Abstract
Human macrophages infected with Mycobacterium tuberculosis may undergo apoptosis. Macrophage apoptosis contributes to the innate immune response against M. tuberculosis by containing and limiting the growth of mycobacteria and also by depriving the bacillus of its niche cell. Apoptosis of infected macrophages is well documented; however, bystander apoptosis of uninfected macrophages has not been described in the setting of M. tuberculosis. We observed that uninfected human macrophages underwent significant bystander apoptosis 48 and 96 h after they came into contact with macrophages infected with avirulent M. tuberculosis. The bystander apoptosis was significantly greater than the background apoptosis observed in uninfected control cells cultured for the same length of time. There was no evidence of the involvement of tumor necrosis factor alpha, Fas, tumor necrosis factor-related apoptosis-inducing ligand, transforming growth factor β, Toll-like receptor 2, or MyD88 in contact-mediated bystander apoptosis. This newly described phenomenon may further limit the spread of M. tuberculosis by eliminating the niche cells on which the bacillus relies.
Mycobacterium tuberculosis infects one in three people globally and is the second most common cause of death due to an infectious disease (16). Following infection M. tuberculosis uses macrophages as a reservoir for continuous bacterial replication and as a vehicle for bacillary dissemination. The success of this infection owes much to the ability of the bacillus to evade the human immune response. Macrophage apoptosis removes niche cells upon which the bacilli rely, augments the adaptive immune response through dendritic cell activation and subsequent antigen presentation, and also reduces bacterial viability (2, 19, 25, 34, 44, 46, 47). In an attempt to deprive M. tuberculosis of its chosen niche cell, macrophages infected with attenuated strains of M. tuberculosis can undergo apoptosis in a manner that involves tumor necrosis factor alpha (TNF-α) (27). This cell death process also results in reduced survival of mycobacteria and leads to cross priming of M. tuberculosis-specific CD8+ T cells (27, 30, 34, 39, 47). Macrophage apoptosis may contribute to the innate immune response against this intracellular infection by containing and limiting the growth of the bacilli, as has been observed for other infectious agents, and may therefore be part of a successful host defense mechanism (15, 28, 52).
Apoptosis is a fundamental process in the development and maintenance of the immune system, and its importance in the host response is now being recognized. We have recently shown that M. tuberculosis causes caspase-independent death of human macrophages, and evidence suggests that both the mitochondria (cleavage of Bid) and lysosomes (cathepsin-mediated DNA fragmentation) are involved in this cell death pathway (38). Death receptors, including Fas, TNF-R, tumor necrosis factor-related apoptosis-inducing ligand RI (TRAIL-RI), and TRAIL-RII, initiate apoptosis in response to ligation with their respective death ligands and induce cell death in some systems by activation of caspases (3).
Previous studies have shown that alveolar macrophages and differentiated THP-1 macrophages undergo apoptosis in similar manners when they are infected with attenuated M. tuberculosis (27, 44). We have also shown that M. tuberculosis strains with increasing virulence prevent macrophage apoptosis in a manner that involves interleukin-10-dependent release of soluble TNF receptor 2 (5). It is now known that following infection with virulent M. tuberculosis, NF-κB activation is seen up to 48 h postinfection, compared to 4 h with attenuated strains; this results in upregulation of the antiapoptotic Bcl2 family member bfl-1/A1 (12). Sly et al. also reported involvement of another antiapoptotic Bcl2 family member, Mcl-1, in H37Rv-infected macrophages (49). Attenuated H37Ra infection at a low multiplicity of infection (MOI) results in macrophage cell death that is characterized by DNA fragmentation, phosphatidylserine exposure, and nuclear condensation and involves the activation of caspase 8, Bid, and also serine protease activity (25, 38, 44).
Bystander apoptosis has been characterized in a number of infectious disease states. In human immunodeficiency virus (HIV) infection, apoptosis of uninfected CD4+ T cells aids T-cell depletion, whereby interactions between HIV type 1 (HIV-1) envelope glycoproteins and CXCR4 and/or CCR5 induce bystander killing (9, 22). Toxoplasma gondii infection of dendritic cells can also induce contact-dependent bystander apoptosis in T lymphocytes by an unknown mechanism (32, 51). Bystander cytotoxicity by avian leukosis virus through the TVB(S3) receptor, a member of the TNF receptor family, has also been demonstrated, and cytotoxic lymphocytes from patients with chronic hepatitis C infection mediate bystander killing through Fas-FasL interactions (13, 20). In mice infected with Mycobacterium bovis Bacille Calmette-Guerin (BCG), infection-induced apoptosis promotes apoptosis of activated nonspecific CD4+ T cells through a mechanism that requires the gamma interferon receptor on CD4+ T cells (11, 36). Bystander apoptosis of macrophages, which are potential niche cells for M. tuberculosis, has not been described.
The similarity between the host responses to viral and bacterial infections led us to hypothesize that elimination of uninfected bystander macrophages may function to remove niche cells from the infectious loci. We investigated the role of bystander apoptosis in response to attenuated M. tuberculosis infection in human macrophages in vitro. Our studies demonstrated that bystander cell death occurs in uninfected macrophages that have been in contact with macrophages infected with M. tuberculosis at a low MOI. Infection at the low MOI probably reflects the physiological situation in vivo, given that only a low number of bacteria are required to establish an infection in humans (4). Bystander apoptosis occurred with the same kinetic timeframe as infected cell apoptosis and occurred through a mechanism independent of TNF-α, Fas, TRAIL, transforming growth factor β (TGFβ), Toll-like receptor 2 (TLR2), or MyD88.
MATERIALS AND METHODS
M. tuberculosis.
M. tuberculosis H37Ra and H37Rv were obtained from the American Type Culture Collection (ATCC 25177; Manassas, VA) and were grown to log phase in Middlebrook 7H9 broth (Difco, Becton Dickenson, Sparks, MD) supplemented with albumin-dextrose-catalase (Becton Dickinson, Oxford, United Kingdom) and 0.05% Tween 80 (Difco) and made up in low-endotoxin water (Sigma, St. Louis, MO). Aliquots were stored at −80°C, thawed, and propagated in Middlebrook 7H9 medium to log phase prior to infection.
Cell culture.
Human monocytic leukemia THP-1 cells (European Collection of Cell Cultures) were cultured in RPMI 1640 medium with l-glutamine (Gibco/BRL; Invitrogen, Carlsbad, CA) supplemented with non-heat-inactivated 10% fetal bovine serum (Gibco/BRL), 1 mM HEPES, 50 U/ml amphotericin B (Gibco), and 50 μg/ml cefotaxime (Melford Laboratories, United Kingdom). For all experiments, THP-1 cells were cultured at an initial density of 0.5 × 105 cells/ml and treated with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO) at a final concentration of 100 nM for 72 h. The medium was then removed and replaced with complete RPMI 1640 medium.
