Abstract
Pneumocystis carinii is an opportunistic fungus causing severe pneumonia in immune-compromised hosts. Recent evidence suggests that Pneumocystis exists as separate sex types, though definitive evidence is currently lacking. These studies were undertaken to determine whether Pneumocystis maintains functional meiotic control molecules, which are required for sexual life stages in eukaryotes. Using the Pneumocystis carinii Genome Project database, two partial sequences for meiotic control molecules were detected, namely, PCRan1, a presumptive meiotic control kinase, and PCMei2, a homologue to a primary activator of meiosis in Schizosaccharomyces pombe. Rapid amplification of cDNA ends was employed to obtain the full open reading frames and to further investigate the functions of these proteins. These presumptive meiotic control molecules were most homologous to molecules present in S. pombe (52% identical and 67% homologous for PCRan1 and 75% identical and 88% homologous for PCMei2 by BLAST analysis). Heterologous expression of these Pneumocystis meiotic genes in corresponding temperature-sensitive and knockout strains of S. pombe, respectively, further verified the functions of the PCRan1 and PCMei2 proteins. These proteins were further shown to control downstream components of the meiotic pathway in S. pombe. Lastly, in vitro kinase assays were used to determine that PCRan1p phosphorylates PCMei2p. These experiments represent the first characterization of any proteins in P. carinii involved in meiosis and indicate the presence of a conserved meiotic pathway in Pneumocystis. Elucidation of this pathway will be essential in gaining a greater understanding of this important opportunistic fungal pathogen.
Pneumocystis carinii is an opportunistic fungal pathogen that causes pneumonia in severely immune-compromised hosts. While historically this genus of pathogens has not been viewed as particularly virulent, Pneumocystis infection became increasingly significant with the onset of the AIDS epidemic during the 1980s. In addition, these opportunistic fungi can also cause severe pneumonia in patients with immune suppression related to malignancies, organ transplantation, and inflammatory disorders requiring chronic immune suppression. The mortality rate of Pneumocystis pneumonia can range anywhere from 10 to 50%, representing a significant healthcare issue worldwide (13).
Interestingly, however, Pneumocystis has remained completely refractory to long-term culture in vitro. Although isolated reports describe Pneumocystis carinii growth in complex, defined medium (19), these approaches have been difficult to reproduce consistently, leaving investigators without a defined in vitro method to study these organisms. As a result, little is known about the fungus' life history or the manner in which it replicates and survives over long periods of time, either inside or outside of the host. Thus, the life cycle of Pneumocystis remains elusive but involves progression of smaller trophic forms into thick wall cyst forms, characteristic of the organism (26). Ultrastructural studies further support the presence of precystic forms that are intermediate between trophic forms and cysts (26).
A central question pertaining to the Pneumocystis life cycle is whether the organism utilizes sexual reproduction and therefore maintains the ability to undergo meiosis. There has been indirect evidence suggesting this may be the case, such as the observation of synaptonemal complexes in freshly isolated organisms by using electron microscopy (16). Later reports have established the presence of genes playing a role in the pheromone-sensing pathway of Pneumocystis, also suggesting the presence of mating and meiosis in these organisms (30). However, to date, no direct evidence of meiotic pathway control molecules has been discovered.
Since Pneumocystis remains genetically intractable in culture, the only devisable method to further study the organism, sans electron microscopy, is via molecular identification of putative Pneumocystis life cycle control genes and heterologous expression in phylogenetically related and culturable fungi, such as in Schizosaccharomyces pombe. This method has been well validated by our lab and others (3, 7, 11). After a thorough in silico investigation of various contigs present in the Pneumocystis genome database (4), we were able to locate two sequence fragments of interest with considerable homology to meiotic regulatory elements in S. pombe. These presumptive Pneumocystis meiotic control elements were renamed PCRan1 and PCMei2.
The two conserved genes used in this study are homologous to the Ran1 (Pat1) kinase, identified by two separate groups in 1985 (2, 6), and the Mei2 activator of meiosis (32). Ran1 has since been identified as encoding a protein that acts as a negative regulator of meiosis by phosphorylating and inactivating its target, Mei2. In S. pombe, a knockout of this gene is sufficient to induce meiosis, since without Ran1p to phosphorylate and thereby inactivate Mei2p, the Mei2 proteins can constitutively initiate meiosis. Thus, the Ran1 knockout initiates meiotic catastrophe while S. pombe is under a haploid background (2, 6). Further studies of Mei2 have determined it to be the primary effector necessary for initiation of premeiotic DNA synthesis and meiosis I. If Mei2 is deleted from the genome, S. pombe is rendered completely unable to execute its meiotic pathway (23, 31, 32). Hence, these two genes encode the proteins central for the initiation and modulation of meiosis in S. pombe.
It is therefore of significant interest to determine whether these genes are indeed fully conserved and functional within Pneumocystis. If so, this would represent the first definitive evidence of functional meiotic control genes in the intractable fungus P. carinii. Defining the main meiotic regulators would also provide crucial and long-sought-after data concerning the life cycle of Pneumocystis.
MATERIALS AND METHODS
Materials and strains.
Pneumocystis carinii was originally derived from ATCC culture collections and grown for 8 to 10 weeks in immunosuppressed, corticosteroid-treated rats, as previously reported (5, 12). P. carinii cells enriched with cysts and trophic forms were purified from the infected rat lung via homogenization of the lungs, followed by filtration through a 10-μm filter. To exclude the presence of other infectious organisms in the P. carinii isolates, the preparations were routinely stained (Diff-Quick modified Wright-Giemsa stain; Dade Diagnostics, Aguada, Puerto Rico) and examined to exclude concurrent infection with bacteria or fungi. Isolates with significant contamination of other microorganisms were discarded (28). The isolates were examined for P. carinii nuclei as described for Diff-Quick-stained smears, and P. carinii trophic forms represented greater than 99% of the material on Diff-Quick-stained smears (35). For experiments requiring separation of the cysts and trophic forms, differential filtration through a 3-μm filter was performed as we previously reported (16). Subsequent 3-μm filtration results in 99.5% pure trophic forms and >40-fold-enriched cysts (9).
For complementation studies, appropriate strains of S. pombe were obtained as described in Table 1. Additionally, the vector pNMT41-TOPO (Invitrogen, Carlsbad, CA) was used to express these proteins under control of the inducible nmt promoter. For protein purification, pYES2.1-TOPO (Invitrogen, Carlsbad, CA) was used to induce expression under control of the GAL1 promoter. Plasmids were transformed intro Saccharomyces cerevisiae INVSC- strains (Invitrogen, Carlsbad, CA).
TABLE 1.
Yeast strains used in this studya
| Strain | Relevant genotype |
|---|---|
| FY13293 h90 pNMT41 | mei2::ura4 + ura4 leu1 ade6-M210 |
| FY13293 h90 pNMT41 + PCMei2 | mei2::ura4 + ura4 leu1 ade6-M210 PCMei2 |
| GP5286 h-pNMT41 | ade6-M216 leu1-32 pat1-114ts |
| GP5286 h-pNMT41 + PCRan1 | ade6-M216 leu1-32 pat1-114ts PCRan1 |
| INVSC-MATa/MATα PCRan1 | his3Δ1 leu2 trp1-289 ura3-52 PCRan1 |
| INVSC-MATa/MATα PCMei2 | his3Δ1 leu2 trp1-289 ura3-52 PCMei2 |
Strain FY13293 was donated by Shikiko Murakami, and strain GP5286 was donated by Gerry Smith.
