. Talmadge and T. Hugli (ed.), XIXth International Complement Workshop, Palermo, Italy.
- 49.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 27850853-50862. [DOI] [PubMed] [Google Scholar]
- 50.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, R. Boden, B. G. Monks, C. O'Connell, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. C4bp binding to porin mediates stable serum resistance of Neisseria gonorrhoeae. Int. Immunopharmacol. 1423-432. [DOI] [PubMed] [Google Scholar]
- 51.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, B. G. Monks, C. O'Connell, R. Boden, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ram, S., S. Gulati, D. P. McQuillen, R. Boden, C. Elkins, M. K. Pangburn, and P. A. Rice. 1999. Interactions between Neisseria gonorrhoeae and C4b-binding protein: a molecular basis for gonococcal serum resistance. Mol. Immunol. 36297. (Abstract.) [Google Scholar]
- 53.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, U. Vogel, M. Frosch, C. Elkins, H. K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 36915-928. [DOI] [PubMed] [Google Scholar]
- 54.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ram, S., U. Vogel, S. Gulati, G. Heinze, L. Wetzler, H.-K. Guttormsen, M. Frosch, and P. A. Rice. 1999. The interaction between factor H and Neisseria meningitidis. Mol. Immunol. 36297. (Abstract.) [DOI] [PubMed] [Google Scholar]
- 56.Reid, K. B., and R. R. Porter. 1981. The proteolytic activation systems of complement. Annu. Rev. Biochem. 50433-464. [DOI] [PubMed] [Google Scholar]
- 57.Rice, P. A. 2005. Gonococcal arthritis (disseminated gonococcal infection). Infect. Dis. Clin. North Am. 19853-861. [DOI] [PubMed] [Google Scholar]
- 58.Rice, P. A., W. M. McCormack, and D. L. Kasper. 1980. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J. Immunol. 1242105-2109. [PubMed] [Google Scholar]
- 59.Rooijakkers, S. H., and J. A. van Strijp. 2007. Bacterial complement evasion. Mol. Immunol. 4423-32. [DOI] [PubMed] [Google Scholar]
- 60.Ross, S. C., and P. Densen. 1984. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 63243-273. [PubMed] [Google Scholar]
- 61.Rowe, P. C., R. H. McLean, R. A. Wood, R. J. Leggiadro, and J. A. Winkelstein. 1989. Association of homozygous C4B deficiency with bacterial meningitis. J. Infect. Dis. 160448-451. [DOI] [PubMed] [Google Scholar]
- 62.Schifferli, J. A., G. Steiger, J. P. Paccaud, A. G. Sjoholm, and G. Hauptmann. 1986. Difference in the biological properties of the two forms of the fourth component of human complement (C4). Clin. Exp. Immunol. 63473-477. [PMC free article] [PubMed] [Google Scholar]
- 63.Schneider, H., J. M. Griffiss, G. D. Williams, and G. B. Pier. 1982. Immunological basis of serum resistance of Neisseria gonorrhoeae. J. Gen. Microbiol. 12813-22. [DOI] [PubMed] [Google Scholar]
- 64.Seppanen, M., M. L. Lokki, T. Timonen, M. Lappalainen, H. Jarva, A. Jarvinen, S. Sarna, V. Valtonen, and S. Meri. 2001. Complement C4 deficiency and HLA homozygosity in patients with frequent intraoral herpes simplex virus type 1 infections. Clin. Infect. Dis. 331604-1607. [DOI] [PubMed] [Google Scholar]
- 65.Shafer, W. M., A. Datta, V. S. Kolli, M. M. Rahman, J. T. Balthazar, L. E. Martin, W. L. Veal, D. S. Stephens, and R. Carlson. 2002. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J. Endotoxin Res. 847-58. [PubMed] [Google Scholar]
- 66.Shafer, W. M., K. Joiner, L. F. Guymon, M. S. Cohen, and P. F. Sparling. 1984. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J. Infect. Dis. 149175-183. [DOI] [PubMed] [Google Scholar]
- 67.Stephens, D. S., and Z. A. McGee. 1983. Association of virulence of Neisseria meningitidis with transparent colony type and low-molecular-weight outer membrane proteins. J. Infect. Dis. 147282-292. [DOI] [PubMed] [Google Scholar]
- 68.Swanson, J. 1978. Studies on gonococcus infection. XII. Colony color and opacity variants of gonococci. Infect. Immun. 19320-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takata, Y., T. Kinoshita, H. Kozono, J. Takeda, E. Tanaka, K. Hong, and K. Inoue. 1987. Covalent association of C3b with C4b within C5 convertase of the classical complement pathway. J. Exp. Med. 1651494-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsokos, G. C., and G. M. Kammer. 2000. Molecular aberrations in human systemic lupus erythematosus. Mol. Med. Today 6418-424. [DOI] [PubMed] [Google Scholar]
- 72.Virji, M., H. Kayhty, D. J. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 51831-1841. [DOI] [PubMed] [Google Scholar]
- 73.Vogel, U., H. Claus, G. Heinze, and M. Frosch. 1999. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect. Immun. 67954-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vogel, U., A. Weinberger, R. Frank, A. Muller, J. Kohl, J. P. Atkinson, and M. Frosch. 1997. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect. Immun. 654022-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walport, M. J. 2001. Complement. N. Engl. J. Med. 3441058-1066. [DOI] [PubMed] [Google Scholar]
- 76.West, S. E., and V. L. Clark. 1989. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin. Microbiol. Rev. 2(Suppl.)S92-S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wetzler, L. M., E. C. Gotschlich, M. S. Blake, and J. M. Koomey. 1989. The construction and characterization of Neisseria gonorrhoeae lacking protein III in its outer membrane. J. Exp. Med. 1692199-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamasaki, R., D. E. Kerwood, H. Schneider, K. P. Quinn, J. M. Griffiss, and R. E. Mandrell. 1994. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection: evidence for a new glycosylation pathway of gonococcal lipooligosaccharide. J. Biol. Chem. 26930345-30351. [PubMed] [Google Scholar]
- 79.Yang, Z., and C. Y. Yu. 2000. Organizations and gene duplications of the human and mouse MHC complement gene clusters. Exp. Clin. Immunogenet. 171-17. [DOI] [PubMed] [Google Scholar]
- 80.Yu, C. Y. 1998. Molecular genetics of the human MHC complement gene cluster. Exp. Clin. Immunogenet. 15213-230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.