Peripheral blood mononuclear cells were isolated from the buffy coat of healthy donors (provided, with permission, by the Irish Blood Transfusion Service) by centrifugation (1,600 × g for 28 min) on a Ficoll gradient using Vacutainer CPT tubes (Becton Dickinson), washed, and resuspended in RPMI 1640 culture medium. The medium was supplemented with 10% pooled type AB human serum (Sigma), 50 μg of cefotaxime/ml, and 50 U of amphotericin B/ml. The cells were seeded onto two-well LabTek glass chamber slides (Nunc, Inc., Naperville, IL). Nonadherent cells were removed by washing the wells with Hanks balanced salt solution after 24 h, and fresh medium was added. The medium was replaced, with washing to remove any remaining nonadherent cells, every 2 to 3 days. Macrophages were cultured for 7 to 10 days before infection with M. tuberculosis.
Infection of macrophages with M. tuberculosis.
Log-phase mycobacteria were centrifuged (3,000 × g) for 10 min, and the pellet was resuspended in complete RPMI 1640 medium. Prior to inoculation of macrophages, mycobacteria were aspirated through a 25-gauge needle 10 times, followed by centrifugation (100 × g) for 3 min. The supernatant was transferred to a fresh tube and used to infect PMA-differentiated THP-1 cells. To ensure an optimal infection ratio, MOIs were determined by adding dilutions of prepared bacilli to differentiated THP-1 cells growing in two-well LabTek glass chamber slides (Nunc, Inc., Naperville, IL). After 4 h of incubation at 37°C in the presence of 5% CO2, extracellular mycobacteria were washed away, and the cells were fixed in 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 5 min. Cells were then stained using a TB fluorescent M stain kit (Becton, Dickinson and Company, Franklin Lakes, NJ) according to the manufacturer's instructions. Briefly, slides were immersed in TB auramine M for 15 min, washed gently in running water, and decolorized with TB Decolorizer TM for 30 s. This was followed by another wash step and Hoechst 33358 (10 μg/ml; Molecular Probes, Leiden, The Netherlands) staining for 15 min in the dark. The slides were finally washed gently with water, and cover slips were added using DAKO fluorescent embedding medium (Dakocytomation, Carpinteria, CA). The MOI was determined by fluorescent microscopy using a Leica photomicroscope (Leica Microscopy Systems, Heerbrugg, Switzerland). In order to quantitate bystander apoptosis, suboptimal infection was required (approximately 50% infection with an MOI of 1 to 5 bacilli per infected cell). For the supernatant, transwell, and PKH67 experiments, this was not necessary, and >70% of the cells were infected at an MOI of 1 to 5.
In situ DNA fragmentation analysis.
DNA fragmentation as a measure of apoptosis was determined by the in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technique using a TMR red in situ cell death detection kit (Roche Applied Science, Lewes, United Kingdom). PMA-differentiated THP-1 cells, plated at a concentration of 0.5 × 105 cells/ml in two-well LabTek glass chamber slides, were infected with M. tuberculosis H37Ra for 4 h as described above, washed to remove extracellular mycobacteria, and then cultured for 1, 2, or 3 days at 37°C in the presence of 5% CO2. The medium was then carefully removed by aspiration, and the cells were fixed with 2% PFA in PBS. TUNEL staining was performed according to the manufacturer's instructions, followed by auramine staining and counterstaining of nuclei using Hoechst 33342, as described above. TUNEL positivity and infection levels were evaluated by fluorescent microscopy. Particular care was taken to count nuclei (blue), M. tuberculosis (green), and TUNEL positivity (red) in separate analyses under three different laser colors and not on composite figures. At least 100 cells were counted for each condition. Cells treated for 4 to 16 h with 200 ng/ml staurosporine (Sigma-Aldrich) were included as a positive apoptosis control.
Cell death detection ELISA.
PMA-differentiated THP-1 cells (0.5 × 105 cells) were seeded in 24-well tissue culture plates and infected with M. tuberculosis H37Ra for 4 h as described above. Cells were washed to remove extracellular mycobacteria and then cultured for 1, 2, or 3 days at 37°C in the presence of 5% CO2. A cell death detection ELISAPLUS enzyme-linked immunosorbent assay (ELISA) (Roche Diagnostics, Lewes, United Kingdom) was performed according to the manufacturer's instructions, and optical density (OD) values were determined using a Wallac Victor2 1420 multilabel counter (Turku, Finland). Uninfected cells treated for 4 to 16 h with 200 ng/ml staurosporine (Sigma-Aldrich) were included as a positive apoptosis control. To compare individual experiments, data were analyzed by setting the basal level of apoptosis to 1.0 based on the OD value for uninfected cells. All other OD values in an experiment were divided by the uninfected-cell OD to provide a relative apoptosis value, which was expressed in arbitrary units (44).
Transwell experiments.
PMA-differentiated THP-1 macrophages (0.5 × 105 cells/ml) were seeded into the lower compartment of a 48-well transwell plate (Costar) and also onto the polycarbonate membrane of the transwell insert (pore size, 0.1 μm). Macrophages in the transwell insert were infected with H37Ra as described above. Following coincubation, TUNEL TMR staining and Hoechst nuclear staining were performed with uninfected cells in the lower chamber and on the transwell membrane. The number of apoptotic TUNEL-positive cells relative to the total number of nuclei per field was determined, and at least 100 cells per condition were counted.
PKH67 staining.
To assess bystander apoptosis, target THP-1 monocytes were labeled using a PKH67 green fluorescent cell linker mini kit (Sigma, St. Louis, MO) according to manufacturer's instructions. THP-1 monocytes were washed twice in Dulbecco's PBS (Gibco/BRL, Grand Island, NY) to remove fetal bovine serum, resuspended in PKH67 staining solution, and incubated at room temperature for 5 min at 300 rpm. Fetal bovine serum was added, and the cell suspension was inverted continuously for 1 min to stop the labeling reaction. Cells were then transferred to a 15-ml conical tube and washed extensively with complete RPMI 1640 medium to remove unbound dye. Successful staining was visualized by fluorescent microscopy using a Leica photomicroscope (Leica Microscopy Systems, Heerbrugg, Switzerland).
PKH67-labeled THP-1 cells (uninfected bystander cells; 0.5 × 105 cells/ml) were added to unlabeled PMA-differentiated THP-1 macrophages infected with H37Ra (infected effector cells; 0.5 × 105 cells/ml) that were seeded into two-well LabTek glass chamber slides. Effector macrophages were infected with M. tuberculosis for 4 h and then extensively washed to remove extracellular bacteria. Cocultures of uninfected PKH67-stained bystander cells and infected cells were maintained at 37°C in the presence of 5% CO2 for 24 or 48 h. After incubation, supernatants were removed, cells were fixed with 2% PFA in PBS and TUNEL TMR (red) stained to indicate apoptosis, and nuclei were stained using Hoechst 33342, as described above. The levels of bystander apoptosis in PKH67-stained green macrophages were determined using fluorescent microscopy. To do this accurately, cells were assessed under separate filters and scored as red or green. When a single cell was scored as both green (bystander) and red (apoptotic), this indicated presence of bystander apoptosis. The number of PKH67-positive, TUNEL-positive cells relative to the total number of nuclei per field was determined, and at least 200 cells per condition were counted.
