Abstract
Dendritic cells (DC) play an essential role in initiating and directing T-cell responses, in part by production of interleukin-12p70 (IL-12p70), IL-23, and IL-27. However, comparative studies on the capacity for cytokine production of DC subsets are rare. Here, we compare splenic CD8α+, CD4+, and double-negative (DN) DC, isolated 5 h to 28 days after Leishmania donovani infection, for (i) production of IL-12p70, (ii) accumulation of IL-12/23p40, IL-12p35, IL-23p19, and IL-27p28 mRNAs, and (iii) their capacity to direct CD4+ T-cell differentiation. At 5 h, conventional DC (cDC) accumulated mRNA for IL-12/23p40 (CD8α>CD4>DN), IL-23p19 (CD4>CD8α>DN), and IL-27p28 (CD8α>CD4>DN), in an infection dose-dependent manner. IL-12p70 was restricted to CD8α+ cDC, reflecting the subset-specific accumulation of IL-12p35 mRNA. In contrast, cDC from mice infected for 14 to 28 days accumulated little mRNA for IL-12p40 and IL-12p19, though IL-27p28 mRNA remained detectable (CD8α>DN>CD4). IL-12p70 secretion by CD8α+ cDC was also absent, reflecting deficient IL-12/23p40, rather than IL-12p35, mRNA accumulation. The capacity of CD8α+ cDC isolated early after infection to direct Th1 cell differentiation was mediated through IL-12/23p40, whereas this ability in CD4+ and DN cDC was independent of IL-12/23p40 and did not result from overexpression of Delta 4 Notch-like ligand. However, DN cDC produced gamma interferon (IFN-γ) and also contained a rare population of CD11chi DX5+ IFN-γ-producing cells. Our data illustrate the extensive diversity in, and temporal regulation of, splenic cDC subsets during infection and suggest caution in interpreting data obtained with unfractionated or minimally purified DC.
Dendritic cells (DC) are central to initiating, maintaining, and directing T-cell immune responses. Murine splenic conventional DC (cDC) can be divided into three subsets based on the expression of CD4 and CD8α (22, 49, 64), namely, CD8α+ CD4−, CD8α− CD4+, and CD8α− CD4− (double-negative [DN]) cDC. In early studies, it had been suggested that different DC subsets were programmed to prime different T helper cell responses. Thus, antigen-pulsed CD8α− DC drove a Th2 phenotype, whereas CD8α+ DC led to Th1 differentiation (32, 44). However, other studies have shown that the capacity of DC to influence T-cell priming depends on the microbial stimulus itself. For example, Pulendran and colleagues (43) showed that DC stimulated by lipopolysaccharide (LPS) derived either from Escherichia coli or Porphyromonas gingivalis induced opposing Th1 or Th2 profiles. While LPS derived from both these microorganisms induced DC maturation based on changes in costimulatory molecules, only LPS from E. coli induced CD8α+ DC to produce interleukin-12p70 (IL-12p70) (43). Additional evidence that microbial stimuli influence the T-cell polarizing capacity by DC comes from in vitro studies demonstrating that CD8α+, CD4+, and DN cDC stimulated with soluble Toxoplasma antigen promoted Th1 differentiation. In contrast, each DC subset stimulated with zymosan particles leads to IL-10-mediated Th2 differentiation (33). In spite of these in vitro data, there is a paucity of analysis dissecting the respective influence of cDC subsets on Th cell development. Although IL-12 production by DC appears to determine Th1/Th2 balance, the recent discovery of IL-12-related cytokines (IL-23 and IL-27) highlights the importance of reexamining the role each cytokine plays in T-cell differentiation. IL-23 shares the p40 subunit also utilized by the heterodimeric cytokine IL-12. Recent findings demonstrate that IL-23 rather than IL-12 is crucial for the pathogenesis of some autoimmune diseases (9, 38). Specifically, disease-promoting IL-17-producing Th17 cells, which develop in the presence of IL-6 and transforming growth factor β, can be expanded by IL-23 (31). In contrast, studies on the role of IL-27 have demonstrated a critical role in early events leading to Th1 polarization. However, infection models using IL-27 knockout mice have observed exaggerated T-cell responses, highlighting an immunoregulatory role for IL-27 (18, 46, 62). To date, few studies have addressed the expression or function of these cytokines during chronic infectious disease.
Leishmania donovani, a causative agent of visceral leishmaniasis (VL), is an intracellular protozoan parasite that primarily infects myeloid cells (27). Control and resolution of this disease rely at least in part on activation and differentiation of naïve CD4+ T cells into gamma interferon (IFN-γ)-secreting cells that activate parasitized macrophages to produce leishmanicidal effectors, such as nitric oxide (47). In humans and experimental mouse models, the development of VL is associated with immunological dysfunction (reviewed in reference 27), and in experimental models, host protective immunity is expressed in an organ-specific manner (12, 27). Th1-associated cytokines are abundant (26, 36), Th2 cytokines also contribute to host protection (53), and both IL-10 (39) and transforming growth factor β (66) have disease-promoting effects. Although some aspects of DC function have been described in this model, the precise role that individual subsets of splenic cDC play in the development and regulation of T helper cell differentiation during different stages of the disease remain unknown.
In this study, therefore, we directly examined the capacity of murine splenic cDC subsets from different stages of infection for the accumulation of cytokine mRNAs and for their capacity to direct T-cell receptor (TCR) transgenic antigen-specific CD4+ T-cell differentiation. We show that CD8α+, CD4+, and DN cDC isolated from mice immediately after infection skew the differentiation of naïve T cells toward the Th1 phenotype, whereas no Th1/Th2 bias was observed in T cells stimulated with splenic cDC derived from chronically infected mice. Furthermore, the polarization of T cells by cDC subsets in vitro appears to be strongly influenced by the distinct cytokine (IL-12, IL-23, and IL-27) profile of each DC subset. This analysis also revealed the importance of ensuring DC purity for such studies, with the function of DN cDC being influenced by rare contaminating interferon-producing NK cells (IKDC) (7, 8, 58).
MATERIALS AND METHODS
Mice and infection.
BALB/c mice (Charles River Laboratories) were used at 6 to 10 weeks of age and housed under specific-pathogen-free conditions. DO11.10.SCID mice were originally provided by F. Powrie, University of Oxford). OT-II.RAG1−/− mice were originally obtained from The National Institute of Medical Research, London, United Kingdom. Parasites of the Ethiopian strain of Leishmania donovani (LV9) were maintained by serial passage in Syrian hamsters. Amastigotes were isolated from infected spleens, as previously described (55), and mice were infected with either 2 × 108 (high-dose [HD]) or 2 × 107 (conventional or low-dose [LD]) L. donovani amastigotes intravenously in 200 μl of RPMI 1640 (Gibco, Paisley, United Kingdom). Mice were killed by cervical dislocation. All animal care and experimental procedures were in accord with United Kingdom Home Office requirements and performed with local ethical approval.
Splenic DC enrichment.