Medium and growth conditions.
For proper selection of transformants and yeast growth, S. pombe dropout base medium (Q-Biogene, Montreal, CA) was used with the appropriate amino acid dropout components. All cultures were grown at 30°C in a shaking incubator for the specified times except for FY13293 plus PCMei2 and FY13293 plus vector, which were grown at 25°C.
Identification of the full ORFs of PCRan1 and PCMei2.
Analysis of the Pneumocystis Genome Project database (http://pgp.cchmc.org/) revealed two partial sequences of potential meiotic control elements, specifically, one 650-bp partial sequence homologous to the meiotic kinase Ran1 and another 400-bp partial sequence homologous to the meiotic activator Mei2. To identify the remaining 5′ and 3′ coding regions of these genes, a rapid amplification of cDNA ends strategy was employed with known antisense primers. The GeneRacer system (Invitrogen, Carlsbad, CA) was used to create a cDNA library with defined 5′ and 3′ ends for amplification using primers to the known target sequence and antisense primers on the known 5′ and 3′ ends coupled with PCR to elucidate the putative coding regions. The coding sequences have been completely sequenced. Furthermore, Southern blotting was used to confirm the presence of PCRan1 and PCMei2 coding regions within Pneumocystis genomic DNA. This was necessary to exclude the possibility of having inadvertently amplified rat nucleic acid, which would contaminate the Pneumocystis preparations to a minor degree. For both PCRan1 and PCMei2, Southern blotting was performed using 300-bp probes hybridized to EcoRI, HindIII, XbaI, or XhoI-digested P. carinii DNA using published methods (8, 11). In addition, to further verify that the identified genetic sequences were of Pneumocystis origin, the PCRan1 and PCMei2 were also hybridized to contour-clamped homogenous field electrophoresis (CHEF)-separated Pneumocystis chromosomes. Again, the relevant 300-bp probes were hybridized using the same Southern blotting protocol previously described.
Complementation of meiotic defects associated with knockout of either Ran1 or Mei2.
To fully evaluate whether PCRan1 or PCMei2 generated functional proteins, we assessed the ability of these identified molecules to complement the effects of a knockout of the corresponding homologues in S. pombe. Specifically the S. pombe Ran1 knockout strain GP5286 h- was transformed with either pNMT41-Topo vector alone or pNMT41-Topo plus PCRan1. Similarly, S. pombe strain FY13293 h90 was transformed with either vector alone or pNMT41 plus PCMei2. For GP5286 h- containing the vector or PCRan1, the strains were grown to log phase overnight before transferring them to essential minimal medium lacking nitrogen, in order to drive the cells into meiosis. Cells were then fixed at 24 h in 4% paraformaldehyde. Fixed cells were then placed on a slide coated with poly-l-lysine and allowed to dry. 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR) was added, and the cells were investigated microscopically at ×100 magnification. The same procedure was utilized for FY13293 h90 cells containing the vector alone or PCMei2; however, SPAS mating medium was used instead of essential minimal medium to introduce proper mating conditions for the h90 cells.
In vitro kinase assay with PCRan1 and PCMei2.
Since the native function of Ran1p is to phosphorylate Mei2p, we next determined whether PCRan1p and PCMei2p proteins interacted similarly. The two gene sequences were cloned into pYES2.1+TOPO containing the GAL1 promoter and a C-terminal V5/His tag. The resulting clones containing the V5/His tag were transformed into an S. cerevisiae INVSC- strain. Cultures were grown in the appropriate dropout medium containing 2% glucose and then switched to 1.5 liters of medium containing 2% galactose in order to induce protein expression. Total protein was isolated as described above. The proteins were purified using the Y-PER His6 purification column (Pierce, Rockford, IL). Fractions were Western blotted with either specific horseradish peroxidase (HRP)-conjugated antibody or V5-HRP-conjugated antibody as previously described to visualize the level of protein expression. Fractions containing PCRan1p or PCMei2p were pooled for the kinase assays. Kinase assays were performed by adding 1 μg PCRAN1p enzyme and 2 μg PCMei2p substrate. PCMei2p substrate was placed at 72°C for 30 min before use to heat inactivate the protein. Enzyme and substrate were mixed with [γ-32P]ATP (MP Biomedicals, Solon, OH) and kinase buffer (150 mM Tris-HCl, 20 mM MgCl2, 10 mM MnCl2, 15 mM β-mercaptoethanol) and incubated at 30°C for 1 h. Reactions were then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were dried for 1.5 h at 80°C and visualized by autoradiography.
Determination of downstream meiotic element regulation with quantitative RT-PCR (qRT-PCR).
To next determine whether the heterologously expressed PCMei2 activated downstream meiotic control elements in S. pombe, yeast strain FY13293 h90 with PCMei2 or vector was treated for 18 h in 10 mM hydroxyurea to synchronize the cells and subsequently grown in SPAS mating medium for 4, 8, and 24 h. Total RNA was extracted, and reverse transcription (RT) was performed using the Superscript III First Strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). Using this approach, we quantified expression of the downstream meiotic control molecule Rem1. Actin was used for relative comparison and normalization of amplification efficiency. To perform these studies, cDNA (100 ng) was added to each well and amplified for 1 cycle at 50°C for 2 min and 45 cycles at 95°C for 2 min, 57°C for 30 seconds, and 72°C for 30 seconds with each of the three following primer sets: actin-1F, TGGAAGAAGAAATCGCAGCG; actin-1R, CAAGGTCAAAATACCACGCTT; PCMei2-F1, CGTGTTGGGACAAAATGG; PCMei2-R2, CCTTCTCGGATTATTAGGTGC; Rem1-1F, ATGAACTCTAACAACAAAAGAGT; and Rem1-1R, CTATCATAGTCCTTTTGTATTTG.
Northern and Western analysis of PCRan1 and PCMei2 expression in Pneumocystis.