In separate experiments, we reversed the cells that were labeled; i.e., the resident infected macrophages were labeled with PHK67, and unlabeled THP-1 cells were added before cells were stained by the TUNEL technique. In brief, THP-1 cells were labeled with PKH67 green stain, as described above, and allowed to differentiate following treatment with PMA. PKH67-stained THP-1 cells were infected with M. tuberculosis H37Ra (infected effector cells) for 4 h and then extensively washed to remove extracellular bacteria. PKH67-labeled infected cells and unlabeled THP-1 cells (uninfected bystander cells; 0.5 × 105 cells/ml) were maintained in culture at 37°C in the presence of 5% CO2 for 48 h. After incubation, supernatants were removed, cells were fixed with 2% PFA in PBS and TUNEL TMR (red) stained to indicate apoptosis, nuclei were stained using Hoechst 33342, and bystander apoptosis was scored as described above.
Antibody blocking experiments.
Anti-Fas monoclonal antibody (human, neutralizing) clone ZB4 (Upstate Cell Signaling Solutions, New York) was used at a concentration of 2.5 μg/ml. Anti-human TNF-α monoclonal antibody (MAB 210; R&D Systems, Minneapolis, MN) was used at a concentration of 5 μg/ml. Anti-human TRAIL (clone RIK-2; ebioscience, San Diego, CA) was used at a concentration of 2.5 μg/ml. Anti-hLAP (TGFβ1) (MAB 246; R&D Systems, Minneapolis, MN) was used at a concentration of 2.5 μg/ml. Anti-TLR2 (clone TL2.1; ebioscience, San Diego, CA) was used at a concentration of 2.5 μg/ml. Immunoglobulin G1 (IgG1) isotype control antibodies were used at corresponding concentrations. Antibodies were added to THP-1 cultures 1 h before infection with H37Ra and also following a 4-h infection period.
The abilities of the blocking antibodies to neutralize their ligands were determined by challenging Jurkat or undifferentiated THP-1 cells with the appropriate ligand in the presence of the antibody at the concentrations indicated above. Fas ligand, TRAIL, TGFβ, and TNF-α were obtained from R&D Systems, and the synthetic bacterial lipopeptide Pam3CysSerLys4 was obtained from Calbiochem. Where appropriate, the cells were incubated with cycloheximide for 15 min before addition of the apoptotic stimulus. After 48 h the cells were stained with propidium iodide (50 μg/ml) and analyzed using a FACSCalibur (Becton Dickinson) flow cytometer to measure viability. To compare individual experiments, data were analyzed by defining the viability of control untreated cells as 1, based on the percentage of propidium iodide-negative (viable) cells in untreated samples. All other viability values in an experiment were divided by the percentage of viable cells in untreated samples to obtain a relative viability value (in arbitrary units). Similarly, the TGFβ antibody was tested to determine its ability to block the growth-inhibiting effect of TGFβ on undifferentiated THP-1 cells. Cell counts were determined by microscopy using a hemocytometer. The results were expressed as cell growth relative to the number of cells in wells treated with dexamethasone alone compared to cells treated with TGFβ plus dexamethasone in the presence or absence of TGFβ-neutralizing antibody.
Measurement of cytokine levels.
PMA-differentiated THP-1 cells (0.5 × 105 cells/ml) were cultured and challenged with M. tuberculosis as described above. Supernatants were harvested, and cytokine levels were quantified by an ELISA using a commercial kit (ebioscience, San Diego, CA) in accordance with the manufacturer's specifications.
Statistical analysis.
The results are expressed as means ± standard errors of the means. Statistical analysis was performed using GraphPad InStat (version 3.00 for Windows 95; GraphPad Software, San Diego, CA) and Microsoft Excel statistical software. Differences were considered significant when the P value was <0.05.
RESULTS
M. tuberculosis strain H37Ra induces bystander apoptosis in uninfected macrophages.
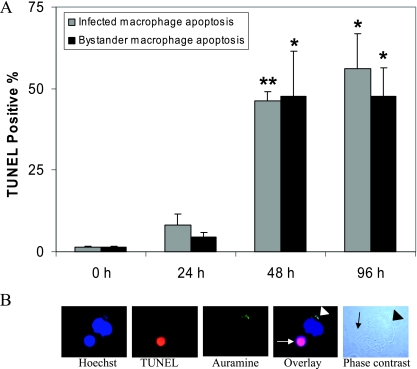
We and others have shown that infection with M. tuberculosis strain H37Ra induces significant macrophage cell death in a dose- and time-dependent manner, and significant apoptosis is seen 48 h after H37Ra infection (38). In order to investigate apoptosis of uninfected bystander human macrophages, PMA-differentiated THP-1 cells were seeded onto tissue culture-treated glass slides for subsequent TUNEL staining. This technique has the advantage of allowing simultaneous monitoring of cell death in infected and uninfected macrophages using fluorescent microscopy (magnification, ×100). In order to observe significant bystander macrophage apoptosis, it was important that approximately 50% of the macrophages were infected. It was observed that 47.95% ± 12.05% (mean ± standard error of the mean) of macrophages were infected with an MOI of 1 to 5 bacilli per cell, as determined by auramine staining and fluorescent microscopy (Fig. 1B). At 24, 48, or 96 h postinfection, macrophages were fixed and processed for TUNEL staining using TMR red-labeled nucleotides, and this was followed by auramine staining to visualize mycobacteria and Hoechst 33342 counterstaining of the nuclei. The percentage of infected cells and the percentage of infected and uninfected cells that were TUNEL positive were determined by fluorescent microscopy. Apoptosis of infected cells was observed 24 h postinfection, and the levels were significantly increased at 48 and 96 h postinfection (46.3% ± 2.7% [P < 0.005] and 56.0% ± 10.7% [P < 0.05], respectively) compared to the control (Fig. 1A). In addition to apoptosis of infected cells, uninfected human macrophages were observed to undergo bystander apoptosis 48 and 96 h postinfection (47.7% ± 13.7% [P < 0.05] and 47.7% ± 8.7% [P < 0.05], respectively) compared to the control (Fig. 1A). As a positive control for apoptosis, cells were treated with staurosporine (200 ng/ml for 16 h), and these cells were uniformly TUNEL positive throughout the experiments (data not shown). The bystander apoptosis was up to 20-fold higher than the background apoptosis observed for uninfected control cells cultured for the same length of time. At least 100 cells per slide were counted at a magnification of ×100 using fluorescent microscopy, as shown in Fig. 1B. In this figure an image shows a TUNEL-positive, apoptotic uninfected bystander macrophage in close proximity to a nonapoptotic infected cell whose nucleus was stained using Hoechst stain. Intracellular auramine-stained (green) bacilli are also shown. As expected, macrophages infected with the virulent M. tuberculosis strain, strain H37Rv, were prevented from undergoing apoptosis; surrounding uninfected bystander macrophages also did not undergo apoptosis (data not shown), indicating that virulent strains of M. tuberculosis can avoid inducing cell death of potential niche cells in close proximity to infected cells. Bystander apoptosis was also evaluated using primary human monocyte-derived macrophages following infection with M. tuberculosis at a low MOI, and primary macrophages lost viability as indicated by propidium iodide staining; however, DNA fragmentation was not observed in any of the donors studied at this MOI (data not shown).