Splenic DC from naïve and infected mice were enriched by digesting the spleens in RPMI 1640 supplemented with 0.2 mg/ml collagenase (10 ml per spleen) for 25 min at room temperature. All subsequent steps were done between 0 and 4°C. Following collagenase digestion, 5 ml of a phosphate-buffered saline (PBS) solution containing 50 mM EDTA was added to the spleen cells, and the digest was passed through a 100-μm strainer to make a single-cell suspension. Dead cells and highly phagocytic cells were then depleted prior to CD11c enrichment by magnetic cell sorting using basic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer's protocol. Briefly, digested splenocytes were incubated with basic magnetic microbeads for 10 min on ice. After the cells were washed, the cells that had nonspecifically bound or phagocytosed microbeads were trapped on a separation column. CD11c+ cells were then enriched by incubating anti-CD11c microbeads for 30 min on ice and then passed over a separation column. CD11chi cells were routinely 85 to 98% pure. To purify splenic cDC subsets, CD11chi cells were stained with CD11c-phycoerythrin (PE), CD8α-fluorescein isothiocyanate (FITC), and CD4-allophycocyanin (APC) (all from BD Pharmingen, San Diego, CA) and sorted on a FACS vantage cell sorter (BD Biosciences, Mountain View, CA). Sort gates are described below in Results. Each subset showed greater than 99% purity.
Flow cytometry.
For flow cytometry, cells were incubated with 10 μg/ml 2.4G2 anti-Fc receptor monoclonal antibody (MAb) (ATCC, Rockville, MD), followed by staining with directly conjugated MAbs. Cells were stained with the following: FITC-conjugated anti-CD8α (53-6.7); anti-CD40 (HM40/3); PE-conjugated anti-CD11c (HL3); anti-CD49b (DX5); biotinylated conjugated, anti- major histocompatibility complex class II (MHC-II) CD80 (16.10A1); CD86 (GL1); DO11.10 (KJ126); anti-CD11c (HL3); and APC-conjugated CD4 (RM4-5) (all from BD Pharmingen, San Diego, CA). Labeling with biotinylated antibodies was visualized with PerCP-streptavidin (BD Pharmingen). Minimal background staining was observed using appropriate fluorochrome-labeled isotype controls. All staining was performed on ice in PBS containing 2% fetal calf serum (FCS) and 5 mM EDTA for 30 min on ice. Flow cytometric analysis was performed with a FACSCalibur (BD Biosciences, Mountain View, CA) on 50,000 cells and analyzed using CellQuest software (BD Biosciences).
Real-time reverse transcription-PCR (RT-PCR).
RNA was isolated from DC subsets by using an RNeasy kit according to the manufacturer's instructions (Qiagen). RNA was then reverse transcribed into cDNA using the first-strand cDNA synthesis kit according to the manufacturer's instructions (Invitrogen). Oligonucleotides (5′→3′) used for the specific amplification of IL-10, IL-12/23p40 and hypoxanthine phosphoribosyltransferase (HPRT) were as described in reference 2. Primers used were as follows: for IL-4, forward, CCTCACAGCAACGAAGAACA; reverse, TGGACTCATTCATGGTGCAG; for IL12p35, forward, CCTCGGCATCCAGCAG; reverse, TCTGGCCGTCCTCACCA; for IFN-γ, forward, CCTCCTGCGGCCTAGCTC; reverse, GTAACAGCCAGAAACAGCCATG; for IL-27(p28), forward, GGCCATGAGGCTGGATCTC; reverse, AACATTTGAATCCTGCAGCCA; for IL-23p19, forward, TGGCATCGAGAAACTGTGAGA; reverse, TCAGTTCGTATTGGTAGTCCTGTTA; and for Delta 4 Notch-like ligand, forward, CAGAGACTTCGCCAGGAAACTC; reverse, GCCAAATCTTACCCACAGCAA. The PCR conditions for all primers were 95°C (15 s), 62°C (30 s), and 72°C (30 s) for 40 cycles.
Real-time quantitative PCR was performed with the SYBR green PCR kit in an ABI Prism 7000 sequence detection system (Applied Biosystems) according to the manufacturer's instructions. Expression of target genes was normalized to HPRT and expressed as either absolute copy number (target molecules/1,000 HPRT molecule) or relative expression using the change in cycle threshold (ΔΔCT) analysis method (relative expression of the gene in activated DC compared to its naïve counterpart).
Differentiation of TCR transgenic CD4+ T cells in vitro.
Splenic naïve CD62Lhi CD44lo cells from DO11.10 ovalbumin (OVA) transgenic mice were prepared as described previously (16). Primary stimulation cultures were established under nonpolarizing conditions by activation of sorted naïve CD4+ T cells (1 × 105/well) with sorted naïve or infected DC subsets (104/well) and OVA323-339 peptide (0.1 μg/ml) in the absence of exogenous cytokines or anti-cytokine MAbs. As indicated, 10 μg/ml blocking anti-IL-12/23p40 MAb (C17.8) or control rat immunoglobulin G2a was added at the initiation of cultures. On day 4, the culture medium for the cells was changed and fresh medium was supplemented with IL-2 (40 U/ml). After 1 week, the T cells were restimulated with unfractionated spleen cells (1 × 105/well) and 2 μg/ml OVA peptide for 7 h at 37°C. Brefeldin A (5 μg/ml) was added during the last 4 h of culture. Cells were washed in ice-cold PBS, surface stained with KJ-126, and fixed with 2% paraformaldehyde for 10 min. Samples were resuspended in PBS containing 2% FCS and 5 mM EDTA and left overnight in the refrigerator before intracellular staining. Dead cells were excluded by forward scatter and side scatter gating. The total number of DO11.10 T cells was determined by spiking samples with beads of known amount and gating on KJ-126+ cells during flow cytometry acquisition.
Intracellular cytokine staining.
Fixed-cell samples were resuspended in PBS-EDTA-FCS containing 0.5% bovine serum albumin and 0.5% saponin (saponin buffer). Cells were then stained with FITC-conjugated anti-IFN-γ (XMG 1.2), and PE-conjugated anti-IL-4 (11B11) for 45 min on ice. FITC- and PE-labeled isotype controls were used to set gates. Samples were washed twice in saponin buffer and once in PBS without saponin. Flow cytometric analysis was performed using a FACSCalibur (BD Biosciences, Mountain View, CA). A total of 50,000 cells were collected and analyzed using CellQuest software (BD Biosciences).
IL-12p70 ELISA.
Sorted naïve or infected DC subsets were cultured for 24 h (1 × 105/well), and IL-12p70 was analyzed in cell culture supernatants. Commercial enzyme-linked immunosorbent assay (ELISA) antibody pairs were used to detect IL-12p70 (eBioscience) according to the manufacturer's instruction. Recombinant cytokines were used to generate standard curves.
Statistics.
Statistical analysis was performed using a Student t test. A P of <0.05 was considered significant.
RESULTS
DC maturation following L. donovani infection.
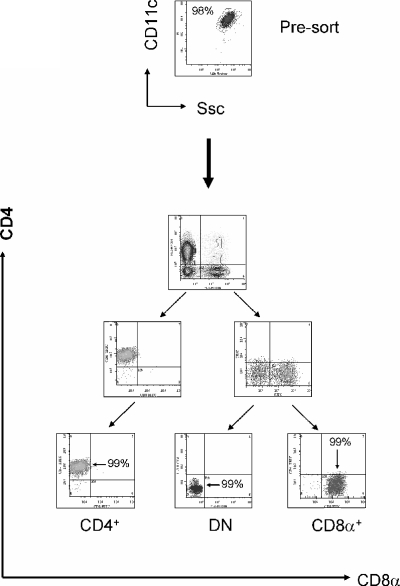
The murine spleen contains three cDC subsets that can be distinguished by their expression of CD4 and CD8α. To isolate these subsets to near homogeneity, macrophages and dead cells were first eliminated from collagenase-digested spleens by magnetic sorting using unconjugated microbeads. In our hands, removal of autofluorescent macrophages was an essential prerequisite for subsequent flow cytometric analysis and/or sorting (data not shown). By combining a subsequent magnetic sort using CD11c microbeads with flow sorting, CD4+, CD8α+, and DN subsets of cDC could be isolated to >99% purity (Fig. 1). Similar degrees of purity were achieved using both naïve and infected mice (data not shown).