In order to further characterize the overall expression patterns of both PCRan1 and PCMei2 mRNA and related proteins, Northern and Western blot analyses were performed. P. carinii cysts and trophic forms were separated by differential filtration as previously described (16). For Northern blotting, RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), and equal cyst and trophic RNA (3 μg) aliquots were electrophoresed through a 1% agarose gel in the presence of 2.2 M formaldehyde and transferred to nitrocellulose. The resulting membrane was then hybridized with 300-bp probes recognizing PCRan1 and PCMei2 radiolabeled with [α-32P]dATP (Amersham, Inc., Piscataway, NJ). Posthybridization, the blots were washed and visualized via autoradiography (9). For the Western blot analysis, protein was isolated using Y-PER extraction buffer (Pierce, Rockford, IL) and quantified with the Coomassie protein reagent protocol (Pierce, Rockford, IL). Equal quantities of cyst and trophic form protein extracts (25 μg) were loaded and separated by 12% SDS-PAGE at 200 V for 1 h. The proteins were then electrophoretically transferred to nitrocellulose membranes at 100 V for 1 h. The transfer membrane was then blotted with specific rabbit polyclonal antibodies for PCRan1p and PCMei2p generated against peptides derived from the predicted sequences (PCRan1p, TPPSFSCKESPLTPSTSKTK; PCMei2p, IMDRSNSVPCYFLQNEKPPY; Bethyl Laboratories, Montgomery, TX). Preimmune serum served as the control. The membranes were incubated with the antibodies for 2 hours, followed by washing and addition of HRP-conjugated goat-anti-rabbit antibodies for 1 hour. Followed washing, bound antibodies were detected with enhanced chemiluminescence substrate (Amersham, Inc., Piscataway, NJ).
Immunoelectron microscopy.
In order to further determine the ultrastructural localization the Ran1p and Mei2p proteins during the various stages of the Pneumocystis life cycle, immunoelectron microscopy was utilized. To prepare these samples, P. carinii cysts and trophic forms were separated by differential staining and fixed in buffer containing 4% formaldehyde and 2% glutaraldehyde in phosphate-buffered saline. Electron microscopy was performed at the Mayo EM core facility (Rochester, MN) using methods we have previously reported (21).
Nucleotide sequence accession numbers.
Nucleotide sequences for PCRan1 and PCMei2 were deposited in the NCBI genome database under accession no. EF612708 and EF612707, respectively.
RESULTS
Pneumocystis carinii contains coding sequences for the putative meiotic control molecules PCRan1 and PCMei2.
Due to our inability to culture these organisms, investigations of the life cycle of Pneumocystis have traditionally been limited to static images obtained via electron microscopy. However, more recent molecular approaches and the availability of the partial sequences through the Pneumocystis Genome Project have offered investigators a new avenue with which to study the fungus. In a search for potential meiotic homologues within the P. carinii genome, two interesting partial sequences were discovered. The first partial sequence, PCRan1, was homologous to a kinase that acts as the central inhibitor of meiosis in the fission yeast S. pombe (17). Of interest, analysis of the available sequences revealed the presence of a second partial sequence present in the Genome Project database, PCMei2. This second sequence represents the described molecular target of the kinase and is the sole activator of the meiotic pathway in fission yeast.
According to prior reports, the S. pombe counterparts act by Ran1 phosphorylating Mei2 to inhibit binding of Mei2 to meiotic RNA (meiRNA). Hence, these fragments represented homologues to the central lock and key mechanism to the activation of meiosis in fission yeast. Using rapid amplification of cDNA ends technology, we next elucidated the complete 5′ and 3′ coding regions of each of the sequence fragments. The complete mRNA sequence of PCRan1 was found to encode a predicted protein of 45 kDa, while the complete mRNA sequence of PCMei2 was found to encode a predicted protein of 85 kDa.
In order to confirm that these sequences isolated from the Pneumocystis Genome Project database were, in fact, of P. carinii origin, we next performed Southern blotting (Fig. 1A and B). These hybridizations suggested that both the PCRan1 and PCMei2 sequences were each represented within a single region of the Pneumocystis genomic DNA. In addition, we performed hybridization of these sequences to CHEF blots (Fig. 1C and D), which indicated that both gene sequences were present on a respective single Pneumocystis chromosome. These hybridizations further confirmed the Pneumocystis origin of these genetic sequences, since low levels of rat host cell contaminants might have been amplified through this approach.
FIG. 1.
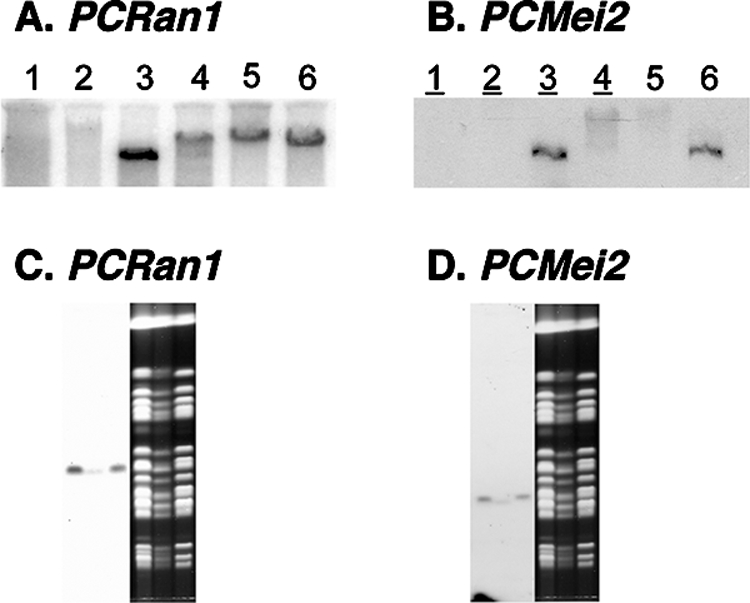
Representation of PCRan1 and PCMei2 within Pneumocystis DNA. (A and B) Southern blotting of PCRan1 (A) and PCMei2 (B) using, in lanes 1 to 6, respectively, rat DNA, uncut P. carinii genomic DNA, and EcoRI, HindIII, XhoI, and XbaI restriction enzyme-digested P. carinii genomic DNA. (C and D) Hybridization of PCRan1 (C) and PCMei2 (D) to P. carinii chromosomes separated by CHEF. Each lane contains P. carinii chromosomes derived from a single rat lung sample.
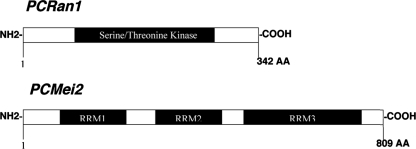
Sequence analysis of these presumptive meiotic control molecules revealed them to be most homologous to those molecules present in S. pombe, with 52% identity and 67% homology for PCRan1 and 75% identity and 88% homology for PCMei2 by BLAST analysis. Additionally, we determined the active domains in each of the proteins. For PCRan1 this included a serine/threonine kinase domain. In PCMei2, analysis revealed three separate RNA recognition motifs (Fig. 2). In fission yeast, the RNA recognition motifs are known to bind meiRNA to activate downstream meiosis (34). By isolating and identifying the full coding region of each of these P. carinii genes, we were able to further investigate the putative functions of the proteins and their effect within the P. carinii life cycle.
FIG. 2.
Schematic drawing of P. carinii PCRan1 and PCMei2 proteins. Schematic drawings of the novel proteins PCRan1p and PCMei2p show conserved active domains of interest and a conserved serine/threonine kinase domain (PCRan1p).
PCRan1 and PCMei2 exhibit functional activity in meiotic control during heterologous expression in S. pombe.