FIG. 1.
M. tuberculosis strain H37Ra induces bystander apoptosis in uninfected human macrophages. (A) Macrophages were infected with M. tuberculosis strain H37Ra (47.95% ± 12.05% cells were infected with an MOI of 1 to 5 bacilli per cell) for 4 h in two-well LabTek chambers. Apoptosis was determined for both infected and uninfected bystander cells after 24, 48, and 96 h by the in situ TUNEL TMR technique. Significant DNA fragmentation occurred in H37Ra-infected macrophages at 48 h (P < 0.005) and 96 h (P < 0.05) postinfection compared to the control. The uninfected bystander apoptosis was significantly greater than the apoptosis of uninfected control cells at 48 h (P < 0.05) and 96 h (P < 0.05) postinfection. The data are expressed as percentages of TUNEL-positive cells (means and standard errors of the means) of triplicate samples, and at least 100 cells per slide were counted at a magnification of ×100. Statistical significance was determined using the Student t test (one asterisk, P < 0.05; two asterisks, P < 0.005). (B) Fluorescent microscopy images showing a TUNEL-positive, apoptotic uninfected bystander macrophage (white arrow) in close proximity to a nonapoptotic infected cell whose nucleus was stained using Hoechst stain. The white arrowhead indicates intracellular auramine-stained (green) bacilli. The phase-contrast light micrograph shows an infected cell (black arrowhead) and uninfected bystander cell (black arrow) in contact.
Induction of apoptosis in bystander cells is not mediated through soluble factors.
To determine whether the observed bystander apoptosis of uninfected THP-1 cells in the presence of cells infected with M. tuberculosis H37Ra was due to the release of a soluble factor, supernatant was harvested from infected apoptotic cells and added to freshly PMA-differentiated uninfected THP-1 cells. This conditioned medium was filtered to remove any mycobacteria. The level of apoptosis in infected cells from which conditioned medium was harvested was assessed by the cell death detection ELISA (Fig. 2A). Infection with mycobacterial strain H37Ra induced significant apoptosis in THP-1 cells compared to the uninfected control (3.62- ± 0.07-fold increase; P < 0.0001; n = 3). Conditioned medium from infected apoptotic cells did not, however, induce apoptosis in THP-1 macrophages in a parallel experiment over 24, 48, and 96 h as determined by the cell death detection ELISA (Fig. 2B). In confirmatory experiments, macrophages were seeded onto both the upper membrane of a transwell membrane (pore size, 0.1 μm) and the lower chamber. Following H37Ra infection of macrophages in the upper chamber, uninfected macrophages in the lower compartment did not undergo apoptosis compared to the uninfected control 48 h postinfection (Fig. 2C and 2D) (2.78% ± 1.78% and 3.84% ± 1.75%, respectively; n = 3). In this transwell experiment, we used a TUNEL assay to assess apoptosis, which we found to be more sensitive than the ELISA. Treatment with staurosporine, as a positive control, induced significant apoptosis in uninfected cells (97.03% ± 1.6%; P < 0.0001; n = 3). At least 100 cells per condition were counted using fluorescent microscopy (magnification, ×20).
FIG. 2.
Soluble factors released from infected apoptotic human macrophages do not induce apoptosis in uninfected bystander macrophages. (A) Macrophages were infected with M. tuberculosis strain H37Ra for 4 h. At 96 h postinfection, the level of apoptosis in infected cells, as indicated by DNA fragmentation, was determined using a cell death detection ELISA. H37Ra infection induced a significant level of apoptosis compared to the uninfected control (P < 0.0001). Treatment with staurosporine was used as a positive control. (B) Supernatants and conditioned medium from infected apoptotic cells were harvested 24, 48, and 96 h after infection and added to a parallel culture of uninfected PMA-differentiated THP-1 cells. The apoptosis levels in uninfected THP-1 cells were determined using the cell death detection ELISA 96 h after the addition of conditioned medium. No significant apoptosis occurred in uninfected cells after exposure to conditioned medium from apoptotic infected cells. The data are expressed as fold increases compared with the uninfected control (means and standard errors of the means) of at least three separate experiments. (C) Transwell culture dishes were seeded with PMA-differentiated THP-1 macrophages (0.5 × 105 cells/ml). Macrophages in the upper chamber were infected with M. tuberculosis strain H37Ra for 4 h and were separated from uninfected macrophages in the lower chamber by a permeable membrane (pore size, 0.1 μm). At 48 h postinfection the uninfected THP-1 macrophages (0.5 × 105 cells) in the lower chamber had not undergone significant levels of apoptosis compared to the uninfected control as determined by TUNEL TMR staining. As a positive control, PMA-differentiated THP-1 macrophages were treated with staurosporine (P < 0.0001). (D) Fluorescent microscopy (magnification, ×20) images of uninfected macrophages from the lower chambers of transwell culture dishes. The left panels show Hoechst and TUNEL staining of uninfected control macrophages. The middle panels show staining of uninfected bystander macrophages that were exposed to culture medium from infected cells for 48 h. The right panels show staining of macrophages that were exposed to staurosporine as a positive control. Statistical significance was determined using Student's t test (one asterisk, P < 0.05; two asterisks, P < 0.001; three asterisks, P < 0.0001). Mtb, M. tuberculosis.
Apoptosis of bystander macrophages requires cell-cell contact with infected macrophages.