FIG. 1.
Purification of splenic cDC subsets. CD11c+ DC from five mice were enriched by magnetic activated cell sorting prior to cell sorting. The purity of each cDC subset used in these studies was >99% and is shown in the top graph and in the three bottom graphs. Ssc,. side scatter.
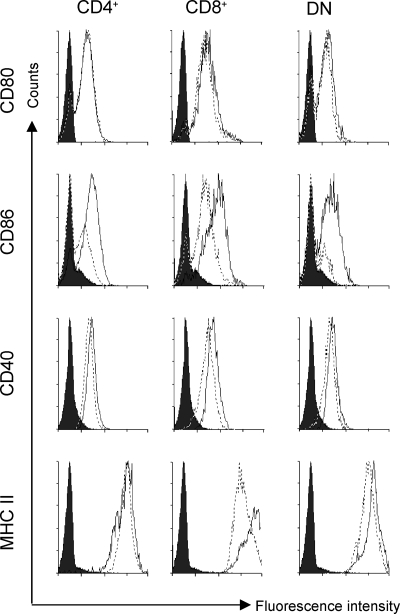
We then investigated the effects of infection on the expression of various cell surface molecules that are associated with DC activation/maturation. As shown in Fig. 2, all cDC subsets isolated from naïve mice expressed similar levels of MHC-II, CD40, CD80, and CD86. DC from mice infected 5 h previously with 2 × 107 L. donovani amastigotes, the conventional infection dose used in this model, had minimally augmented levels of MHC-II, CD86, and to a lesser extent, CD40 (data not shown). Somewhat more pronounced effects, particularly with CD86, were seen when the infection dose was increased to 2 × 108 amastigotes (Fig. 2), but even at this dose, while reproducible, the response was generally not striking, e.g., relative to responses to strong TLR agonists, such as LPS (data not shown). Moreover, as reported by us and others (2, 11), upregulation of these surface molecules was observed at a population level, suggesting that it was largely mediated by inflammatory signals rather than by infection of cDC per se. Parallel experiments using carboxyfluoroscein succinimidyl ester-labeled parasites determined that <1% of isolated cDC subsets were infected, with no bias among subsets for uptake of parasites (data not shown). In contrast, expression of CD80 was not increased as a consequence of infection, irrespective of dose (Fig. 2 and data not shown). The absolute numbers of each subset did not alter appreciably over 5 h of infection [(9.1 ± 0.19) × 105, (3.0 ± 0.6) × 105, and (2.2 ± 0.4) × 105 versus (8.8 ± 1.1) × 105, (3.3 ± 0.4) × 105, and (3.1 ± 0.4) × 105 CD4+, CD8+, and DN cDC per spleen in naïve mice and mice infected with a LD for 5 h, respectively]. Thus, in terms of the regulation of these cell surface molecules, only modest activation of splenic cDC occurs following infection of BALB/c mice with L. donovani amastigotes.
FIG. 2.
L. donovani infection induces maturation of splenic cDC subsets. CD11chi DC subsets from naïve (broken line) or 5-h-infected (2 × 108) (solid line) BALB/c mice were examined for changes using a panel of costimulation markers as well as MHC-II. Subsequent to magnetic activated cell sorting enrichment of CD11c+ cells, samples were stained with CD11c, CD4, and CD8 in combination with the markers examined above. Histograms were gated on CD11chi cells. Black histograms represent isotype controls. Data represent cDC subsets isolated and pooled from five mice per group and are representative of three experiments.
cDC from infected mice induce weak CD4+ T-cell polarization.
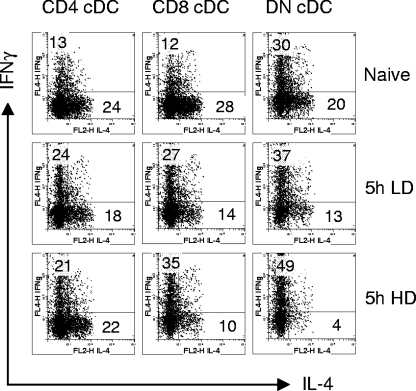
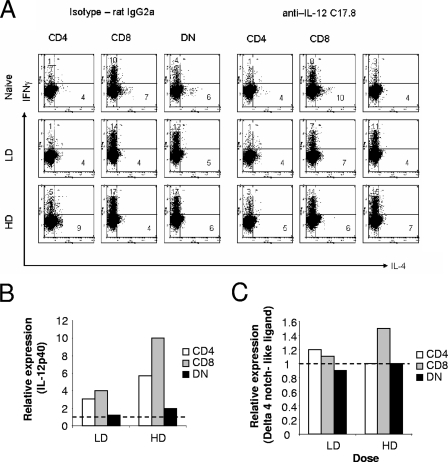
Experimental VL following infection with L. donovani amastigotes is associated with a mixed cytokine response, though with some bias toward IFN-γ production, particularly early on during infection (14). To determine whether this was reflected by the capacity of splenic cDC to polarize CD4+ T cells, we isolated cDC subsets from the spleens of naïve and 5-h-infected mice and assayed their ability to direct the differentiation of OVA-specific TCR transgenic T cells (DO11.10) under nonpolarizing conditions (Fig. 3). Under our experimental conditions, CD4+ and CD8α+ cDC from naïve mice induced a mixed cytokine response in DO11.10 T cells, with a bias toward the production of IL-4 (IFN-γ/IL-4 ratios of approximately 1:2 and 1:2.5 for CD4+ and CD8α+ cDC, respectively). These same subsets isolated from mice 5 h after a conventional infection with L. donovani had heightened capacity to stimulate IFN-γ responses (IFN-γ/IL-4 ratios of 1.3:1 and 2:1 for CD4+ and CD8α+ cDC, respectively). Importantly, the absolute number of cytokine-producing DO11.10 cells changed little between naïve and infected mice, suggesting that this result arises from skewing of the differentiation of activated DO11.10 cells rather than heightened antigen presentation per se. To determine whether we could drive this response more effectively, we infected mice with a higher dose of L. donovani (2 × 108 amastigotes). As shown in Fig. 3, skewing toward IFN-γ became more prominent when CD8α+ cDC, but not CD4+ cDC, were used, suggesting that CD8α+ cDC are more sensitive to increasing parasite dose.
FIG. 3.
L. donovani-activated cDC polarize antigen-specific DO11.10 T-cell differentiation. Sorted DC subsets (1 × 104) from naïve and mice infected with 2 × 107 (LD) or 2 × 108 (HD) amastigotes were cultured with purified naïve DO11.10 T cells (1 × 105/well) in the presence of 0.1 μg/ml OVA peptide. One week later, cells were restimulated with whole spleen cells and 2 μg/ml OVA peptide for 7 h and subsequently stained for IFN-γ and IL-4. Plots were gated on CD4+/KJ1-26+ cells. Data are representative of three independent experiments. The numbers indicate the percentage of events within the two quadrants.