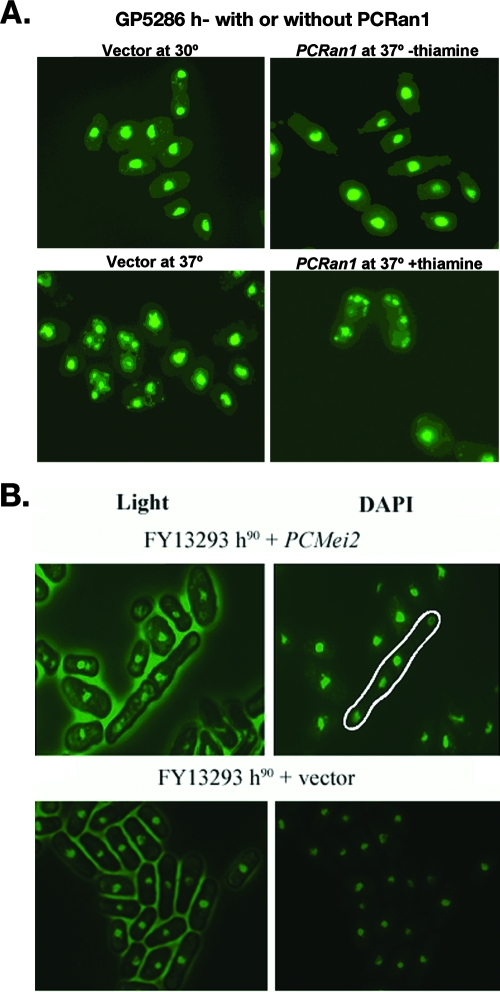
Since PCRan1 and PCMei2 genes were found to be homologous to two fission yeast meiotic regulatory proteins, we next sought to determine whether the Pneumocystis proteins encoded by these sequences would be able to exert their function during heterologous expression in the phylogenetically related fungus S. pombe. To achieve this, we cloned these full-length open reading frames into S. pombe expression plasmids and transformed the vectors into two independent knockout strains (Table 1). The GP5286 h− strain contains a temperature-sensitive knockout for S. pombe Ran1. In the h− (haploid) background, this process causes constitutive meiosis, and the fission yeast undergo meiotic catastrophe (31). Through the use of DAPI staining, we were thus able to determine that complementation of the temperature-sensitive mutant with PCRan1p was sufficient to inhibit the meiotic catastrophe and restore the wild-type phenotype with nuclei that were healthy in appearance (Fig. 3A). This strongly indicated that PCRan1p was sufficient to inhibit the initiation of meiotic events in this fungal strain.
FIG. 3.
PCRan1 and PCMei2 complement meiotic defects in mutant fission yeast strains. A. S. pombe strain GP5286 h− containing a meiotic defect related to Ran1 was transformed with either vector containing PCRan1 or vector alone and incubated at either the permissive 30°C or restrictive 37°C followed by DAPI staining to demonstrate either inhibition or progression of haploid meiosis. Expression of the vector insert was under control of the nmt (not made in thiamine) promoter. B. S. pombe strain FY13293 h90 containing a meiotic defect related to Mei2 was transformed with PCMei2 or vector alone, incubated at 25°C, and stained with DAPI to observe the occurrence of premeiotic DNA synthesis, demonstrated as organisms containing four nuclear forms (encircled in white). In contrast, S. pombe strain FY13293 h90 with vector alone did not exhibit any complementation.
Similarly, we next used the FY13293 h90 Mei2 knockout strain of fission yeast to determine whether PCMei2 could also restore wild-type function. A knockout strain of Mei2 is rendered completely unable to undergo meiosis, regardless of the conditions (32). In this manner, we verified that PCMei2 can functionally complement the mutant, with restoration of meiosis. This was determined by looking for cells that had undergone premeiotic DNA synthesis and contained four nuclei (Fig. 3B). Again, this strongly supported our contention that PCMei2p functioned similarly to its homologous counterpart in S. pombe, lending further evidence to our premise that a sexual meiotic life cycle is conserved in P. carinii.
PCRan1 encodes a protein with kinase activity capable of phosphorylating PCMei2p.
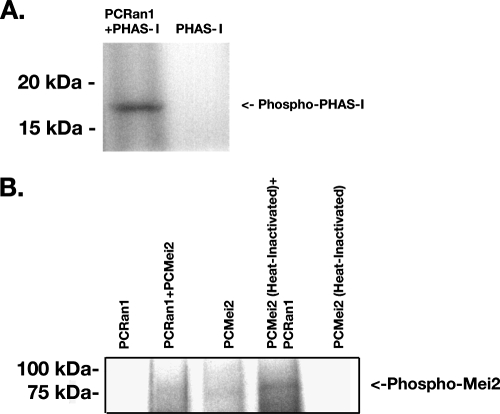
Since these complementation assays demonstrated that both PCRan1 and PCMei2 could exert their intended activity and complement deficiencies of the corresponding knockout strains, we next evaluated whether PCRan1p exhibited protein kinase activity in vitro. In addition, we further sought to verify whether PCMei2p could serve as a potential target of that kinase. To determine this, V5/His-tagged PCRan1p and V5/His-tagged PCMei2p were expressed in yeast strain INVSC− with the relevant genes inserted into the pYES2.1 expression vector. These proteins were purified over a nickel column and immunoblotted to ensure the proteins were expressed at the expected molecular weights (Fig. 4A and B). The purified PCRan1p was then analyzed with in vitro kinase assays, first with the broad mitogen-activated protein kinase target protein PHAS-I, which resulted in strong phosphorylation of the target protein (Fig. 5A). This verified that PCRan1p was indeed an active protein kinase.
FIG. 4.
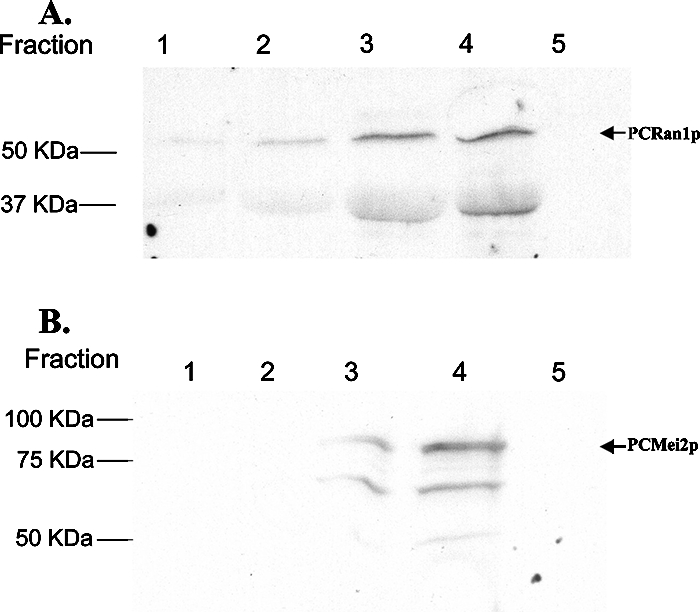
Expression and purification of PCRan1p and PCMei2p. A. S. cerevisiae strain INVSC− with PCRan1 was cultured in 1.5 liters of dropout base medium lacking uracil and containing 2% galactose to induce expression of the transgene for 24 h. The protein was purified by His-affinity using a nickel-charged column and collected into five separate fractions. These fractions were separated on a 12% SDS-PAGE and Western blotted using the specific PCRan1p antibody. B. Similarly, S. cerevisiae strain INVSC− with PCMei2 was cultured, and expression of the recombinant protein was induced as described above. Culture supernatants were purified as described above and collected into five separate fractions. These fractions were separated on a 12% SDS-PAGE and Western blotted using an anti-V5 antibody.