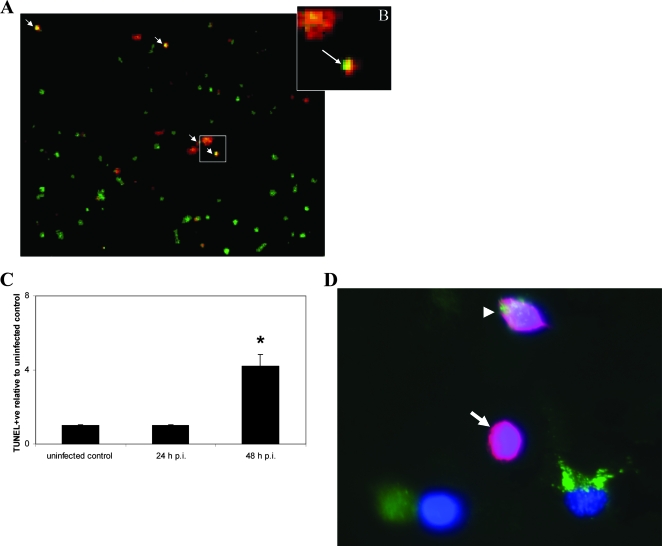
Our previous results demonstrated that soluble factors alone do not induce bystander apoptosis in uninfected cells. To assess whether cell-cell contact was required for bystander cell death to occur, we examined whether addition of fluorescently labeled uninfected monocytes to infected macrophages initiated apoptosis in the naïve monocytic cells on contact. Target monocytic THP-1 cells were labeled with the green fluorescent dye PKH67, which is incorporated into lipid regions of the plasma membrane. Effector (infected macrophage) and target PKH67-labeled cells were coincubated for 24 to 48 h. Bystander apoptosis was quantified following TUNEL TMR (red) staining, and bystander cells undergoing apoptosis were labeled with both the red and green stains; Fig. 3A and 3B show examples of apoptotic bystander macrophages. Hoechst staining was used to determine the total number of cells. Following addition of uninfected cells to the infected cell population, the level of apoptosis in the uninfected bystander cell population increased more than fourfold from 0.5% to 8.7% ± 1.77% (Fig. 3C), demonstrating that contact of the uninfected macrophages with infected macrophages induced significant cell death in the bystander population. In the experiment shown in Fig. 1, uninfected cells and infected cells were cocultured, providing more opportunity for cell-cell contact and resulting in a higher rate of bystander apoptosis.
FIG. 3.
Uninfected bystander apoptosis for macrophages that were in contact with infected macrophages. (A) Macrophages were infected for 4 h with M. tuberculosis strain H37Ra. Uninfected PKH67-labeled green THP-1 cells (0.5 × 105 cells/ml) were added to infected macrophages for 24 and 48 h. Adherent cells were then stained with Hoechst 33342 and with TUNEL TMR (red) to determine apoptosis levels. Superimposition of PKH67-labeled (green) bystander cell fields and TUNEL-positive (red) fields showed bystander apoptotic macrophages (orange). Nuclear staining (blue) (not shown) revealed the total number of cells per field. One representative field at a magnification of ×20 is shown. The arrows indicate apoptotic bystander macrophages. (B) Close-up of apoptotic bystander macrophage (arrow). (C) Uninfected PKH67-labeled (green)THP-1 macrophages (0.5 × 105 cells/ml) were cocultured with infected PMA-differentiated THP-1 macrophages (0.5 × 105 cells/ml) for 24 and 48 h. Supernatants were removed, and adherent cells were TUNEL TMR stained and counterstained using Hoechst 33342. The levels of bystander macrophage apoptosis were >4-fold greater (P < 0.01) than the uninfected control levels. The data are expressed as the fold increases compared with the control (one asterisk, P < 0.01), and at least 100 cells per slide were counted at magnification of ×20. Statistical significance was determined using Student's t test. (D) Reverse staining experiment in which bystander cells were not labeled: superimposition of PKH67-stained (green) infected THP-1 cell, nuclear staining (blue), and TUNEL-positive (red) fields allowed quantification of unlabeled (no green) bystander apoptotic macrophages. The image is one representative field (magnification, ×40). The arrow indicates an apoptotic bystander macrophage, and the arrowhead indicates an apoptotic PKH67-stained effector cell. The data are representative of at least three separate experiments.
Similar levels of bystander apoptosis were observed in reverse staining experiments in which the infected effector cells were labeled with PKH67 and the uninfected bystander cells were not labeled, as shown in Fig. 3D. The levels of bystander apoptosis seen after 48 h of coculture showed that there was a >4-fold increase from 3.8% ± 0.3% to 16.5% ± 1.0%, further demonstrating that contact of the uninfected macrophages with infected macrophages induced significant cell death in the bystander population.
Bystander apoptosis in response to M. tuberculosis H37Ra-infected macrophages is not dependent on classical cytotoxic proteins.
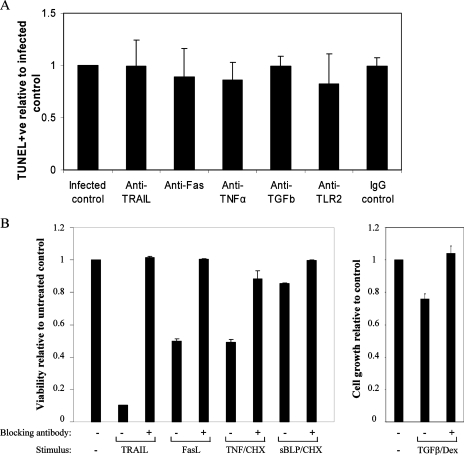
The results described thus far indicate that bystander apoptosis occurs in uninfected macrophages that are in close proximity to infected cells and undergo apoptosis without the actions of soluble factors alone. To further delineate the mechanism by which bystander apoptosis occurs, neutralizing antibodies to the main cytotoxic proteins were added to cultures of macrophages before and after infection with H37Ra. We and others have previously shown that apoptosis of infected macrophages with H37Ra requires TNF-α (26, 27). To further determine if TNF-α was a key apoptotic mediator in bystander apoptosis of THP-1 cells, macrophages were challenged with M. tuberculosis H37Ra in the presence of anti-TNF-α antibody or an IgG1 isotype control antibody. It was found that bystander apoptosis of uninfected macrophages was not dependent on TNF-α (Fig. 4), even though the levels of TNF-α in supernatants from H37Ra-infected macrophages were significantly higher than the levels in supernatants from uninfected controls (1,587.3 ± 8.6 and 37.55 ± 0.25 pg/ml, respectively; P < 0.0001; n = 3).
FIG. 4.