Compared to CD4+ and CD8α+ cDC, DN cDC were both more efficient at stimulating DO11.10 cells (reflected by the total number of cytokine-producing cells) and most inherently biased toward inducing IFN-γ (IFN-γ/IL-4 ratio of 1.5:1 in naïve mice). More unexpectedly, DN cDC from 5-h-infected mice were highly efficient at polarizing DO11.10 cells toward IFN-γ production, and at a high infection dose, this led to a dramatic loss of IL-4-producing cells (IFN-γ/IL-4 ratio of 12:1). Collectively, these data demonstrate for the first time using ex vivo-isolated cells that cDC subsets activated as a consequence of L. donovani infection have different capacities to induce Th cell polarization but that only under extreme conditions of HD infection, and notably with DN cDC, does this lead to a pronounced reduction in IL-4-producing cells. These results are thus consistent with the measured CD4+ T-cell cytokine responses observed in the early phase of experimental VL (36).
Accumulation of IL-12-related subunit mRNA differs among cDC subsets after L. donovani infection.
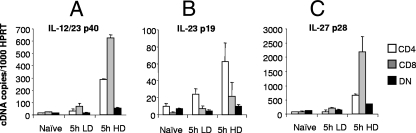
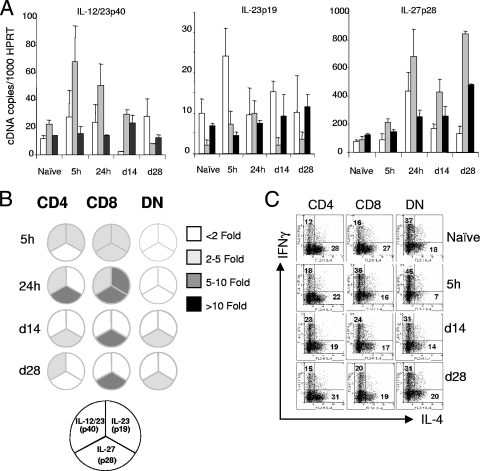
Although IL-12 has been attributed with a major role in the differentiation of CD4+ T cells (60), there is increasing evidence that the IL-12-related molecule IL-27 can also play a role (20). By competition for IL-12/23p40, IL-23p19 may also indirectly regulate IL-12 production. Although previous reports have shown that human monocyte-derived DC and murine bone marrow-derived DC have different capacities to produce IL-12, IL-23, and IL-27, to date, a formal comparative analysis of the accumulation of IL-12-related cytokine subunit mRNAs has not been extended to cDC subsets isolated ex vivo during the course of experimental microbial infection. Therefore, to address this question and to determine whether the pattern of DO11.10 cytokine production induced by each cDC subset was a reflection of differing expression of IL-12-related subunits, we analyzed cytokine mRNA accumulation using quantitative real-time RT-PCR. As shown in Fig. 4, all cDC subsets isolated from naïve mice had low levels of transcripts for IL-12/23p40, IL-27p28, and IL-23p19. Five hours following conventional, and more strikingly following HD infection, considerable heterogeneity in subunit mRNA accumulation was observed between the different cDC subsets. CD8α+ cDC accumulated the most IL-12/23p40, as expected from the literature (17), and also the most IL-27p28 mRNA of the cDC subsets tested. In contrast, while CD4+ cDC from infected mice showed a less pronounced IL-12/23p40 and IL-127p28 response, this subset showed the highest accumulation of IL-23p19 mRNA among the cDC subsets tested. Strikingly, given the results reported above for DO11.10 differentiation, DN cDC from infected mice had only minimal change in mRNA accumulation for each of the three IL-12-related subunits studied.
FIG. 4.
Differential accumulation of IL-12-related subunit mRNAs in cDC subsets. CD11chi DC subsets were sorted on the basis of CD4 and CD8α expression from naïve and infected (2 × 107 [LD] or 2 × 108 [HD] parasites/mouse). (A) IL-12/23p40 mRNA, (B) IL-23p19 mRNA, and (C) IL-27p28 mRNA accumulation in CD4+, CD8α+, and DN DC were examined by real-time RT-PCR. Target genes were normalized against HPRT. Data represent the means plus standard errors of the means (error bars) for three separate experiments.
In order to confirm and extend these results obtained with cDC subsets from infected BALB/c mice and DO11.10 T cells, we also analyzed the cDC response in infected C57BL/6 mice using OVA-specific OT-II TCR transgenic T cells. In our hands, cytokine production by OT-II cells was less extensive compared to DO.11.10 cells, but nevertheless, a similar pattern of cDC-driven responses was observed (Fig. 5A, left panels). Maximal skewing toward IFN-γ was observed with CD8+ and DN cDC subsets, though the ability of DN and CD4+ cDC to skew OT-II cells toward IFN-γ was less marked than in the BALB/c-DO.11.10 system (cf. Fig. 3). Similarly, as shown for cDC from BALB/c mice (Fig. 4), the IL-12/23p40 response of DN cDC isolated from infected C57BL/6 mice was also markedly lower than that of CD8+ cDC (Fig. 5B), in spite of their capacity to induce IFN-γ-producing T cells. To explore further the mechanism by which cDC subsets regulated IFN-γ production by OT-II cells, we blocked IL-12/23p40 function (Fig. 5A, right panels). As expected, anti-IL-12/23p40 MAb significantly inhibited the capacity of CD8+ cDC to skew T-cell differentiation toward IFN-γ (e.g., IFN-γ/IL-4 ratio of 3.5:1 reduced to 1:1 in the presence of anti-IL-12/23p40 for cDC isolated from LD infection). However, blockade of this cytokine had no impact on the functional activity of DN cDC (e.g., IFN-γ/IL-4 ratio of 2.4 versus 2.7 in the absence and presence of blocking MAb, respectively, with LD infection) or CD4+ cDC. A recent report has suggested that CD8− cDC may drive Th cell differentiation in an IL-12-independent manner via a pathway involving Delta 4 Notch-like ligand, with 20-fold-increased accumulation of Delta 4 Notch-like ligand mRNA being observed in this subset in response to LPS (50). In contrast, following L. donovani infection, we observed little change in Delta 4 Notch-like ligand mRNA accumulation by any of the cDC subsets examined (Fig. 5C).
FIG. 5.
Regulation of Th cell differentiation by IL-12/23p40 and Delta 4 Notch-like ligand. (A) Sorted DC subsets (1 × 104) from naïve C57BL/6 mice and C57BL/6 mice infected with 2 × 107 (LD) or 2 × 108 (HD) amastigotes were cultured with purified naïve OT-II T cells (1 × 105/well) in the presence of 0.1 μg/ml OVA peptide and in the presence of either a control rat immunoglobulin G2a (IgG2a) MAb (left panels) or anti-IL-12/23p40 MAb (right panels). One week later, the cells were restimulated with whole spleen cells from B6.CD45.1 mice and 2 μg/ml OVA peptide for 7 h and subsequently stained for IFN-γ and IL-4. Plots were gated on CD4+ CD45.1− cells. The numbers indicate the percentage of events within the two quadrants. (B and C) IL-12/23p40 (B) and Delta 4 Notch-like ligand (C) mRNA accumulation for each cDC subset from infected mice is shown relative to mRNA accumulation in the same subset isolated from naïve mice. Data are derived from one experiment using cells obtained from five mice as cDC donors.