FIG. 5.
PCRan1p exhibits protein kinase activity and is capable of phosphorylating PCMei2p. A. In vitro phosphorylation assay performed using the broad-spectrum serine-threonine kinase target PHAS-I, incubated either alone or in conjunction with purified PCRan1p at 30°C for 1 h in the presence of [γ-32P]ATP. The products were separated on a 12% SDS-PAGE and visualized by autoradiography. B. In vitro phosphorylation assay performed as described above using either recombinantly expressed PCMei2p and heat-inactivated recombinantly expressed PCMei2p incubated either alone or in the presence of PCRan1p. The products were separated on a 7.5% SDS-PAGE and visualized as described above. PCRan1p was also incubated alone as a control for autophosphorylation.
The next series of experiments focused on whether PCRan1 could directly phosphorylate its putative target protein PCMei2p. Initial experiments demonstrated that PCMei2p exhibited autophosphorylation activity, a surprising phenomenon. However, this activity has also been noted in relation to the early meiotic gene Rim15 (21). Rim15p is also a serine/threonine kinase responsible for the upregulation of several early meiotic genes (29). Therefore, in order to determine whether PCRan1p could phosphorylate PCMei2p, PCMei2p was heat inactivated, as described above, prior to its use as a substrate in the in vitro kinase assay. The resulting heat-inactivated PCMei2p was successfully phosphorylated by PCRan1p kinase (Fig. 5B). In addition, these results were independently confirmed by generating PCRan1p using the in vitro Rapid Translation system (Roche, Indianapolis, IN) and applying it to the kinase assay (data not shown). These findings strongly suggest that PCRan1p can indeed phosphorylate PCMei2p and may therefore represent the proximate substrate for the kinase.
Expression of PCMei2 results in an enhanced expression of a downstream meiotic marker.
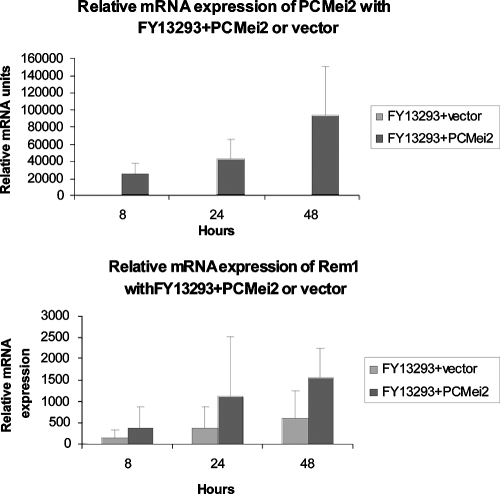
Since we demonstrated phosphorylation of PCMei2p by PCRan1p and since our complementation studies demonstrated that PCMei2p activity was necessary for meiosis to occur, we next wanted to definitively determine the downstream effect of PCMei2 on activating the meiotic control system. To accomplish this, qRT-PCR was utilized by amplifying PCMei2 as well as Rem1, a downstream meiotic regulatory gene, in FY13293 h90 with PCMei2 S. pombe. Rem1 is a meiotic fission yeast cyclin that is present only during meiosis and is essential for premeiotic DNA synthesis and meiotic recombination (15). These studies demonstrated that Rem1 in SPAS mating medium had a basal level of expression over the course of 24 h in FY13293 h90 with vector. However, in FY13293 h90 with PCMei2, when PCMei2 mRNA was overexpressed, Rem1 levels demonstrated a direct correlation and the expression of this downstream cyclin was increased robustly (Fig. 6). The enhanced expression of this downstream meiotic cyclin is shown here as a direct result of the overexpression of PCMei2, demonstrating that PCMei2 does act positively to activate the meiotic pathway in a heterologous expression system.
FIG. 6.
Overexpression of PCMei2 mRNA results in up-regulation of the downstream meiotic cyclin Rem1. S. pombe strain FY13293 h90 containing PCMei2 or FY13293 h90 with vector alone was cultured in SPAS medium for the indicated times, after synchronization with hydroxyurea. Total RNA was extracted from the cultures, and the expression levels of PCMei2 and Rem1 were assayed by qRT-PCR. Overexpression of PCMei2 correlated directly with enhanced expression of the downstream Rem1 meiotic cyclin.
Determination of the expression profile of PCRan1 and PCMei2 in Pneumocystis.
Once we determined that PCRan1p phosphorylated PCMei2p and that PCMei2p could activate the downstream meiotic pathway heterologously, we sought to next determine the expression profile of these proteins in Pneumocystis in order to better understand how the expression of these proteins may modulate the progression of the Pneumocystis life cycle, namely, during transitions from the trophic form to the cystic form. Northern blot assays were performed on total RNA with probes for both transcripts, using separated populations of these various life cycle forms. We observed that PCRan1 mRNA was highly expressed in the cyst form, while it demonstrated low overall expression in the trophic form (Fig. 7A). A similar profile for PCRan1p protein was further noticed in the Western blot assay at roughly 45 kDa (Fig. 7C). Since the presumed central function of PCRan1 is to prevent continuation of a meiotic pathway, these observations may suggest that meiosis is prevented in the intracystic bodies of Pneumocystis.
FIG. 7.