Mechanism of bystander macrophage apoptosis is independent of TRAIL, Fas, TNF-α, TGFβ, and TLR2. (A) Macrophages were treated with anti-Fas antibody (neutralizing; ZB4; 2.5 μg/ml), anti-TNF-α antibody (10 ng/ml), anti-TGFβ antibody (2.5 μg/ml), anti-TLR2 antibody (2.5 μg/ml), or an IgG1 isotype control (10 ng/ml) before and after infection with M. tuberculosis strain H37Ra (MOI, 1 to 5). At 72 h postinfection a TUNEL analysis was performed, and apoptosis levels were assessed using fluorescent microscopy. There was no significant difference between control bystander apoptosis levels and the apoptosis levels of preparations treated with neutralizing antibodies. (B) As positive controls for the neutralizing antibody experiments, Jurkat cells were incubated in the presence or absence of Fas ligand (10 ng/nl), TRAIL (20 ng/ml), and TNF-α (5 ng/ml) and/or cycloheximide (CHX) (0.2 μg/ml) for 48 h with or without the appropriate neutralizing antibody at the concentrations described above for panel A; the TLR2 antibody was tested to determine its ability to block apoptosis of THP-1 cells induced by the synthetic bacterial lipopeptide (sBLP) Pam3CysSerLys4 (20 ng/ml) in combination with cycloheximide (0.5 μg/ml). The cells were then stained with propidium iodide (50 μg/ml) and analyzed by flow cytometry to measure apoptosis. To validate the neutralizing effect of the anti-TFGβ antibody, THP-1 cells were incubated with dexamethasone (Dex) (25 nM) or dexamethasone plus TGFβ (2 ng/ml) for 72 h in the presence or absence of anti-TGFβ. Cell counts were determined by microscopy using a hemocytometer. The values are the means and standard errors of the means for three experiments, and the results are expressed relative to the appropriate control for each stimulus.
In addition, infected and uninfected cells cultured in the presence of saturating concentrations of blocking or neutralizing antibodies to Fas, TRAIL, TGF-β, or TLR2 exhibited levels of apoptosis similar to those of infected control cells, as determined using TUNEL staining (Fig. 4A). The abilities of these antibodies to neutralize signaling by their respective ligands were confirmed in parallel experiments (Fig. 4B). PMA-differentiated THP-1 cells (0.5 × 105 cells/ml) were also incubated with an intracellular inhibitory MyD88 peptide or scrambled control peptide for 24 h prior to infection with H37Ra; however, at 72 h postinfection, no significant difference between the bystander apoptosis levels in control and treated cells was observed (data not shown).
DISCUSSION
Following infection with attenuated strains of M. tuberculosis, macrophages undergo an apoptosis-like death that involves, at low MOIs, the actions of active caspases, cathepsins, and serine proteases (27, 38). Apoptosis of infected macrophages may be part of a successful host defense mechanism that limits the growth of the mycobacteria, aiding antigen cross presentation to CD8+ T cells (47) and inducing bactericidal mechanisms. To date, studies of the host response to M. tuberculosis infection have focused predominantly on apoptosis of infected cells; however, here we present data on apoptosis of uninfected bystander macrophages occurring in response to M. tuberculosis infection in macrophages. Data presented here show that macrophages infected with M. tuberculosis can induce a contact-dependent apoptotic response in uninfected bystander macrophages. Bystander apoptosis may represent a mechanism whereby the innate immune response mediates killing of uninfected potential niche cells, limiting progression of the infectious disease. Within 48 h of infection, significant macrophage apoptosis occurs. Infected cells induce apoptosis of uninfected macrophages by a cell-cell contact-dependent mechanism. The death signal that is transmitted to bystander cells does not depend upon soluble mediators or upon TNF-α, Fas, TRAIL, TGFβ, or TLR2. Previously, we have shown that even though the tuberculosis granuloma is a paucibacillary environment, significantly high levels of macrophage apoptosis occur within the cellular structure (25). With this in mind, bystander apoptosis tuberculosis may be relevant in tuberculosis granuloma homeostasis and could represent a host response for defense against further bacterial intracellular growth and infection.
The process of apoptosis has adapted to function as a defense mechanism against intracellular infection (6). As an intracellular microbe, M. tuberculosis depends on viable macrophages as niche cells in which to replicate and survive. A recent report suggested that the newly identified murine ipr gene is important in susceptibility to tuberculosis and proposed that macrophage apoptosis in vivo also plays a role in host defense against M. tuberculosis (40). This emphasizes the importance of apoptosis in influencing the host response to infection.
The present study documents a novel aspect of the host response, whereby bystander apoptosis occurs in human macrophages that have been in contact with macrophages undergoing apoptosis following infection. The dependence on contact between the two populations of cells was determined by addition of PKH-67 green-labeled uninfected bystander macrophages to infected macrophages and subsequent apoptosis analysis. The kinetics of apoptosis seen in this study correlate with previous findings which showed that significant fragmentation of infected cell and bystander DNA occurred 48 h postinfection (38).
Bystander apoptosis has been described for a number of infectious diseases, including HIV infection (33, 41). The envelope glycoprotein complex (Env) of HIV-1 can induce apoptosis in a variety of ways. Infected cells express gp120/gp41 on the plasma membrane and can induce apoptosis of uninfected bystander cells expressing CD4 and CXCR4 by at least three different mechanisms (41). In this manner the virus predominantly kills uninfected cells and maintains an apoptosis-resistant phenotype in infected cells, allowing viral replication and chronic infection (48). Infection with the intracellular protozoan T. gondii also results in apoptosis of the host cells of the parasite, T lymphocytes, and also uninfected leukocytes. It is thought that this T-cell apoptosis is orchestrated mainly through a Fas-dependent mechanism (33). In mice infected with M. bovis BCG, Fas and FasL are upregulated in T lymphocytes, and it is thought that Fas-mediated apoptosis of T lymphocytes contributes to a reduction in T-cell proliferation and delayed-type hypersensitivity (29). It has also been shown that in a murine model of tuberculosis, directly infected macrophages within the macrophage-rich granuloma can express FasL (35). Human monocyte-derived macrophages infected with M. tuberculosis are also partially susceptible to Fas-mediated apoptosis (37). However, in the current study, addition of Fas-neutralizing antibodies to human macrophage cultures infected with the attenuated H37Ra strain did not reduce the bystander apoptosis levels compared to the control, indicating that Fas has little or no role in mediating cell death of uninfected macrophages in this system.
TNF-α is a critically important cytokine in tuberculosis infection. Not only is it produced at the site of disease in tuberculosis patients (7, 43), but it is also crucial for the structural maintenance of the granuloma, as demonstrated after the use of neutralizing anti-TNF monoclonal antibody treatment resulted in reactivation of latent tuberculosis via disruption of the granuloma (17, 24). Previously, we and others have demonstrated a role for TNF-α cytotoxicity in alveolar macrophage apoptosis following M. tuberculosis infection (5, 25, 26, 27). However, data presented here indicate that in this model, TNF-α is not required in order for bystander apoptosis to occur.
TRAIL (Apo-2L) is a type II membrane-bound TNF family ligand that is homologous to FasL and is expressed on many cell types, including THP-1 cells (42). TRAIL, whose the main function is to induce apoptosis, is upregulated on macrophages following mycobacterial infection (14). Emerging evidence indicates that TRAIL may induce cell death in HIV-1-infected macrophages (23). We attempted to identify a role for TRAIL in induction of apoptosis in uninfected macrophages that were in contact with infected macrophages. However, addition of monoclonal antibodies to TRAIL did not inhibit bystander apoptosis, indicating that TRAIL does not play a mechanistic role in bystander apoptosis.