Collectively, these data for the first time illustrate the diversity and complexity of IL-12-related subunit regulation in cDC subsets during infection with L. donovani. Furthermore, while IL-12/23p40 largely governed the capacity of CD8α+ cDC to induce IFN-γ skewing of CD4+ T cells in vitro, this was not the case for CD4+ and DN cDC. Rather, our data suggest that CD4+ and DN cDC, following infection of mice with L. donovani, acquire an IL-12/23p40- and Delta 4 Notch-like ligand-independent capacity to induce IFN-γ production in CD4+ T cells.
Dynamic changes in IL-12-related subunit mRNA accumulation during progressive infection with L. donovani.
Functional alterations in splenic cDC have previously been described during the later stages of L. donovani infection, including changes to MHC-I/II expression, reduced IL-12/23p40, and downregulation of CCR7 (3, 4). To evaluate whether distinct cDC subsets behaved differently during infection and might differentially regulate IL-12-related cytokines, we first extended our analysis to include cDC from mice infected for 24 h and 14 and 28 days. Over the course of longer infection, the absolute number of each cDC subset continued to increase with time [(16.6 ± 2.7) × 105, (4.7 ± 0.8) × 105, and (8.8 ± 1.4) × 105 versus (37.5 ± 7.5) × 105, (10.3 ± 1.6) × 105, and (19.1 ± 2.9) × 105 CD4+, CD8+, and DN cDC at days 14 and 28 postinfection (p.i.), respectively]. These data are shown in Fig. 6A and are also presented as relative to the levels in naïve mice in Fig. 6B. The capacity of cDC subsets isolated from mice at these different times after infection to induce CD4+ T-cell differentiation are compared in Fig. 6C. A number of trends emerge from this analysis. First, on the basis of the total number of cytokine-producing DO11.10 cells, we observed no deficit in antigen presentation by isolated cDC subsets from mice infected for 14 or 28 days compared to those from naïve mice. These data suggest that any changes to MHC-II or costimulatory molecule expression associated with APC dysfunction during long-term L. donovani infection (25) are likely negated by optimal stimulation in vitro with peptide antigen. Second, compared to the ex vivo analysis after a 5-h infection, IL-12/23p40 expression by CD8α+ cDC waned over time, commensurate with a decline in the capacity of these cells to drive IFN-γ production. Thus, whereas CD8α+ cDC from 5-h-infected mice stimulated DO11.10 cells to differentiate with an IFN-γ/IL-4 ratio of 2.25:1, this was reduced to 1:1 with CD8α+ cDC from mice infected for 28 days. Third, the early IL-23p19 response made by CD4+ cDC was more transient than that of CD8α+ cDC, but nevertheless both subsets returned to baseline levels of mRNA for this IL-12-related subunit by day 14 of L. donovani infection. Last, although evident in both CD4+ and CD8α+ cDC early after infection, IL-27p28 mRNA accumulation was sustained only in CD8α+ and DN cDC isolated from long-term-infected mice. However, these increases in IL-27p28 mRNA accumulation do not correlate with the capacity to induce IFN-γ and IL-4 production in DO11.10 T cells. Together, these data illustrated a complex and dynamic regulation of cDC function during the evolution of experimental VL.
FIG. 6.
Cytokine subunit mRNA accumulation and T-cell differentiating capacity of cDC subsets are altered during the course of infection. (A) Splenic CD11chi DC subsets were isolated from naïve and LD-infected mice (2 × 107) at different times (5 h, 24 h, day 14 [d14], and day 28 [d28]). Ex vivo analysis of IL-12/23p40, IL-23p19, and IL-27p28 mRNA accumulation in CD4+ (white bars), CD8α+ (gray bars), and DN (black bars) DC were examined by real-time RT-PCR. Target genes were normalized against HPRT. (B) Data from panel A are summarized as changes in the accumulation of cytokine mRNA from infected DC subsets compared to their naïve counterpart. (C) Sorted DC subsets from naïve and infected mice (5 h, day 14, and day 28) were cultured with purified naïve DO11.10 T cells in the presence of 0.1 μg/ml OVA peptide. Expanded cells were subsequently restimulated with whole spleen cells and 2 μg/ml OVA peptide and stained for IFN-γ and IL-4. Plots were gated on CD4+/KJ1-26+. The numbers indicate the percentage of events within the two quadrants. Data are representative of three independent experiments.
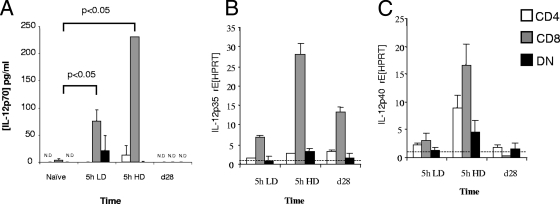
Regulation of IL-12/23p40 rather than IL-12p35 dictates the level of bioactive IL-12p70 in chronically infected mice.
The regulation of IL-12p70 expression has been attributed to regulation of either IL-12p40 or IL-12p35, depending upon the cell type examined (24, 52). As shown in Fig. 7A, cDC isolated from mice 5 h p.i. secreted IL-12p70, a response that was largely restricted to CD8α+ cDC and that was induced in vivo in an infection dose-dependent manner. In contrast, none of these cDC subsets produced IL-12p70 when isolated from mice at day 28 p.i. In addition, IL-12p35 mRNA accumulation was clearly observed in CD8α+ cDC in a dose-dependent manner at 5 h p.i. and was also readily detectable above the levels seen in naïve mice in CD8α+ cDC isolated at day 28 p.i. (Fig. 7B). In contrast, IL-12/23p40 mRNA accumulation was near or below basal levels in all cDC subsets examined at day 28 p.i. (Fig. 7C). Analysis of mRNA accumulation in these ex vivo cDC therefore demonstrated that regulation of IL-12/23p40 was the dominant means of controlling IL-12p70 protein production. These data are supported by the paucity of IL-12/23p40+ cells detectable in the spleen of long-term-infected mice by immunohistochemistry (13).
FIG. 7.
Lack of bioactive IL-12p70 in cDC from chronically infected mice reflects the absence of IL-12/23p40 rather than IL-12p35 mRNA accumulation. Cytokine production by purified splenic CD11chi cell subsets from naïve mice and mice infected for different time periods (5 h or 28 days [d28]) were examined using ELISA to determine secreted IL-12p70 (A) and real-time RT-PCR methods (B and C). (A) cDC subsets were seeded at a concentration of 1 × 106/ml, and samples were collected after 24 h of culture to determine IL-12p70 levels. (B and C) For ex vivo cytokine mRNA expression, relative quantitation analysis of target genes was normalized against HPRT, and each DC subset was calibrated against its naïve counterpart. Data are shown as means plus standard errors of the means (error bars) for the five mice in each group. rE, relative expression; N.D., not detectable.
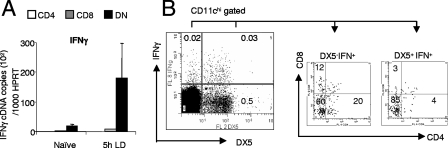
Rare IFN-γ-producing CD11chi DX5+ cells copurify with DN cDC.