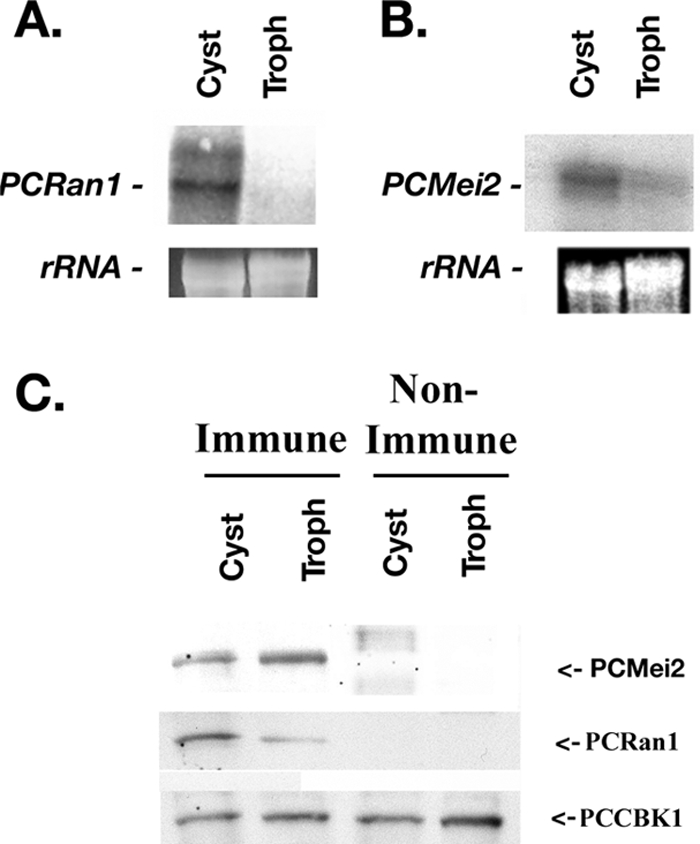
Differential expression of PCRan1 and PCMei2 in various life cycle forms of P. carinii. A. Northern blot analysis for PCRan1 revealed differential mRNA expression in separated populations of P. carinii cystic and trophic forms. Pneumocystis rRNA is shown as a loading control. B. Northern blot analysis of PCMei2 also demonstrated differential mRNA expression in separated populations of P. carinii cystic and trophic forms. C. Western blot analysis of separated P. carinii cyst and trophic forms using specific PCRan1p antibodies, specific PCMei2p antibodies, antibodies to the control protein PCCBK1, or nonimmune serum were next performed. This analysis further confirmed differential expression of the PCRan1p protein kinase in the separate life cycle forms of the organism, with greater PCRan1p being present in cystic forms. However, Western blotting for PCMei2p demonstrated expression in both the cystic and trophic life forms. PCCBK1p confirmed equal loading throughout. (Note that the PCCBK1 nitrocellulose was always assayed with specific PCCBK1 antibody, not under nonimmune conditions, to confirm equal loading of protein under every condition tested.)
Interestingly, a coordinate expression profile was not observed for PCMei2. In Northern blot analysis, PCMei2 mRNA was more highly expressed in cysts than in trophic forms (Fig. 7B). However, Western blotting demonstrated that PCMei2p protein expression was roughly equivalent when comparing these two major life cycle forms of the organism (Fig. 7C). For both the PCRan1 and PCMei2 Western blot assays, a loading control, PCCBK1, was included in both the immune and nonimmune lanes, respectively, to demonstrate equal loading. PCCBK1 is a Pneumocystis-specific protein that has been demonstrated in our findings to be equally expressed in both the cyst and trophic forms of P. carinii (9). These observations further suggested that while PCMei2p is the likely target of PCRan1p phosphorylation, the overall regulation of this control system appears to be synchronized by the relative up- and down-regulation of the PCRan1p protein kinase.
Localization of PCRan1p and PCMei2p in life cycle forms of Pneumocystis.
In order to further understand how these two proteins might interact over the course of the Pneumocystis life cycle, we finally investigated the localization of PCRan1p and PCMei2p using our respective primary antibodies, followed by gold-labeled secondary antibodies and immunoelectron microscopy. In both the trophic and precystic forms, we were able to determine that the overall localization of PCRan1p was in the cytoplasm of P. carinii. However, within the mature cystic forms, the PCRan1p protein was selectively localized to the intracystic bodies (Fig. 8).
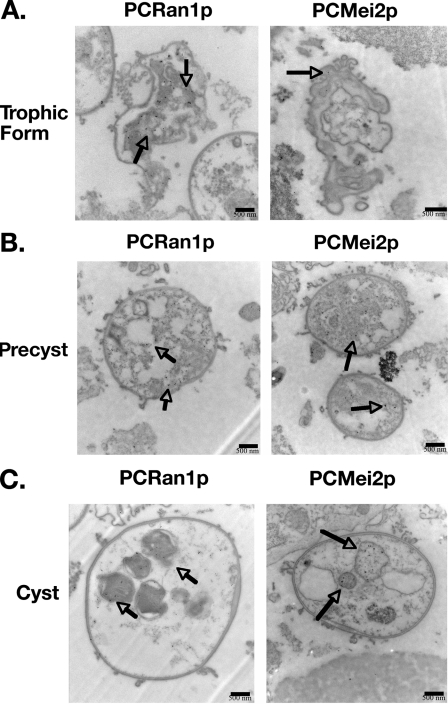
FIG. 8.
Immunoelectron microscopy using specific antibodies to PCRan1p and PCMei2p antibodies shows localization of these meiotic control proteins over the life cycle of the organism. PCRan1p antibodies demonstrate localization of the protein as the life cycle progresses from trophic (A) to precyst (B) to mature cysts (C) within the intracystic bodies. PCRan1p is localized in trophic form and precyst cytoplasm but seems to selectively localize to the intracystic bodies in the mature cyst. In comparison, gold-labeled PCMei2p antibodies demonstrate protein localization in the trophic, precyst, and maturing cyst. Again, PCMei2p appears to localize predominantly to the intracystic bodies of maturing cysts.
Similarly, PCMei2p was also localized to the nuclei in the precystic bodies (Fig. 8). This localization of PCMei2p to the nuclei was interesting, because it is known that in S. pombe Mei2p binds with meiRNA and then must localize to the nuclei in order for meiosis to occur (20, 22). These data further suggest that meiosis in Pneumocystis may in fact be occurring at the end of this precystic stage. In addition, these data for PCRan1p and PCMei2p ultrastructural localization further support that these molecules are present at key sites necessary for a molecular pathway regulating meiotic division in Pneumocystis.
DISCUSSION
Pneumocystis pneumonia remains a significant cause of mortality and morbidity in immunocompromised patients. Even with effective treatment, the mortality of an individual episode of Pneumocystis pneumonia continues to range between an unacceptable 15 to 40% (27). Furthermore, patients who are successfully treated remain under threat of relapse, necessitating long-term chemoprophylaxis in many. Concerns have arisen due to the emergence of resistance mutations in the major drug targets used to treat Pneumocystis, including dihydropteroate synthase, a target of trimethoprim-sulfamethoxazole therapy (27). Such concerns necessitate continued investigations to discover new and innovative means to prevent and treat this infection. Development of new drugs to treat this challenging infection has been limited by our inability to culture Pneumocystis and our poor understanding of the organism's life cycle. Our discovery of two functional proteins that serve a direct role in the inhibition and activation of meiosis sheds new light on this significant problem and indicates that P. carinii utilizes a meiotic and sexual phase during its life cycle. Although meiosis had been previously inferred after the observation of what appeared to be synaptonemal complexes in Pneumocystis through the use of electron microscopy (16), our current observations represent the first direct evidence that meiosis not only is likely present in this intractable fungus but also is highly regulated.
PCRan1 and PCMei2 are of considerable interest because of their significant homology to the key control proteins regulating meiosis in S. pombe. Ran1 and Mei2 encode proteins that are active in controlling the initiation of the meiotic pathway in fission yeast (31). Our finding that these functional homologues are expressed by Pneumocystis strongly suggests a parallel regulatory system activating meiosis in Pneumocystis. During our investigation of potential conserved domains in both PCRan1p and PCMei2p, we found key domains with extensive homology to those described in S. pombe. This suggested similar functions, with PCRan1p acting as a kinase to phosphorylate and suppress the activity of PCMei2p. Our data further provide evidence of such kinase activity of PCRan1p phosphorylating PCMei2p, demonstrating directly this specific phosphorylation event. Additionally, PCMei2p also demonstrated three putative RNA recognition motifs. This further suggests that, like in S. pombe, PCMei2p binds meiRNA in order to activate meiosis. Although a search for Pneumocystis meiRNA in P. carinii is ongoing, its existence is strongly supported by the structure and functional activity of PCMei2p.