Bronchoalveolar lavage fluid cells from tuberculosis patients contains elevated levels of bioactive TGFβ, and the levels of mRNA transcripts of TGFβ RΙ and TGFβ RΙΙ are significantly increased in alveolar macrophages compared to controls (8, 21). TGFβ produced from macrophages has also been shown to interfere with the bioactivity of interleukin-12 during enhancement of M. tuberculosis-induced gamma interferon mRNA expression and protein production (50). Apoptotic cells release a network of anti-inflammatory cytokines, including TGFβ, and can also trigger TGFβ production by apoptotic macrophages (10, 18). The potential role of TGFβ in enhancing bystander apoptosis was examined in our system, and TGFβ was found to have no effect on bystander apoptosis levels compared to the control.
There is evidence which suggests that TLR2 is linked to M. tuberculosis-induced macrophage apoptosis (1, 32) and that TLR4 mediates lipopolysaccharide-induced macrophage apoptosis (45). Lee et al. showed that there was no significant difference in the levels of propidium iodide-stained macrophages from wild-type, MyD88−/−, and TLR4−/− mice infected with M. tuberculosis Erdman at a high MOI (31). Similarly, our study suggests that human macrophages infected with H37Ra do not mediate bystander apoptosis in a TLR2- or MyD88-dependent fashion.
Following M. tuberculosis infection of niche macrophages with attenuated bacilli, the host may respond by inducing apoptosis in the infected cell population in order to prevent further growth of the bacteria and dissemination to other cells in the local area. We present data which support another mechanism by which the host innate response can defend against further bacterial intracellular growth and infection. Bystander apoptosis of uninfected macrophages in close proximity to infected cells allows the host to eliminate potential niche cells in the infectious foci. We determined that bystander apoptosis occurs through an apoptotic pathway that does not include Fas, TNF-α, TGFβ, TLR2, or MyD88. Macrophage apoptosis in itself results in a more effective innate immune response. Delineation of molecular mechanisms of the host response to M. tuberculosis may allow design of more effective therapeutics or vaccine options in the future.
Acknowledgments
This work was supported by grants from Science Foundation Ireland (grant HL-03964), the Health Research Board (grants CSA/2004/7 and PR070/2001), PRTLI/HEA, and The Royal City of Dublin Hospital Trust.
We thank Hardy Kornfeld for helpful discussions.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 22 October 2007.
REFERENCES
- 1.Aleman, M., P. Schierloh, S. S. de la Barrera, R. M. Musella, M. A. Saab, M. Baldini, E. Abbate, and M. C. Sasiain. 2004. Mycobacterium tuberculosis triggers apoptosis in peripheral neutrophils involving Toll-like receptor 2 and p38 mitogen protein kinase in tuberculosis patients. Infect. Immun. 725150-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., and P. D. Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 2811305-1308. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian, V., E. H. Wiegeshaus, B. T. Taylor, and D. W. Smith. 1994. Pathogenesis of tuberculosis: pathway to apical localization. Tuber. Lung Dis. 75168-178. [DOI] [PubMed] [Google Scholar]
- 5.Balcewicz-Sablinska, M., J. Keane, H. Kornfeld, and H. Remold. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. Immunol. 1612636-2641. [PubMed] [Google Scholar]
- 6.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8113-126. [DOI] [PubMed] [Google Scholar]
- 7.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 613482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonecini-Almeida, M. G., J. L. Ho, N. Boechat, R. C. Huard, S. Chitale, H. Doo, J. Geng, L. Rego, L. C. Lazzarini, A. L. Kritski, W. D. Johnson, Jr., T. A. McCaffrey, and J. R. Silva. 2004. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect. Immun. 722628-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbonari, M., A. M. Pesce, M. Cibati, A. Modica, L. Dell'Anna, G. D'Offizi, A. Angelici, S. Uccini, A. Modesti, and M. Fiorilli. 1997. Death of bystander cells by a novel pathway involving early mitochondrial damage in human immunodeficiency virus-related lymphadenopathy. Blood 90209-216. [PubMed] [Google Scholar]
- 10.Chen, W., M. E. Frank, W. Jin, and S. M. Wahl. 2001. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14715-725. [DOI] [PubMed] [Google Scholar]
- 11.Dalton, D. K., L. Haynes, C. Q. Chu, S. L. Swain, and S. Wittmer. 2000. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhiman, R., M. Raje, and S. Majumdar. 2007. Differential expression of NF-κB in mycobacteria infected THP-1 affects apoptosis. Biochim. Biophys. Acta 1770649-658. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Griffero, F., S. A. Hoschander, and J. Brojatsch. 2003. Bystander killing during avian leukosis virus subgroup B infection requires TVB(S3) signaling. J. Virol. 7712552-12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl, G. E., H. H. Yue, K. Hsieh, A. A. Kuang, M. Ho, L. A. Morici, L. L. Lenz, D. Cado, L. W. Riley, and A. Winoto. 2004. TRAIL-R as a negative regulator of innate immune cell responses. Immunity 21877-889. [DOI] [PubMed] [Google Scholar]
- 15.Dockrell, D. H., M. Lee, D. H. Lynch, and R. C. Read. 2001. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184713-722. [DOI] [PubMed] [Google Scholar]
- 16.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282677-686. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers, S. 2005. Why does tumor necrosis factor targeted therapy reactivate tuberculosis? J. Rheumatol. Suppl. 7435-39. [PubMed] [Google Scholar]
- 18.Fadok, V. A., D. L. Bratton, D. M. Rose, A. Pearson, R. A. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 40585-90. [DOI] [PubMed] [Google Scholar]
- 19.Gan, H., X. He, L. Duan, E. Mirabile-Levens, H. Kornfeld, and H. G. Remold. 2005. Enhancement of antimycobacterial activity of macrophages by stabilization of inner mitochondrial membrane potential. J. Infect. Dis. 1911292-1300. [DOI] [PubMed] [Google Scholar]
- 20.Gremion, C., B. Grabscheid, B. Wolk, D. Moradpour, J. Reichen, W. Pichler, and A. Cerny. 2004. Cytotoxic T lymphocytes derived from patients with chronic hepatitis C virus infection kill bystander cells via Fas-FasL interaction. J. Virol. 782152-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch, C. S., Z. Toossi, G. Vanham, J. L. Johnson, P. Peters, A. Okwera, R. Mugerwa, P. Mugyenyi, and J. J. Ellner. 1999. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J. Infect. Dis. 179945-953. [DOI] [PubMed] [Google Scholar]