An unexpected outcome of this study was the finding that DN cDC were very efficient at inducing skewing of CD4+ T-cell differentiation, even when isolated from naïve mice, and yet this subset accumulated little mRNA compared to CD4+ and CD8α+ cDC for the IL-12-related members analyzed here. Another factor known to direct the differentiation of naïve T cells is IFN-γ (51), and transcripts for this cytokine have previously been found in human and murine DC subsets (17). However, this literature has recently become less clear following the identification of a subset of NK cells (CD11c+ DX5+) that share DC markers, so-called IKDC (7, 8, 58). As shown in Fig. 8, by RT-PCR, CD4+ and CD8α+ cDC isolated from either naïve or 5-h-infected mice had minimal IFN-γ transcript accumulation. In contrast, IFN-γ mRNA accumulation was clearly evident in DN cDC and notably elevated in infected mice (Fig. 8A). To determine whether this finding reflected IFN-γ production by DN cDC or by IKDC, we used intracellular cytokine staining to directly assess IFN-γ protein production. As shown in Fig. 8B, a significant population of IFN-γ-producing cells was observed in ex vivo CD11chi cells that coexpressed DX5, the CD49b antigen found on both NK cells and IKDC. By gating the IFN-γ-producing CD11chi DX5+ (0.03% of total CD11chi cells) and CD11chi DX5− cells (0.02% of total CD11chi cells), we were able to determine their expression of CD4 and CD8α. Notably, the majority of CD11chi DX5+ IFN-γ+ cells were negative for both CD4 and CD8α (85%), indicating that DN cDC contained appreciable contamination with IFN-γ-producing IKDC. Furthermore, within the CD11chi DX5− IFN-γ+ population, DN cDC remained the major producer of IFN-γ, with only modest contributions from CD4+and CD8α+ DC. These results mirrored results of our mRNA analysis and indicated that true DN cDC and to a much lesser extent other cDC subsets isolated from 5-h-infected mice can accumulate IFN-γ transcripts. Nevertheless, the ability of DN cDC to skew CD4+ T-cell differentiation (Fig. 3, 5, and 6) cannot be distinguished from the impact of IKDC in the assays reported above.
FIG. 8.
CD11c+ DX5+ DC are the major producers of IFN-γ. (A) CD11chi DC subsets from naïve mice and mice infected with a low dose for 5 h were sorted on the basis of CD4 and CD8 expression, and ex vivo IFN-γ mRNA accumulation in sorted CD4+, CD8+, and DN DC was examined by real-time RT-PCR. Target genes were normalized against HPRT. Data represent the means plus standard errors of the means (error bars) from three separate experiments. (B) Splenic CD11chi cells from mice infected for 5 h with L. donovani were enriched using CD11c microbeads. Enriched CD11chi cells were then cultured in brefeldin A (10 μg/ml) for 4 h and surface stained with antibodies for CD11c, CD4, CD8, and CD49b (DX5). Fixed-cell samples were then permeabilized and stained for intracellular IFN-γ. Dot plots were gated on CD11chi cells. Quadrant gates were set on isotype controls. The numbers indicate the percentage of events within the three quadrants. This figure shows the results of one experiment that was representative of three independent experiments.
DISCUSSION
The interaction between phenotypically and functionally heterogenous DC and naïve T cells in secondary lymphoid organs shapes the type and magnitude of an immune response to an invading pathogen. There are numerous reports demonstrating the capabilities of DC in directing CD4+ T-cell differentiation (29, 32, 45, 67), but fewer experiments have addressed the function of DC subsets ex vivo. Furthermore, despite the fact that interactions of DC subsets with other species of Leishmania have been reported (37, 65), relatively little is known about the function of DC subsets in relation to long-term L. donovani infection.
In this study, we examined the changes in IL-12-related subunit mRNA accumulation and IL-12p70 protein in cDC subsets during various stages of infection and the relative capacity of each subset to shape the differentiation of TCR transgenic CD4+ T cells in vitro. We showed that cDC subsets have different capacities to secrete IL-12p70 and accumulate each subunit mRNA following infection of BALB/c mice and that the overall response profile of each DC subset was modified during the course of infection. During the early phase of infection (5 h to 24 h), cDC accumulated mRNA for IL-12/23p40, IL-12p35, and IL-23p19 to modest levels. However, accumulation of these mRNAs and the secretion of IL-12p70 was dramatically reduced during the course of infection. Furthermore, we showed that mRNA accumulation in cDC broadly correlated with CD4+ T-cell differentiation in vitro. While CD8α+ and DN splenic cDC subsets isolated from the acute phase of infection efficiently polarized naïve CD4+ T cells into IFN-γ-secreting cells, this bias was lost when the same subsets were isolated from chronic stages of infection. There is now strong evidence that the cytokine milieu has an overriding influence in shaping adaptive immune responses (10, 19, 21, 32, 41, 56, 64, 67). Consistent with previous reports on other pathogens (41, 45, 64, 67), we confirmed CD8α+ cDC as the dominant producer of IL-12p70 in response to L. donovani infection after 5 h, although CD4+ cDC also express low levels of this cytokine after a HD infection. In experimental VL, cDC were shown to be a critical source of early IL-12 production following infection (13, 15), and IL-12/23p40−/− mice on a B6 (Slc11a1 mutant) background were more permissive of hepatic (13, 40, 48) and splenic (13, 48) parasite growth. Nevertheless, whereas IL-12/23p40−/− mice had hepatic parasite burdens similar to those of IFN-γ−/− mice by week 12 p.i., this was not the case for IL-12p35−/− mice, suggesting a late-acting (12 weeks p.i.) role of IL-23 in the liver (40). Neither the outcome of L. donovani infection in mice lacking IL-23p19 nor the comparative effects of IL-12/23p40 and IL-12p35 deficiency in the spleen have been described. The mechanism(s) by which IL-12/23p40 mRNA accumulation and IL-12p70 protein secretion by splenic cDC was reduced during infection remain to be identified. Leishmania parasites are known to actively inhibit IL-12 production in infected macrophages, in addition to making macrophages refractory to normal IL-12-inducing stimuli (5, 15), but few DC are infected either early or late during infection (11, 30). However, IL-10, a factor known to suppress DC function and IL-12 production (35), is abundant in later stages of splenic infection (39, 54, 57).
Whereas the central role of IL-12 in the generation of Th1 cells has long been appreciated, IL-27 and IL-23, two cytokines structurally related to IL-12, were initially reported to play important roles in the regulation of IFN-γ production from naïve and memory T cells, respectively (23, 42, 61). Our real-time PCR analysis of the IL-27p28 subunit revealed that this mRNA accumulates in DC from infected mice compared to naïve mice and is maintained in CD8α+ cDC even at day 28 p.i.. Although preliminary data described a role for IL-27 in IFN-γ responses, it is now clear that IL-27 can also limit the intensity and duration of innate and adaptive immune responses (20). TCCR−/− mice, which lack IL-27 signaling, fail to mount an efficient Th1-type response in a Leishmania major model (1). In contrast, TCCR−/− mice mount a robust Th1 response after L. donovani infection and control parasite burdens significantly faster than wild-type mice do, although this was associated with development of liver pathology (46). In addition, we also showed that IL-23p19 mRNA accumulated transiently in DC, primarily in the CD8α+ subset. While IL-23 has been implicated in the expansion of IL-17-producing CD4+ T cells, to date, we have failed to detect CD4+ IL-17+ T cells in the spleens of infected mice (data not shown). In addition to cytokine-mediated mechanisms governing Th cell differentiation, other factors may also have played a role. Enhanced levels of costimulation may have altered the thresholds for T-cell activation, though no strict correlations between CD80 and CD86 expression have been found (34). Delta-Notch interactions have also recently received attention in inducing Th cell subset bias (50). However, as reported here, we have found no correlation between expression of Delta 4 Notch-like ligand and cDC subset behavior.