Our study provides new evidence that more closely aligns Pneumocystis with S. pombe. Although the exact phylogenetic relationships of P. carinii are not fully known, it is generally classified as am Ascomycetous fungus with closest homology to S. pombe and Saccharomyces cerevisiae (26). Findings from the partially complete Pneumocystis Genome Project indicate that this organism exhibits closest DNA sequence homology to S. pombe (24). Unlike S. pombe, S. cerevisiae does not regulate meiosis through a Ran1-like protein kinase acting to block initiation of this life cycle event. Establishment of a PCRan1p and PCMei2p activation system provides additional support that S. pombe may represent the closest-related organism to Pneumocystis.
Since direct genetic manipulations of Pneumocystis are currently impossible, we have developed heterologous expression as a reliable method to study function of proteins originally isolated from Pneumocystis (9). Our prior studies showed S. pombe to be a particularly useful system to study cell and life cycle control proteins (12, 25). Complementation analyses with PCRan1 and PCMei2 verified that PCRan1p and PCMei2p were functional in a similar meiotic pathway. In addition, to further demonstrate that the interaction of PCRan1p and PCMei2p is part of a conserved pathway regulating the activation of meiosis, we demonstrated that up-regulation of PCMei2p, in combination with the introduction of meiotic conditions, was sufficient to induce upregulation of the downstream meiotic kinase Rem1 in the S. pombe heterologous system. We further utilized in vitro kinase assays to demonstrate that PCRan1p can directly phosphorylate PCMei2p. These investigations establish that PCRan1p and PCMei2p are functional and exhibit specific kinase enzyme-substrate interactions and that their interaction can directly regulate downstream meiotic pathway elements. Taken together, these data strongly indicate a highly regulated inhibition of meiosis by PCRan1 in Pneumocystis until conditions are favorable under which such a meiotic pathway should be activated.
We further sought to investigate how these proteins are expressed over the life cycle of Pneumocystis. Accordingly, we next studied the expression and localization of PCRan1p and PCMei2p using immunoelectron microscopy. Western blotting had revealed that PCRan1p was highly expressed during the cyst stage with low overall expression in the trophic form. In contrast, PCMei2p appeared to be equally expressed in both life cycle stages. In addition to confirming those earlier findings, electron microscopy revealed a fascinating pattern of subcellular localization. Although PCRan1p is expressed in the cytoplasm throughout all life cycle stages, it does seem to exhibit greater local accumulation in the intracystic bodies present within maturing cysts. In comparison, PCMei2p exhibited mainly cytoplasmic localization in the trophic forms and early precyst forms. However, when distinct nuclei were present in the organism forms, PCMei2p seemed to localize almost entirely within the nucleus. These observations of nuclear localization (PCMei2p) and strong localization to the forming intracystic bodies (PCRan1p) further support a role for these proteins in meiotic and life cycle regulation.
Earlier work indicated that Pneumocystis trophic forms are haploid and that this form avidly attaches to alveolar epithelial cells, promoting proliferation of the organism (1, 8, 14, 33). Prior studies from our lab further indicated that attachment of Pneumocystis trophic forms to lung epithelium leads to enhanced expression of PCSte20, which promotes activation of mating (conjugation) pathways in the organism (8, 10). We have further postulated that after conjugation, the organisms detach from the alveolar cells and enter the precyst stage, where they undergo two rounds of DNA synthesis and begin meiosis, forming eight separate nuclei which later mature into eight intracystic bodies. These bodies eventually exit the cyst again as haploid trophic forms. The ultrastructural localization observations and functional activity of PCRan1 and PCMei2 are consistent with such a proposed model life cycle progression.
Based on our findings, we can propose the following meiotic regulatory model. PCRan1 is highly expressed within cystic forms and is predominantly localized to the maturing intracystic bodies. We propose that in each such intracystic body, PCRan1p must be present to phosphorylate PCMei2p and prevent the activation of additional rounds of meiosis. When conditions are favorable, the mature cyst forms rupture, releasing the eight intracystic bodies as new trophic forms. PCRan1 expression is generally lower in these trophic forms. However, as conditions become more unfavorable, P. carinii mates, and the precyst and cysts begin to form. PCMei2 remains at static levels of transcription and translation throughout the life cycle. However, once PCRan1 levels are lowered and the associated kinase activity is reduced, PCMei2 can then bind to meiRNA and activate meiosis following translocation to the nucleus. This process then stimulates the formation of eight new intracystic bodies. In S. pombe, Mei3 acts as a pseudo-substrate which blocks phosphorylation of Mei2, providing an additional mechanism of regulation (18). At present, a Mei3 homologue has not yet been discovered in Pneumocystis.
We believe the current investigation provides important new information on life cycle regulation in Pneumocystis. In this study, we have identified and characterized two novel interacting proteins and their specific functions. We have been able to ascertain their function in the life cycle and their expression patterns and localization in vivo. We have, for the first time, shown direct evidence for the meiotic progression of the Pneumocystis life cycle and the manner in which expression of these proteins drives that life cycle. Moreover, our study provides important new tools to begin to unravel control of the complex meiotic process present in this enigmatic organism.
Acknowledgments
We thank Gerry Smith, Fred Hutchinson Cancer Research Center, and Shikiko Murakami, Osaka, Japan, for the yeast strains used in this study. Also, we also thank Joseph Standing for his assistance with the animal studies. We further appreciate Margaret Springett's efforts in obtaining the electron microscopy images.
This study was supported by National Institutes of Health grant RO1-HL55934 to A.H.L.
Editor: A. Casadevall
Footnotes
Published ahead of print on 5 November 2007.