- 22.Holm, G. H., C. Zhang, P. R. Gorry, K. Peden, D. Schols, E. De Clercq, and D. Gabuzda. 2004. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J. Virol. 784541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, Y., N. Erdmann, H. Peng, S. Herek, J. S. Davis, X. Luo, T. Ikezu, and J. Zheng. 2006. TRAIL-mediated apoptosis in HIV-1-infected macrophages is dependent on the inhibition of Akt-1 phosphorylation. J. Immunol. 1772304-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keane, J. 2005. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford) 44714-720. [DOI] [PubMed] [Google Scholar]
- 25.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane, J., S. Gershon, and M. Braun. 2002. Tuberculosis and treatment with Infiximab. N. Engl. J. Med. 346623-626. [Google Scholar]
- 27.Keane, J., H. G. Remold, and H. Kornfeld. 2000. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 1642016-2020. [DOI] [PubMed] [Google Scholar]
- 28.Khelef, N., A. Zychlinsky, and N. Guiso. 1993. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect. Immun. 614064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremer, L., J. Estaquier, I. Wolowczuk, F. Biet, J. C. Ameisen, and C. Locht. 2000. Ineffective cellular immune response associated with T-cell apoptosis in susceptible Mycobacterium bovis BCG-infected mice. Infect. Immun. 684264-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laochumroonvorapong, P., S. Paul, K. B. Elkon, and G. Kaplan. 1996. H2O2 induces monocyte apoptosis and reduces viability of Mycobacterium avium-M. intracellulare within cultured human monocytes. Infect. Immun. 64452-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, J., H. G. Remold, M. H. Ieong, and H. Kornfeld. 2006. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J. Immunol. 1764267-4274. [DOI] [PubMed] [Google Scholar]
- 32.Lopez, M., L. M. Sly, Y. Luu, D. Young, H. Cooper, and N. E. Reiner. 2003. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J. Immunol. 1702409-2416. [DOI] [PubMed] [Google Scholar]
- 33.Luder, C. G., and U. Gross. 2005. Apoptosis and its modulation during infection with Toxoplasma gondii: molecular mechanisms and role in pathogenesis. Curr. Top. Microbiol. Immunol. 289219-237. [DOI] [PubMed] [Google Scholar]
- 34.Molloy, A., P. Laochumroonvorapong, and G. Kaplan. 1994. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 1801499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mustafa, T., S. Phyu, R. Nilsen, G. Bjune, and R. Jonsson. 1999. Increased expression of Fas ligand on Mycobacterium tuberculosis infected macrophages: a potential novel mechanism of immune evasion by Mycobacterium tuberculosis? Inflammation 23507-521. 29. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor, R. A., S. Wittmer, and D. K. Dalton. 2005. Infection-induced apoptosis deletes bystander CD4+ T cells: a mechanism for suppression of autoimmunity during BCG infection. J. Autoimmun. 2493-100. [DOI] [PubMed] [Google Scholar]
- 37.Oddo, M., T. Renno, A. Attinger, T. Bakker, H. MacDonald, and P. Meylan. 1998. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J. Immunol. 1605448-5454. [PubMed] [Google Scholar]
- 38.O'Sullivan, M. P., S. O'Leary, D. M. Kelly, and J. Keane. 2007. A caspase-independent pathway mediates macrophage cell death in response to Mycobacterium tuberculosis infection. Infect. Immun. 751984-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pais, T. F., and R. Appelberg. 2000. Macrophage control of mycobacterial growth induced by picolinic acid is dependent on host cell apoptosis. J. Immunol. 164389-397. [DOI] [PubMed] [Google Scholar]
- 40.Pan, H., B. S. Yan, M. Rojas, Y. V. Shebzukhov, H. Zhou, L. Kobzik, D. E. Higgins, M. J. Daly, B. R. Bloom, and I. Kramnik. 2005. Ipr1 gene mediates innate immunity to tuberculosis. Nature 434767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perfettini, J. L., M. Castedo, T. Roumier, K. Andreau, R. Nardacci, M. Piacentini, and G. Kroemer. 2005. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 12(Suppl. 1)916-923. [DOI] [PubMed] [Google Scholar]
- 42.Phillips, T. A., J. Ni, G. Pan, S. M. Ruben, Y. F. Wei, J. L. Pace, and J. S. Hunt. 1999. TRAIL (Apo-2L) and TRAIL receptors in human placentas: implications for immune privilege. J. Immunol. 1626053-6059. [PubMed] [Google Scholar]
- 43.Portales-Perez, D. P., L. Baranda, E. Layseca, N. A. Fierro, H. de la Fuente, Y. Rosenstein, and R. Gonzalez-Amaro. 2002. Comparative and prospective study of different immune parameters in healthy subjects at risk for tuberculosis and in tuberculosis patients. Clin. Diagn. Lab. Immunol. 9299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riendeau, C. J., and H. Kornfeld. 2003. THP-1 cell apoptosis in response to mycobacterial infection. Infect. Immun. 71254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruckdeschel, K., G. Pfaffinger, R. Haase, A. Sing, H. Weighardt, G. Hacker, B. Holzmann, and J. Heesemann. 2004. Signaling of apoptosis through TLRs critically involves Toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J. Immunol. 1733320-3328. [DOI] [PubMed] [Google Scholar]
- 46.Russell, D. G. 2007. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 539-47. [DOI] [PubMed] [Google Scholar]
- 47.Schaible, U. E., F. Winau, P. A. Sieling, K. Fischer, H. L. Collins, K. Hagens, R. L. Modlin, V. Brinkmann, and S. H. Kaufmann. 2003. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 91039-1046. [DOI] [PubMed] [Google Scholar]
- 48.Selliah, N., J. Shackelford, J. F. Wang, F. Traynor, J. Yin, and T. H. Finkel. 2003. T cell signaling and apoptosis in HIV disease. Immunol. Res. 27247-260. [DOI] [PubMed] [Google Scholar]
- 49.Sly, L. M., S. M. Hingley-Wilson, N. E. Reiner, and W. R. McMaster. 2003. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 170430-437. [DOI] [PubMed] [Google Scholar]
- 50.Toossi, Z., M. Mincek, E. Seeholtzer, S. A. Fulton, B. D. Hamilton, and C. S. Hirsch. 1997. Modulation of IL-12 by transforming growth factor-beta (TGF-beta) in Mycobacterium tuberculosis-infected mononuclear phagocytes and in patients with active tuberculosis. J. Clin. Lab. Immunol. 4959-75. [PubMed] [Google Scholar]
- 51.Wei, S., F. Marches, J. Borvak, W. Zou, J. Channon, M. White, J. Radke, M. F. Cesbron-Delauw, and T. J. Curiel. 2002. Toxoplasma gondii-infected human myeloid dendritic cells induce T-lymphocyte dysfunction and contact-dependent apoptosis. Infect. Immun. 701750-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358167-169. [DOI] [PubMed] [Google Scholar]