Finally, it has been reported by several laboratories that DC have the capacity to produce IFN-γ (17, 41, 56). By using intracellular cytokine staining, we directly identified that 60% of CD11chi IFN-γ+ cells in the spleens of L. donovani-infected mice are positive for DX5, likely representing a subset of NK cells defined as IKDC (6-8, 58, 63). These data highlight the fact that even under stringent sorting conditions, the functions attributed to cDC subsets may be biased by minor contaminant populations. Nevertheless, mirroring data from real-time RT-PCR, some CD11chi IFN-γ+ cells were DX5−, indicating a capacity to accumulate IFN-γ transcripts within the cDC population. Likewise, a small but significant contribution to IFN-γ production was made by CD8α+ and CD4+ DC. The early IL-12/23p40 and IL-27p28 produced by CD8α+ and CD4+ DC may suggest an autocrine pathway regulating IFN-γ in these subsets (28, 59). However, the signals required for IFN-γ production in DN cDC are as yet unknown.
In conclusion, we have shown that in response to infection with L. donovani, splenic cDC subsets differentially accumulated IL-12/23p40, IL-23p19, and IL-25p28 mRNA and secreted IL-12p70 during the course of the infection in a manner progressively likely to favor parasite survival. Further studies will be required to determine the functional importance of each cDC subset to the eventual outcome of infection.
Acknowledgments
This work was funded by a grant from the Wellcome Trust to P.M.K.
We thank M. Kullberg for critical reading of the manuscript and F. Powrie for providing DO11.10.SCID mice.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 12 November 2007.
REFERENCES
- 1.Artis, D., L. M. Johnson, K. Joyce, C. Saris, A. Villarino, C. A. Hunter, and P. Scott. 2004. Early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J. Immunol. 1724672-4675. [DOI] [PubMed] [Google Scholar]
- 2.Ato, M., A. Maroof, S. Zubairi, H. Nakano, T. Kakiuchi, and P. M. Kaye. 2006. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J. Immunol. 1765486-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ato, M., S. Stager, C. R. Engwerda, and P. M. Kaye. 2002. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat. Immunol. 31185-1191. [DOI] [PubMed] [Google Scholar]
- 4.Basu, A., G. Chakrabarti, A. Saha, and S. Bandyopadhyay. 2000. Modulation of CD11C+ splenic dendritic cell functions in murine visceral leishmaniasis: correlation with parasite replication in the spleen. Immunology 99305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 281389-1400. [DOI] [PubMed] [Google Scholar]
- 6.Blasius, A. L., W. Barchet, M. Cella, and M. Colonna. 2007. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J. Exp. Med. 2042561-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caminschi, I., F. Ahmet, K. Heger, J. Brady, S. L. Nutt, D. Vremec, S. Pietersz, M. H. Lahoud, L. Schofield, D. S. Hansen, M. O'Keeffe, M. J. Smyth, S. Bedoui, G. M. Davey, J. A. Villadangos, W. R. Heath, and K. Shortman. 2007. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J. Exp. Med. 2042579-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, C. W., E. Crafton, H. N. Fan, J. Flook, K. Yoshimura, M. Skarica, D. Brockstedt, T. W. Dubensky, M. F. Stins, L. L. Lanier, D. M. Pardoll, and F. Housseau. 2006. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat. Med. 12207-213. [DOI] [PubMed] [Google Scholar]
- 9.Cua, D. J., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421744-748. [DOI] [PubMed] [Google Scholar]
- 10.De Smedt, T., E. Butz, J. Smith, R. Maldonado-Lopez, B. Pajak, M. Moser, and C. Maliszewski. 2001. CD8alpha− and CD8alpha+ subclasses of dendritic cells undergo phenotypic and functional maturation in vitro and in vivo. J. Leukoc. Biol. 69951-958. [PubMed] [Google Scholar]
- 11.De Trez, C., M. Brait, O. Leo, T. Aebischer, F. A. Torrentera, Y. Carlier, and E. Muraille. 2004. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect. Immun. 72824-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engwerda, C. R., M. Ato, and P. M. Kaye. 2004. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 20524-530. [DOI] [PubMed] [Google Scholar]
- 13.Engwerda, C. R., M. L. Murphy, S. E. Cotterell, S. C. Smelt, and P. M. Kaye. 1998. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur. J. Immunol. 28669-680. [DOI] [PubMed] [Google Scholar]
- 14.Engwerda, C. R., S. C. Smelt, and P. M. Kaye. 1996. An in vivo analysis of cytokine production during Leishmania donovani infection in scid mice. Exp. Parasitol. 84195-202. [DOI] [PubMed] [Google Scholar]
- 15.Gorak, P. M., C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28687-695. [DOI] [PubMed] [Google Scholar]
- 16.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J. E. de Vries, and M. G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389737-742. [DOI] [PubMed] [Google Scholar]
- 17.Hochrein, H., K. Shortman, D. Vremec, B. Scott, P. Hertzog, and M. O'Keeffe. 2001. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 1665448-5455. [DOI] [PubMed] [Google Scholar]
- 18.Honda, K., K. Nakamura, N. Matsui, M. Takahashi, Y. Kitamura, T. Mizutani, N. Harada, H. Nawata, S. Hamano, and H. Yoshida. 2005. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm. Bowel Dis. 111044-1052. [DOI] [PubMed] [Google Scholar]
- 19.Huang, L. Y., C. Reis e Sousa, Y. Itoh, J. Inman, and D. E. Scott. 2001. IL-12 induction by a TH1-inducing adjuvant in vivo: dendritic cell subsets and regulation by IL-10. J. Immunol. 1671423-1430. [DOI] [PubMed] [Google Scholar]
- 20.Hunter, C. A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5521-531. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki, A., and B. L. Kelsall. 2001. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J. Immunol. 1664884-4890. [DOI] [PubMed] [Google Scholar]
- 22.Kamath, A. T., J. Pooley, M. A. O'Keeffe, D. Vremec, Y. Zhan, A. M. Lew, A. D'Amico, L. Wu, D. F. Tough, and K. Shortman. 2000. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 1656762-6770. [DOI] [PubMed] [Google Scholar]
- 23.Kamiya, S., T. Owaki, N. Morishima, F. Fukai, J. Mizuguchi, and T. Yoshimoto. 2004. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J. Immunol. 1733871-3877. [DOI] [PubMed] [Google Scholar]
- 24.Kato, T., R. Hakamada, H. Yamane, and H. Nariuchi. 1996. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J. Immunol. 1563932-3938. [PubMed] [Google Scholar]
- 25.Kaye, P. M. 1995. Costimulation and the regulation of antimicrobial immunity. Immunol. Today 16423-427. [DOI] [PubMed] [Google Scholar]
- 26.Kaye, P. M., A. J. Curry, and J. M. Blackwell. 1991. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J. Immunol. 1462763-2770. [PubMed] [Google Scholar]
- 27.Kaye, P. M., M. Svensson, M. Ato, A. Maroof, R. Polley, S. Stager, S. Zubairi, and C. R. Engwerda. 2004. The immunopathology of experimental visceral leishmaniasis. Immunol. Rev. 201239-253. [DOI] [PubMed] [Google Scholar]
- 28.Kelleher, P., and S. C. Knight. 1998. IL-12 increases CD80 expression and the stimulatory capacity of bone marrow-derived dendritic cells. Int. Immunol. 10749-755. [DOI] [PubMed] [Google Scholar]