REFERENCES
- 1.Anders, R. A., M. Gustafson, M. Edens, A. H. Limper, and E. B. Leof. 1996. Pneumocystis carinii modulates cyclin-dependant kinase activity in a lung epithelial cell line. J. Eukaryot. Microbiol. 4313S. [DOI] [PubMed] [Google Scholar]
- 2.Beach, D., L. Rodgers, and J. Gould. 1985. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr. Genet. 10297-311. [DOI] [PubMed] [Google Scholar]
- 3.Broomall, K., M. Collins, and A. G. Smulian. 1997. Pneumocystis carinii promoter analysis in a heterologous Saccharomyces cerevisiae assay system. J. Eukaryot. Microbiol. 4410S-11S. [DOI] [PubMed] [Google Scholar]
- 4.Cushion, M. T., and J. Arnold. 1997. Proposal for a Pneumocystis genome project. J. Eukaryot. Microbiol. 447S. [DOI] [PubMed] [Google Scholar]
- 5.Hahn, P. Y., S. E. Evans, T. J. Kottom, J. E. Standing, R. E. Pagano, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 2782043-2050. [DOI] [PubMed] [Google Scholar]
- 6.Iino, Y., and M. Yamamoto. 1985. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 822447-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneshiro, E. S., J. A. Rosenfeld, M. Basselin, S. Bradshaw, J. R. Stringer, A. G. Smulian, and J. L. Giner. 2001. Pneumocystis carinii erg6 gene: sequencing and expression of recombinant SAM:sterol methyltransferase in heterologous systems. J. Eukaryot. Microbiol. Suppl. 2001144S-146S. [DOI] [PubMed] [Google Scholar]
- 8.Kottom, T. J., J. R. Kohler, C. F. Thomas, Jr., G. R. Fink, and A. H. Limper. 2003. Lung epithelial cells and extracellular matrix components induce expression of Pneumocystis carinii STE20, a gene complementing the mating and pseudohyphal growth defects of STE20 mutant yeast. Infect. Immun. 716463-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kottom, T. J., and A. H. Limper. 2004. Pneumocystis carinii cell wall biosynthesis kinase gene CBK1 is an environmentally responsive gene that complements cell wall defects of cbk-deficient yeast. Infect. Immun. 724628-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kottom, T. J., and A. H. Limper. 2002. Subtractive hybridization analysis of Pneumocystis carinii gene activation induced by interaction with lung epithelial cells and matrix. Chest 12178S-79S. [DOI] [PubMed] [Google Scholar]
- 11.Kottom, T. J., C. F. Thomas, Jr., and A. H. Limper. 2001. Characterization of Pneumocystis carinii PHR1, a pH-regulated gene important for cell wall Integrity. J. Bacteriol. 1836740-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kottom, T. J., C. F. Thomas, Jr., K. K. Mubarak, E. B. Leof, and A. H. Limper. 2000. Pneumocystis carinii uses a functional cdc13 B-type cyclin complex during its life cycle. Am. J. Respir. Cell Mol. Biol. 22722-731. [DOI] [PubMed] [Google Scholar]
- 13.Limper, A. H., K. P. Offord, T. F. Smith, and W. J. Martin II. 1989. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 1401204-1209. [DOI] [PubMed] [Google Scholar]
- 14.Limper, A. H., C. F. Thomas, Jr., R. A. Anders, and E. B. Leof. 1997. Interactions of parasite and host epithelial cell cycle regulation during Pneumocystis carinii pneumonia. J. Lab. Clin. Med. 130132-138. [DOI] [PubMed] [Google Scholar]
- 15.Malapeira, J., A. Moldon, E. Hidalgo, G. R. Smith, P. Nurse, and J. Ayte. 2005. A meiosis-specific cyclin regulated by splicing is required for proper progression through meiosis. Mol. Cell. Biol. 256330-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto, Y., and Y. Yoshida. 1984. Sporogony in Pneumocystis carinii: synaptonemal complexes and meiotic nuclear divisions observed in precysts. J. Protozool. 31420-428. [DOI] [PubMed] [Google Scholar]
- 17.McLeod, M., and D. Beach. 1988. A specific inhibitor of the ran1+ protein kinase regulates entry into meiosis in Schizosaccharomyces pombe. Nature 332509-514. [DOI] [PubMed] [Google Scholar]
- 18.McLeod, M., M. Stein, and D. Beach. 1987. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 6729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merali, S., U. Frevert, J. H. Williams, K. Chin, R. Bryan, and A. B. Clarkson, Jr. 1999. Continuous axenic cultivation of Pneumocystis carinii. Proc. Natl. Acad. Sci. USA 962402-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohno, M., and I. W. Mattaj. 1999. Meiosis: meiRNA hits the spot. Curr. Biol. 9R66-R69. [DOI] [PubMed] [Google Scholar]
- 21.O'Riordan, D. M., J. E. Standing, K. Y. Kwon, D. Chang, E. C. Crouch, and A. H. Limper. 1995. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J. Clin. Investig. 952699-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato, M., S. Shinozaki-Yabana, A. Yamashita, Y. Watanabe, and M. Yamamoto. 2001. The fission yeast meiotic regulator Mei2p undergoes nucleocytoplasmic shuttling. FEBS Lett. 499251-255. [DOI] [PubMed] [Google Scholar]
- 23.Shimoda, C., M. Uehira, M. Kishida, H. Fujioka, Y. Iino, Y. Watanabe, and M. Yamamoto. 1987. Cloning and analysis of transcription of the mei2 gene responsible for initiation of meiosis in the fission yeast Schizosaccharomyces pombe. J. Bacteriol. 16993-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slaven, B. E., J. Meller, A. Porollo, T. Sesterhenn, A. G. Smulian, and M. T. Cushion. 2006. Draft assembly and annotation of the Pneumocystis carinii genome. J. Eukaryot. Microbiol. 53(Suppl. 1)S89-S91. [DOI] [PubMed] [Google Scholar]
- 25.Thomas, C. F., R. A. Anders, M. P. Gustafson, E. B. Leof, and A. H. Limper. 1998. Pneumocystis carinii contains a functional cell-division-cycle Cdc2 homologue. Am. J. Respir. Cell. Mol. Biol. 18297-306. [DOI] [PubMed] [Google Scholar]
- 26.Thomas, C. F., Jr., and A. H. Limper. 2007. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat. Rev. Microbiol. 5298-308. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, C. F., Jr., and A. H. Limper. 2004. Pneumocystis pneumonia. N. Engl. J. Med. 3502487-2498. [DOI] [PubMed] [Google Scholar]
- 28.Vassallo, R., J. E. Standing, and A. H. Limper. 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol. 1643755-3763. [DOI] [PubMed] [Google Scholar]
- 29.Vidan, S., and A. P. Mitchell. 1997. Stimulation of yeast meiotic gene expression by the glucose-repressible protein kinase Rim15p. Mol. Cell. Biol. 172688-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vohra, P. K., V. Puri, T. J. Kottom, A. H. Limper, and C. F. Thomas, Jr. 2003. Pneumocystis carinii STE11, an HMG-box protein, is phosphorylated by the mitogen activated protein kinase PCM. Gene 312173-179. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe, Y., S. Shinozaki-Yabana, Y. Chikashige, Y. Hiraoka, and M. Yamamoto. 1997. Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature 386187-190. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe, Y., and M. Yamamoto. 1994. S. pombe mei2+ encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell 78487-498. [DOI] [PubMed] [Google Scholar]
- 33.Wyder, M. A., E. M. Rasch, and E. S. Kaneshiro. 1998. Quantitation of absolute Pneumocystis carinii nuclear DNA content. Trophic and cystic forms isolated from infected rat lungs are haploid organisms. J. Eukaryot. Microbiol. 45233-239. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita, A., Y. Watanabe, N. Nukina, and M. Yamamoto. 1998. RNA-assisted nuclear transport of the meiotic regulator Mei2p in fission yeast. Cell 95115-123. [DOI] [PubMed] [Google Scholar]
- 35.Yong, S.-J., Z. Vuk-Pavlovic, J. E. Standing, E. C. Crouch, and A. H. Limper. 2003. Surfactant protein D aggregation of Pneumocystis impairs phagocytosis by macrophages. Infect. Immun. 711662-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]