- 29.Kirby, A. C., U. Yrlid, M. Svensson, and M. J. Wick. 2001. Differential involvement of dendritic cell subsets during acute Salmonella infection. J. Immunol. 1666802-6811. [DOI] [PubMed] [Google Scholar]
- 30.Lang, T., P. Ave, M. Huerre, G. Milon, and J. C. Antoine. 2000. Macrophage subsets harbouring Leishmania donovani in spleens of infected BALB/c mice: localization and characterization. Cell. Microbiol. 2415-430. [DOI] [PubMed] [Google Scholar]
- 31.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maldonado-Lopez, R., C. Maliszewski, J. Urbain, and M. Moser. 2001. Cytokines regulate the capacity of CD8alpha+ and CD8alpha− dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 1674345-4350. [DOI] [PubMed] [Google Scholar]
- 33.Manickasingham, S. P., A. D. Edwards, O. Schulz, and C. Reis e Sousa. 2003. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur. J. Immunol. 33101-107. [DOI] [PubMed] [Google Scholar]
- 34.Manzotti, C. N., M. K. Liu, F. Burke, L. Dussably, Y. Zheng, and D. M. Sansom. 2006. Integration of CD28 and CTLA-4 function results in differential responses of T cells to CD80 and CD86. Eur. J. Immunol. 361413-1422. [DOI] [PubMed] [Google Scholar]
- 35.McBride, J. M., T. Jung, J. E. de Vries, and G. Aversa. 2002. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell. Immunol. 215162-172. [DOI] [PubMed] [Google Scholar]
- 36.Miralles, G. D., M. Y. Stoeckle, D. F. McDermott, F. D. Finkelman, and H. W. Murray. 1994. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect. Immun. 621058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moll, H., and S. Flohe. 1997. Dendritic cells induce immunity to cutaneous leishmaniasis in mice. Adv. Exp. Med. Biol. 417541-545. [DOI] [PubMed] [Google Scholar]
- 38.Murphy, C. A., C. L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R. A. Kastelein, J. D. Sedgwick, and D. J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 1981951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray, H. W., C. M. Lu, S. Mauze, S. Freeman, A. L. Moreira, G. Kaplan, and R. L. Coffman. 2002. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 706284-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray, H. W., C. W. Tsai, J. Liu, and X. Ma. 2006. Responses to Leishmania donovani in mice deficient in interleukin-12 (IL-12), IL-12/IL-23, or IL-18. Infect. Immun. 744370-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J. Exp. Med. 1891981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13715-725. [DOI] [PubMed] [Google Scholar]
- 43.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 1675067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulendran, B., J. L. Smith, G. Caspary, K. Brasel, D. Pettit, E. Maraskovsky, and C. R. Maliszewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA 961036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R. N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1861819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosas, L. E., A. A. Satoskar, K. M. Roth, T. L. Keiser, J. Barbi, C. Hunter, F. J. de Sauvage, and A. R. Satoskar. 2006. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am. J. Pathol. 168158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacks, D., and A. Sher. 2002. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 31041-1047. [DOI] [PubMed] [Google Scholar]
- 48.Satoskar, A. R., S. Rodig, S. R. Telford III, A. A. Satoskar, S. K. Ghosh, F. von Lichtenberg, and J. R. David. 2000. IL-12 gene-deficient C57BL/6 mice are susceptible to Leishmania donovani but have diminished hepatic immunopathology. Eur. J. Immunol. 30834-839. [DOI] [PubMed] [Google Scholar]
- 49.Shortman, K., and S. H. Naik. 2007. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 719-30. [DOI] [PubMed] [Google Scholar]
- 50.Skokos, D., and M. C. Nussenzweig. 2007. CD8− DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J. Exp. Med. 2041525-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smeltz, R. B., J. Chen, R. Ehrhardt, and E. M. Shevach. 2002. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J. Immunol. 1686165-6172. [DOI] [PubMed] [Google Scholar]
- 52.Snijders, A., C. M. Hilkens, T. C. van der Pouw Kraan, M. Engel, L. A. Aarden, and M. L. Kapsenberg. 1996. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J. Immunol. 1561207-1212. [PubMed] [Google Scholar]
- 53.Stäger, S., J. Alexander, K. C. Carter, F. Brombacher, and P. M. Kaye. 2003. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect. Immun. 714804-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stager, S., A. Maroof, S. Zubairi, S. L. Sanos, M. Kopf, and P. M. Kaye. 2006. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur. J. Immunol. 361764-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stager, S., D. F. Smith, and P. M. Kaye. 2000. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J. Immunol. 1657064-7071. [DOI] [PubMed] [Google Scholar]
- 56.Stober, D., R. Schirmbeck, and J. Reimann. 2001. IL-12/IL-18-dependent IFN-gamma release by murine dendritic cells. J. Immunol. 167957-965. [DOI] [PubMed] [Google Scholar]
- 57.Svensson, M., A. Maroof, M. Ato, and P. M. Kaye. 2004. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity 21805-816. [DOI] [PubMed] [Google Scholar]
- 58.Taieb, J., N. Chaput, C. Menard, L. Apetoh, E. Ullrich, M. Bonmort, M. Pequignot, N. Casares, M. Terme, C. Flament, P. Opolon, Y. Lecluse, D. Metivier, E. Tomasello, E. Vivier, F. Ghiringhelli, F. Martin, D. Klatzmann, T. Poynard, T. Tursz, G. Raposo, H. Yagita, B. Ryffel, G. Kroemer, and L. Zitvogel. 2006. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 12214-219. [DOI] [PubMed] [Google Scholar]
- 59.Takeda, A., S. Hamano, A. Yamanaka, T. Hanada, T. Ishibashi, T. W. Mak, A. Yoshimura, and H. Yoshida. 2003. Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J. Immunol. 1704886-4890. [DOI] [PubMed] [Google Scholar]
- 60.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13251-276. [DOI] [PubMed] [Google Scholar]
- 61.Trinchieri, G., S. Pflanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19641-644. [DOI] [PubMed] [Google Scholar]
- 62.Villarino, A., L. Hibbert, L. Lieberman, E. Wilson, T. Mak, H. Yoshida, R. A. Kastelein, C. Saris, and C. A. Hunter. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19645-655. [DOI] [PubMed] [Google Scholar]
- 63.Vosshenrich, C. A., S. Lesjean-Pottier, M. Hasan, O. R. Goff, E. Corcuff, O. Mandelboim, and J. P. Di Santo. 2007. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J. Exp. Med. 2042569-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vremec, D., J. Pooley, H. Hochrein, L. Wu, and K. Shortman. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 1642978-2986. [DOI] [PubMed] [Google Scholar]
- 65.Williams, R. O. 1988. Invasion of murine dendritic cells by Leishmania major and L. mexicana mexicana. J. Parasitol. 74186-187. [PubMed] [Google Scholar]
- 66.Wilson, M. E., B. M. Young, B. L. Davidson, K. A. Mente, and S. E. McGowan. 1998. The importance of TGF-beta in murine visceral leishmaniasis. J. Immunol. 1616148-6155. [PubMed] [Google Scholar]
- 67.Yrlid, U., and M. J. Wick. 2002. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J. Immunol. 169108-116. [DOI] [PubMed] [Google Scholar]